Abstract
Measuring dissolved concentrations of emerging contaminants, such as polybrominated diphenyl ethers (PBDEs) and triclosan, can be challenging due to their physicochemical properties resulting in low aqueous solubilities and association with particles. Passive sampling methods have been applied to assess dissolved concentrations in water and sediments primarily for legacy contaminants. Although the technology is applicable to some emerging contaminants, the use of passive samplers with emerging contaminants is limited. In the present study, the performance of three common passive samplers was evaluated for sampling PBDEs and triclosan. Passive sampling polymers included low density polyethylene (PE) and polyoxymethylene (POM) sheets, and polydimethylsiloxane (PDMS) coated solid phase microextraction (SPME) fibers. Dissolved concentrations were calculated using measured sampler concentrations and laboratory derived partition coefficients. Dissolved tri-, tetra-, and pentabrominated PBDE congeners were detected at several of the study sites at very low pg/L concentrations using PE and POM. Calculated dissolved water concentrations of triclosan ranged from 1.7 to 18 ng/L for POM and 8.8 to 13 ng/L for PE using performance reference compound (PRC) equilibrium adjustments. Concentrations in SPME were not reported due to lack of detectable chemical in the PDMS polymer deployed. Although both PE and POM were found to effectively accumulate emerging contaminants from the water column, further research is needed to determine their utility as passive sampling devices for emerging contaminants.
Keywords: Passive sampling, Emerging contaminants, Polyethylene, Polyoxymethylene, SPME
INTRODUCTION
The occurrence of new or emerging contaminants in the aquatic environment has been a growing concern [1]. These emerging contaminants are not regularly monitored in the environment, but many are biologically active and suspected of causing adverse ecological and/or human effects (http://toxics.usgs.gov/regional/emc). Emerging contaminants encompass a wide range of compounds and may be classified as pharmaceuticals, pesticides/insecticides, personal care products or hormones [2]. More specifically, this includes polybrominated diphenyl ethers (PBDEs), which have been used for years as flame retardants in clothing textiles and furniture [3] and triclosan, an antimicrobial compound used in various consumer products [4].
Polybrominated diphenyl ethers are typically produced commercially as flame retardants in three mixtures. Pentabromodiphenyl ether (penta-BDE) is a product consisting primarily of PBDE congeners 47, 99, 100, 153 and 154. Octabromodiphenyl ether (octa-BDE) is a mixture of hexa- through nona-brominated congeners. The deca-product is composed almost entirely of PBDE congener 209 [5]. Due to their widespread global use, PBDEs have been detected in numerous media including air, water, sediments, aquatic organisms and humans [3, 6–7]. They persist in the environment, have the capability to bioaccumulate and biomagnify in the food chain, and have been found to cause a variety of adverse ecotoxicological effects including enzyme disruption, hepatic disease, thyroid modifications and acute toxicity [6–7].
The antimicrobial agent triclosan is used in household products, such as shampoo, toothpaste, soap and deodorant. Additionally, triclosan is a component of plastic products, including kitchen utensils and children’s toys [4]. Given its extensive use, triclosan has been detected in wastewater, surface water, sewage sludge and sediments [1, 8–14]. It has also been found to cause acute and chronic effects in aquatic organisms [15–16].
Measuring dissolved concentrations of emerging contaminants, such as PBDEs and triclosan, can be challenging due to their physicochemical properties resulting in low aqueous solubilities and association with particles. For example, the aqueous solubility of PBDE congeners range from approximately 2 × 10−3 to 0.1 mg/L [17–18] and have log KOW values of 5.74 to almost 10 [18–20]. Triclosan is more soluble with a solubility of 12 mg/L and log KOW of 4.8 [21]. As a consequence of their physicochemical properties, both PBDEs and triclosan would be expected to occur primarily in sediments within estuarine systems and, in comparison to other contaminants of concern (e.g., polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs)) relatively few investigations have measured these emerging contaminants dissolved in the estuarine water column. The dissolved concentration is critical since it is considered to be the best measure of bioavailability in aquatic systems [22] and is necessary to perform accurate risk assessments. Recently, passive sampling methods have been applied for measuring dissolved concentrations in water and sediments using semi-permeable membrane devices (SPMDs), polyethylene (PE), polydimethylsiloxane (PDMS) and polyoxymethylene (POM) [23–26]. To date, the emphasis of passive sampler development and use has been on monitoring legacy contaminants (e.g., PAHs, PCBs, chlorinated pesticides), but the technology should be applicable to some emerging contaminants although data for this class of chemicals is currently very limited [27–29].
The results presented in the present study are components of a two part assessment of the use of passive samplers in the marine environment. For the present study, the performance of three common passive samplers (PE, PDMS, and POM) was evaluated for sampling PBDEs (congeners 17, 47, 71, 99, 100, 183, 209) and triclosan in the natural waters of several stations in the temperate estuary Narragansett Bay (Rhode Island, USA). Narragansett Bay has a history of industrial activity, which has contaminated the sediments in portions of the bay with legacy contaminants [30–31]. Results of the performance of the passive samplers with legacy contaminants are discussed in Perron et al. [32]. Narragansett Bay is surrounded by suburban and urban areas and contamination by emerging contaminants is highly likely. Dissolved concentrations of each emerging contaminant were determined using partitioning calculations described in Perron et al. [32] based on relationships developed by others (e.g., Huckins et al. [23]). This investigation had two specific objectives: (1) compare passive sampler effectiveness for water column monitoring of selected emerging contaminants in the marine environment, and (2) derivation of partition coefficients for triclosan and PBDEs.
Methods
Materials
Chemical stock solutions of triclosan and methyl triclosan were prepared by Ultra Scientific in acetone. Neat PBDEs were purchased from Cambridge Isotope Laboratories. Fluorinated PBDEs (FBDEs) in methanol were purchased from Sigma-Aldrich. Labeled methyl triclosan in nonane was purchased from Wellington Laboratories. Supplemental Data, Table S1 provides a list of target chemicals, performance reference compounds (PRCs) and internal standards.
Low-density PE (25 μm thickness; Covalence Plastics) and POM (75 μm; CS Hyde Company) were cut into strips of 15 cm × 40 cm and 6 cm × 40 cm, respectively. Strips of PE and POM were pre-cleaned by soaking in acetone for 24 h and then in dichloromethane (DCM) for 24 h. Solid-phase microextraction fibers (200 μm inner silica core with 10 μm outer PDMS; Fiberguide Industries) were cut to 2.5 cm in length and pre-cleaned as described above for PE and POM.
Field Site Locations and Deployment
Strips of PE and POM were soaked in a PRC solution (80:20 methanol:water with FBDEs and 13C-methyl triclosan) for at least 28 days on an orbital shaking table. Each PRC jar contained four sampler strips and 900 mL aqueous PRC solution. After soaking, strips of PE and POM were removed from the PRC solution, one strip of each sampler was taken from each PRC solution jar for measuring pre-deployment PRC concentrations, and the remaining strips attached to stainless steel wire (diameter = 0.032 in.; Malin) inside galvanized extended minnow traps (diameter = 22 cm, length = 30 in.; Tackle Factory). Minnow traps were used to protect samplers from biotic and abiotic threats. Fifteen pre-cut SPME fibers were placed inside a fine copper mesh (TWP) hand-made envelope and the envelope attached to the inside of the trap by stainless steel wire. Inside each trap, three strips of PE and POM were attached to maximize their surface area. Three SPME envelopes were also attached to the inside of each trap. Passive sampler traps were deployed approximately one meter above the sediment surface for 21 days at six site locations in Narragansett Bay (RI, USA) (Supplemental Data, Table S2). Four deployments (Greenwich Bay, Bristol Harbor, Mount Hope Bay and Newport Harbor) were from water-based U.S. Coast Guard navigational buoys. Two deployments (U.S. EPA and Providence River) were from docks at site locations.
Partition Coefficient Derivation
Partition coefficients were obtained by plating chemical solutions onto the sides of a 250 mL amber jar before adding 250 ml of Milli-Q water and approximately 5 mg of a sampler. Plating involved discharging chemical solutions prepared in an organic solvent onto the jar interior wall while slowly rotating the jar and allowing the solvent to evaporate leaving the chemical residue. All operations with the stock solutions were performed under low level laboratory lighting away from direct sunlight to avoid any photodegradation and sodium azide was added to each jar to prevent contaminant microbial degradation. Jars were then placed on an orbital shaking table for 56 days, which was found to be sufficient for equilibration in prior kinetics studies (data not shown). Water and samplers were extracted as described below.
Extraction
Passive samplers were weighed and extracted sequentially with acetone and DCM for 24 hours each. Supplemental Data, Table S1 indicates the internal standards used for each contaminant type. Samplers retrieved from the field were wiped clean of water and epiphytes before extraction. Acetone and DCM extracts were combined, solvent exchanged to hexane, and volume reduced to 1 ml under a stream of nitrogen gas. Water samples from partition coefficient derivation studies were extracted twice with pentane. Extracts were combined, treated with sodium sulfate, solvent exchanged to hexane, and volume reduced to 1 ml. For triclosan analysis, extract subsamples (100 μL) were evaporated to dryness under a nitrogen gas stream and derivatized for 1 hr with 50 μL of N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide. Extract volumes were brought up to 100 μL using ethyl acetate and analyzed within 24 hr of derivatization [16].
Instrumental Analysis
Analyses of triclosan and methyl triclosan were performed on an Agilent 7890 gas chromatograph equipped with a 5975 mass selective detector (Agilent Technologies) operated in selective ion monitoring mode. Analytes and internal standards were separated using an Agilent DB-5MS capillary column (30 m length; 250 μm diameter; 0.25 μm thickness) and quantified with a five or six point calibration curve. Analyses of PBDEs and FBDEs were performed on an Agilent 6890 gas chromatograph equipped with a 5973 mass selective detector (Agilent Technologies) operated in selective ion monitoring mode utilizing negative chemical ionization. Analytes and internal standards were separated using an Agilent DB-5 capillary column (15 m length; 250 μm diameter; 0.1 μm thickness) and quantified with a five point calibration curve.
Calculation of Dissolved Concentrations
Dissolved water concentrations (CD) were calculated using the following equation:
where Ksampler is the chemical-specific passive sampler-water partition coefficient adjusted for the presence of salt using Setschenow constants [33], Csampler is the measured sampler concentrations, and ke is the mass transfer coefficients obtained from PRC equilibration [32]. FBDEs and labeled methyl triclosan were used as PRCs for PBDEs and triclosan, respectively (Supplemental Data, Table S1). In cases where PRC corrections were not used (see discussion below), the equation above is simplified by having the (1 − e−ket) term removed from the denominator and CD is calculated as the ratio of the measured sampler concentration (Csampler) and chemical-specific passive sampler-water partition coefficient (Ksampler) adjusted for the presence of salt using Setschenow constants.
RESULTS AND DISCUSSION
Partition coefficients
Partition coefficients for PE, POM and SPME were obtained for PBDEs, triclosan and methyl triclosan (Table 1). For PBDEs, Ksampler values for each sampler are plotted against log KOW values and the findings discussed below. Values obtained for triclosan and methyl triclosan are discussed individually.
Table 1.
Log octanol-water (log Kow) and log passive sampler-water partition coefficient values (log KPS; L/kg) derived in the laboratory for polyethylene (PE), polyoxymethylene (POM) and solid phase microextraction (SPME) fibers with polydimethylsiloxane (PDMS) coating. Mean and one standard deviation are presented. Statistical p-values are presented for the comparison of passive sampler partition coefficients for each congener (i.e., log KPE vs. log KPOM vs. log KSPME).
| Chemical | Octanol-water partition coefficient (log Kow) | PE-water partition coefficient (log KPE) | POM-water partition coefficient (log KPOM) | SPME-water partition coefficient (log KSPME) | ANOVA p-value |
|---|---|---|---|---|---|
| Polybrominated diphenyl ethers (PBDEs) | |||||
|
| |||||
| PBDE 17 | 5.74a | 5.17±0.04 | 5.42±0.03 | 5.92±0.15 | 0.0002 |
| PBDE 47 | 6.81a | 6.20±0.20 | 6.29±0.13 | 6.61±0.42 | 0.26 |
| PBDE 71 | 6.54a | 5.98±0.15 | 6.03±0.10 | 6.52±0.37 | 0.059 |
| PBDE 99 | 7.32a | 6.74±0.54 | 6.78±0.27 | 7.16±0.35 | 0.42 |
| PBDE 100 | 7.24a | 6.55±0.49 | 6.60±0.23 | 7.01±0.35 | 0.33 |
| PBDE 183 | 8.27a | 6.22±1.16 | 6.54±0.62 | 7.63±0.13 | 0.14 |
| PBDE 209 | 9.97b | 5.11±1.04 | 5.76±0.19 | 6.79±0.42 | 0.054 |
|
| |||||
| Triclosan | |||||
|
| |||||
| Triclosan | 4.80c | 2.44±0.40 | 3.79±0.26 | 4.06±0.13 | |
|
| |||||
| Methyltriclosan | |||||
|
| |||||
| Methyltriclosan | 5.20d | 4.41±0.10 | 5.27±0.18 | 4.90±0.15 | |
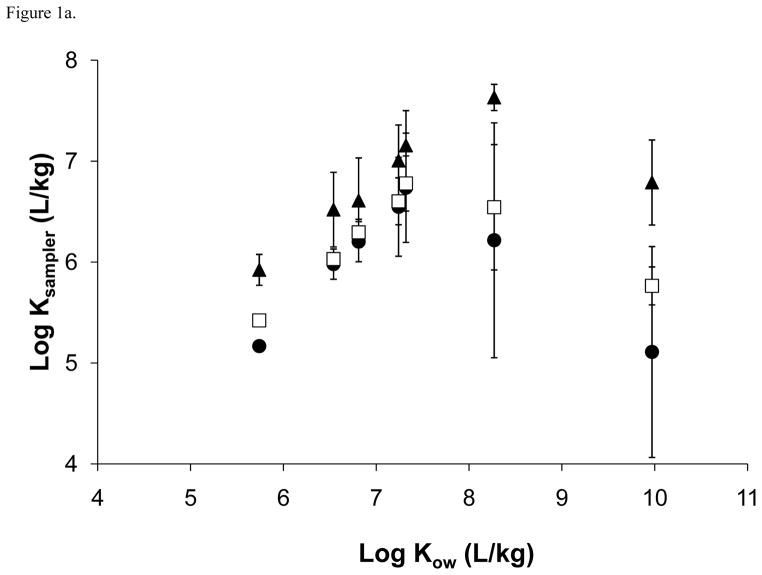
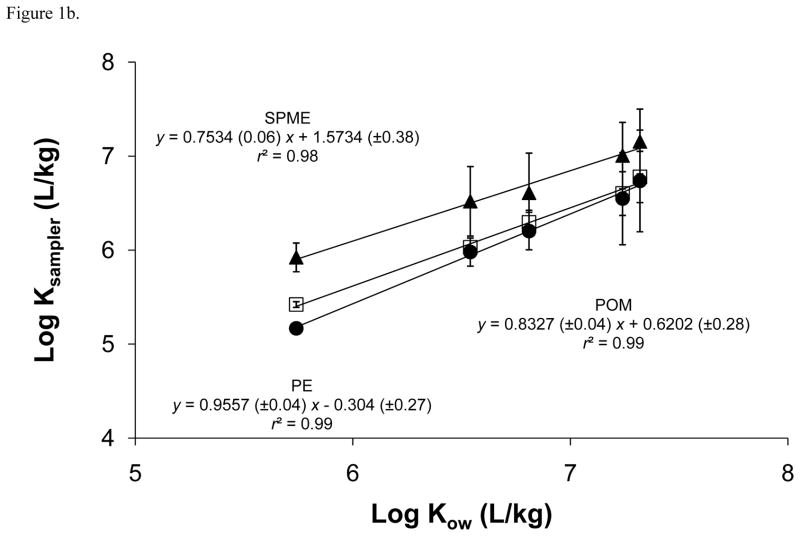
For PE, a good correlation between chemical-specific PE partition coefficients (KPE) and KOW was observed for most of the PBDE congeners with the exception of 183 and 209 (r = 0.997 with removal of these congeners; Figure 1). These two congeners have log KOW values over 8 and would be susceptible to energy differences needed for cavity formation in octanol versus the passive sampler polymer. That is, the Gibbs free energy required for cavity formation in the passive sampler polymer becomes much higher for larger chemicals while the amount of energy needed for cavity formation in octanol changes little for the same chemicals [34]. Bao et al. [20] observed a similar linear relationship for most of the 23 PBDE congeners they studied (Table 2), such that log KPE values increased with increasing log KOW until a plateau at log KOW of ~8.3 was reached followed by a decrease in log KPE with increasing log KOW. Sacks and Lohmann [29] obtained log KPE values for individual PBDE congeners (Table 2), but due to the presence of a third nonane phase in their partitioning experiments apparent partition coefficients were much lower than those derived in the present study and Bao et al. [20]. After accounting for partitioning into this third nonane phase, adjusted values from Sacks and Lohmann [29] were much higher than the range observed in the present study (Table 1) and Bao et al. [20].
Figure 1.
(a) PBDE sampler-water partition coefficients (logKsampler; L/kg) for polyethylene (PE; ●), polyoxymethylene (POM; □), and solid phase microextraction fibers (SPME; ▲) plotted against octanol-water partition coefficients (logKOW; L/kg) and (b) the linear portions of the logKsampler and log KOW relationships.
Table 2.
Experimentally derived polyethylene-water partition coefficients (log KPE; L/kg) measured for polybrominated diphenyl ethers (PBDEs) obtained from the literature. For values from the present study, see Table 1.
| PBDE congener | Bao et al. [20] | Sacks and Lohmann [29] (apparent)a | Sacks and Lohmann [29] (accounting for third phase)a |
|---|---|---|---|
| 17 | 5.43 | ||
| 47 | 6.25 | 5.01 | 7.18 |
| 71 | 6.02 | ||
| 99 | 6.88 | 5.02 | 7.79 |
| 100 | 6.82 | 5.02 | 8.05 |
| 183 | 7.39 | 5.07 | 9.11 |
| 209 | 5.61 |
Converted to L/kg using ρPE = 0.92 g/mL
A linear relationship was observed again with SPME for PBDEs with a plateau at log KOW of ~8.3 (Figure 1a). Good correlation was observed for congeners below this log KOW (r = 0.992; Figure 1b). ter Laak et al. [35] reported log SPME-water partition coefficient (KSPME) values for three of the same congeners measured in the present study. All three values were found to be approximately a magnitude lower than the values obtained in the current study. A similar linear relationship was again observed using POM with the same plateau as PE and SPME (Figure 1a) and a good correlation found below the plateau (r = 0.996; Figure 1b). To our knowledge, this is the first study to report POM-water partition coefficient (KPOM) values for PBDEs, therefore we were unable to compare our values to those obtained in other studies.
When comparing partition coefficients for each congener across passive sampler polymers (e.g., KPE vs. KPOM vs. KSPME for PBDE congener 100), no significant differences (p=0.05) are observed except for PBDE congener 17 (Table 1). This indicates that for most of the PBDE congeners measured, congener specific partition coefficients were approximately the same regardless of the type of passive sampler polymer utilized. Since these partition coefficients were derived within the same laboratory, inter-laboratory variability was removed and this comparison can be performed. The lack of statistical significance between passive sampler partition coefficients suggests that despite substantial differences in their chemical structures and compositions, the partitioning of nonionic organic chemicals from aqueous solution occurs with the same degree of affinity between polymers. For example, relative to chemical composition, POM contains oxygen along with carbon and hydrogen, the only atoms in PE, while PDMS also contains silicon and oxygen atoms.
Although triclosan and methyl triclosan values were not plotted, it should be noted that only one other investigation has published partition coefficients for these chemicals. Sacks and Lohmann [28] found log KPE values of 3.34 and 4.53 for triclosan and methyl triclosan, respectively. The value obtained in the present study for methyl triclosan was only slightly lower at 4.41, but the log KPE for triclosan was much lower at 2.44. This difference may be due to the polarity of the chemical and/or differences in chemical analysis used in the two studies. In the current study, derivatization was used to analyze triclosan, where as Sacks and Lohmann [28] did not derivatize. Derivatization is known to improve instrument sensitivity when measuring triclosan [36–38]. Of the chemicals investigated in the present study, triclosan is the only one which has an ionic form (i.e., pKa = 8.2). It is possible that this characteristic may result in greater variability and differences between studies. As far as we know, this is the first study to report KPOM and KSPME values for triclosan and methyl triclosan, therefore we are unable to compare our values with those obtained in other studies.
Dissolved Concentrations of PBDEs
Since PBDEs are used in a wide range of commercial products, the waste resulting from these materials is believed to be the main source of PBDEs into the environment [6]. This includes discharge from waste water treatment plants, waste incineration and leaching from landfill sites [6]. In Narragansett Bay, all of these may be considered potential sources of PBDEs. Additionally, when in the aquatic environment, it is known that these types of organic contaminants, which over time have accumulated in the sediments, can be released into the water column under the right conditions (e.g., storms, shipping-related resuspension) making the sediments a non-point source.
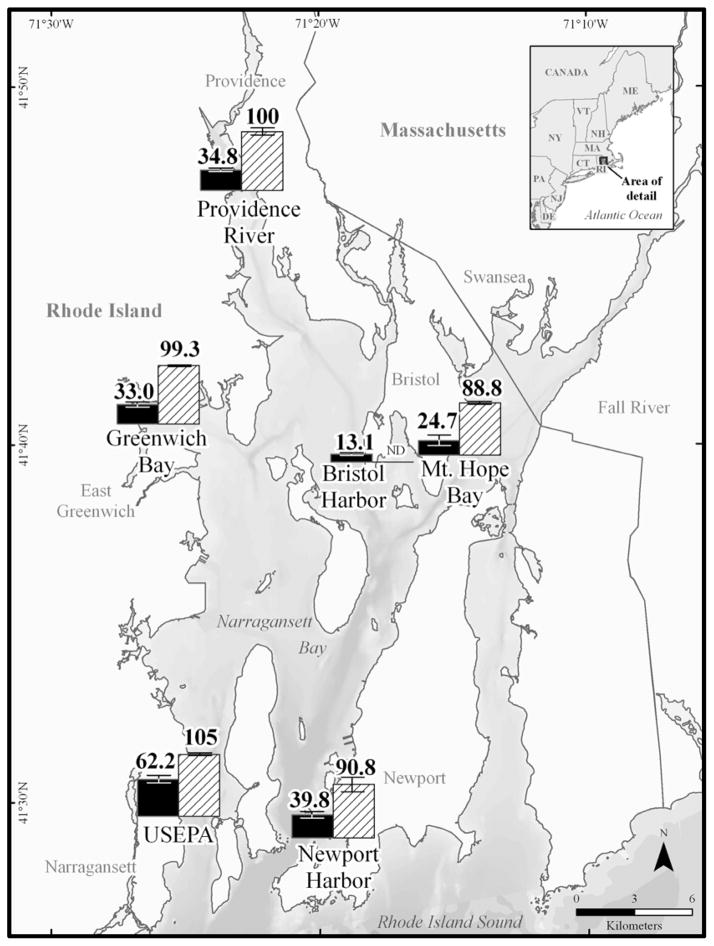
Dissolved PBDE concentrations calculated using the measured PE sampler concentrations (Supplemental Data, Figure S1) with PRC adjustments and the chemical-specific partition coefficients described above (Supplemental Data, Table S1) yielded very low levels (pg/L) (Figure 2, Supplemental Data, Table S3). Results are only shown for PE and POM because contaminants were not detectable in the SPME samplers. The lack of detectable chemicals in the SPME replicates suggests that not enough PDMS polymer was deployed. As a result of a tropical storm-damaged cage, POM replicates were lost at the Bristol Harbor site and no data are shown. Tri-, tetra-, and pentabrominated congeners (17, 47, 71, 99 and 100) were detected at several of the study sites while the hepta- and decabrominated congeners (183 and 209) were not detected at any of them. A study by Sacks and Lohmann [29] in the same estuary measured dissolved PBDE concentrations using PE (25 μm thickness) and found similar results. The majority of the congeners detected in their study were tetra- and pentabrominated congeners. Dissolved concentrations of their most abundant congener (PBDE 47) ranged from 0.18 to 2.3 pg/L. In the present study, dissolved concentrations of the same congener based on PE were measured at slightly higher concentrations (1.94–11.9 pg/L) (Supplemental Data, Table S3).
Figure 2.
Calculated mean dissolved concentrations (pg/L) of total polybrominated diphenyl ethers (PBDEs) in polyethylene (PE) and polyoxymethylene (POM) at the six study sites in Narragansett Bay, RI. Concentrations derived from PE adjusted with performance reference compounds (PRCs) (black bars), and non-PRC adjusted POM (hashed bars) are presented. ND denotes no data for the Bristol Harbor site where POM replicates were lost due to a tropical storm. Error bars represent one standard deviation (SD).
Although water concentrations could be calculated for five PBDE congeners using PRC-adjusted PE data, concentrations could not be obtained in the same manner using POM due to poor PRC behavior indicating equilibration was less than 10%. Three fluorinated PBDEs (FBDE 28, 100 and 208) were used as PRCs to account for non-equilibration of the samplers after retrieval from the field. With POM, issues arose with these PRCs, such that very low or no equilibration was observed for all of the deployments. As a result, dissolved concentrations could not be calculated using the measured PBDE concentrations in the POM and PRC adjustments. The cause of this abnormal behavior of FBDEs with POM is unknown, but could be attributed to the structure of the POM. The POM contains a repeating oxygen-containing group (i.e., ether), which may result in unexpected intermolecular interactions with compounds like FBDEs. For example, in general, nonionic organic chemical and polymer intermolecular interactions are dominated by van der Waals dispersive forces [33]. This is especially the case for PE which is composed of only carbon and hydrogen. In contrast, the ether groups in the POM may result in electron donor-acceptor interactions between the oxygens and the FBDEs that are stronger than the dispersive forces [33]. Consequently, the rate of PRC release from the polymer would not be the same as the uptake of measured contaminants by the polymer. Perron et al. [32] reported similar behavior by PRCs with PCBs using POM. Recognizing that the PRCs were not functioning with the PBDEs, non-PRC adjusted POM concentrations, based on the assumption of equilibrium conditions, yielded dissolved concentrations ranging from 89 to 105 pg/L, which were 1.7 to 3.6 times higher than those observed with PE.
Dissolved Concentrations of Triclosan
Many personal care products and consumer goods that are most often disposed of in household wastewater drains contain triclosan. As a result, triclosan primarily enters the environment through municipal wastewater treatment effluent [9]. Removal efficiencies vary depending on the type of removal processes used by the wastewater treatment plant [39]. There are 35 wastewater treatment plants that discharge into the Narragansett Bay watershed (http://www.savebay.org/page.aspx?pid=335). For the present study, two stations were located near wastewater treatment plants. The state’s largest wastewater treatment facility, Fields Point, provides preliminary, primary, and secondary treatment to Providence and the surrounding area. This facility’s effluent discharge is located near the Providence River site. The East Greenwich Wastewater Treatment Plant is located near the Greenwich Bay site. This smaller facility provides up to tertiary treatment and discharges directly into Greenwich Bay.
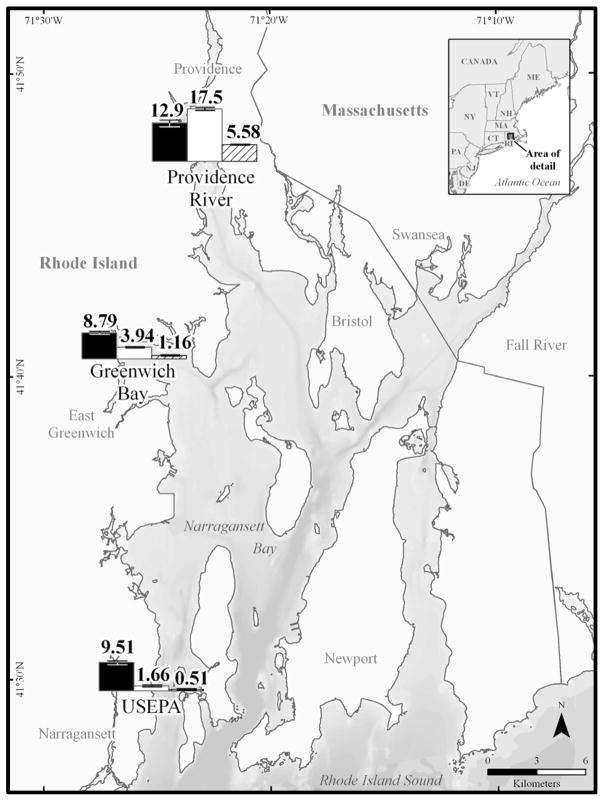
Due to cost restraints, the PRC for triclosan (13C-labeled methyl triclosan) was only used at three stations (U.S EPA, Greenwich Bay, and Providence River); therefore triclosan concentrations are only shown for these stations. As noted with PBDEs, contaminants were not detectable in SPME and POM replicates were lost at the Bristol Harbor site therefore no data are shown. Calculated triclosan water concentrations using measured PE concentrations (Supplemental Data, Figure S2) and PRC adjustments were approximately the same at all three sites (8.8 to 13 ng/L). Conversely, calculated dissolved water concentrations of triclosan ranged from 1.7 to 18 ng/L for POM adjusted with PRC data (Figure 3, Supplemental Data, Table S3). Non-PRC adjusted POM resulted in lower triclosan concentrations at all three sites (~70% decrease from PRC adjusted POM), but still displayed a range from 0.5 to 5.6 ng/L. Water concentrations of triclosan would be anticipated to vary between sites given the Providence River and Greenwich Bay sites are near waste water treatment plant outfalls, which are direct sources of triclosan into Narragansett Bay [40]. On the other hand, expected triclosan levels would be lower at the U.S. EPA site, which is not located near a waste water treatment plant and is closer to open ocean conditions. The differences between PE and POM for this compound are likely due again to the polymer structure of the samplers. As mentioned above, POM contains a repeating polar ether group (and the potential for the associated electron donor-acceptor interactions) that is not present in the exclusively carbon and hydrogen structure of PE, which may result in POM having a higher affinity for polar chemicals such as triclosan [27]. Consequently, it may be a more analytically sensitive sampler for this compound because of its preferential accumulation in the polymer. This is also displayed by the higher partition coefficient for triclosan with POM (log KPOM = 3.79) as compared to PE (log KPE = 2.44). Whether POM is acting as an equilibrium sampler for relatively polar organic contaminants could be arguable. Given these interactions, it might be behaving as an integrative sampler [23].
Figure 3.
Calculated mean dissolved concentrations (ng/L) of triclosan in polyethylene (PE) and polyoxymethylene (POM) at three of the study sites in Narragansett Bay, RI. Concentrations derived from PE adjusted with performance reference compounds (PRCs) (black bars), PRC- adjusted POM (white bars), and non-PRC adjusted POM (hashed bars) are presented. Error bars represent one standard deviation (SD).
Sacks and Lohmann [28] conducted a study in Narragansett Bay at similar study sites and were unable to detect triclosan in the water using PE. We would expect that triclosan would be detected by the passive sampler. Dissolved concentrations of triclosan have been measured in the water column of the same study area using large volume water extraction methods [40] at levels ranging from 0.5–7.4 ng/L, which are comparable to dissolved concentrations calculated in the present study. As discussed earlier, derivatization was not utilized in triclosan analyses by Sacks and Lohmann [28], which can result in reduced analytical sensitivity. Additionally, as observed in the present study, PE may not be the best suited sampler for triclosan due to its relatively low affinity for the polymer.
CONCLUSIONS
Water column deployments demonstrated the strengths and weaknesses of the passive samplers evaluated. First, relative to the selection of the type of passive sampler for water column deployments, both PE and POM were effective at accumulating emerging contaminants, but the use of SPME in the fiber configuration was problematic due to their small size, fragility and large masses needed to achieve acceptable analytical sensitivity. Use of SPME, and more specifically PDMS, in configurations with greater surface areas and masses (e.g., sheets, rings) is worth investigating for water column deployments. Second, to insure confidence in the dissolved concentrations, it is critical that the contaminants and samplers achieve equilibrium in the water column or that equilibrium conditions can be estimated. Here, equilibrium conditions were estimated based on the use of PRCs; however, we observed that issues can arise when using PRCs with certain polymers. For example, although POM accumulated contaminants, using PRCs with POM was problematic with the PBDEs. Application of PRCs with POM is not as commonly practiced as with PE and typically equilibrium is assumed for calculating dissolved concentrations with POM. Given the importance of establishing equilibrium conditions, the performance of water column studies comparing dissolved concentrations from passive samplers to measured dissolved concentrations from large volume extractions would be beneficial. Finally, analytical sensitivity to and polymer affinity for the target chemicals can influence the selection of the type of passive sampler; for example, POM very effectively accumulated triclosan as compared to PE which improved the analytical sensitivity. Understanding the strengths and weaknesses of passive samplers for specific applications will assist in designing scientifically-robust and effective studies while also advancing the acceptance of passive samplers as environmental monitoring tools.
Supplementary Material
Acknowledgments
The authors would like to thank D Katz, J Sullivan, P Pelletier, and D Cobb for their help with field and laboratory work. We appreciate the technical reviews provided by K Ho, P Pelletier, and B Bergen of this manuscript. This project was supported by Grant Number P42ES013660 (Brown University Superfund Research Program) from the National Institute of Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institute of Environmental Health Sciences or the National Institutes of Health. This report has been reviewed by the U.S. EPA’s Office of Research and Development, National Health and Environmental Effects Research Laboratory, Atlantic Ecology Division and approved for publication (ORD Tracking No. ORD-003227). Approval does not signify that the contents necessarily reflect the views of the Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
References
- 1.Novak PJ, Arnold WA, Blazer VS, Halden RU, Klaper RD, Kolpin DW, Kriebel D, Love NG, Martinović-Weigelt D, Patisaul HB, Snyder SA, vom Saal FS, Weisbrod AV, Swackhamer DL. On the need for a national (U.S.) research program to elucidate the potential risks to human health and the environment posed by contaminants of emerging concern. Environ Sci Technol. 2011;45:3829–3830. doi: 10.1021/es200744f. [DOI] [PubMed] [Google Scholar]
- 2.Richardson SD. Water analysis: emerging contaminants and current issues. Anal Chem. 2007;79:4295–4324. doi: 10.1021/ac070719q. [DOI] [PubMed] [Google Scholar]
- 3.Rahman F, Langford KH, Scrimshaw MD, Lester JM. Polybrominated diphenyl ether (PBDE) flame retardants. Sci Total Environ. 2001;275:1–17. doi: 10.1016/s0048-9697(01)00852-x. [DOI] [PubMed] [Google Scholar]
- 4.Daughton CG, Ternes T. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect. 1999;107:907–937. doi: 10.1289/ehp.99107s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta- analysis of concentrations. Environ Sci Technol. 2004;38:945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- 6.Darnerud PO, Eriksen GS, Johannesson T, Larsen PB, Viluksela M. Polybrominated diphenyl ethers: occurrence, dietary exposure and toxicology. Environ Health Perspect. 2001;109:49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- 8.McAvoy DC, Schatowitz B, Jacob M, Hauk A, Eckhoff WS. Measurement of triclosan in wastewater treatment systems. Environ Toxicol Chem. 2002;21:1323–1329. [PubMed] [Google Scholar]
- 9.Halden RU, Paull DH. Co-occurrence of triclocarban and triclosan in US water resources. Environ Sci Technol. 2005;39:1420–1426. doi: 10.1021/es049071e. [DOI] [PubMed] [Google Scholar]
- 10.Kinney CA, Furlong ET, Kolpin DW, Burkhardt MR, Steven D, Zaugg SD, Werner SL, Bossio JP, Benotti MJ. Bioaccumulation of pharmaceuticals and other anthropogenic waste indicators in earthworms from agricultural soil amended with biosolid or swine manure. Environ Sci Technol. 2008;42:1863–1870. doi: 10.1021/es702304c. [DOI] [PubMed] [Google Scholar]
- 11.Singer H, Muller S, Tixier C, Pillonel L. Triclosan: Occurrence and fate of a widely used biocide in the aquatic environment: field measurements in wastewater treatment plants, surface waters and lake sediments. Environ Sci Technol. 2002;36:4998–5004. doi: 10.1021/es025750i. [DOI] [PubMed] [Google Scholar]
- 12.Morales S, Canosa P, Rodriguez I, Rubi E, Cela R. Microwave assisted extraction followed by gas chromatography with tandem mass spectrometry for the determination of triclosan and two related chlorophenols in sludge and sediments. J Chromatogr A. 2005;1082:128–135. doi: 10.1016/j.chroma.2005.05.059. [DOI] [PubMed] [Google Scholar]
- 13.Miller TR, Heidler J, Chillrud SN, DeLaquil A, Ritchie JC, Mihalic JN, Bopp R, Halden RU. Fate of triclosan and evidence for reductive dechlorination of triclocarban in estuarine sediments. Environ Sci Technol. 2008;42:4570–4576. doi: 10.1021/es702882g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantwell MG, Wilson BA, Zhu J, Wallace GT, King JW, Olsen CR, Burgess RM, Smith JP. Temporal trends of triclosan contamination in dated sediment cores from four urbanized estuaries: evidence of preservation and accumulation. Chemosphere. 2010;78:347–352. doi: 10.1016/j.chemosphere.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Dann AB, Hontela A. Triclosan: environmental exposure, toxicity and mechanisms of action. J Appl Toxicol. 2011;31:285–311. doi: 10.1002/jat.1660. [DOI] [PubMed] [Google Scholar]
- 16.Perron MM, Ho KT, Cantwell MG, Burgess RM, Pelletier MC. Effects of triclosan on marine benthic and epibenthic organisms. Environ Chem Toxicol. 2012;31:1861–1866. doi: 10.1002/etc.1884. [DOI] [PubMed] [Google Scholar]
- 17.Tittlemier SA, Halldorson T, Stern GA, Tomy GT. Vapor pressures, aqueous solubilities, and Henry’s law constants of some brominated flame retardants. Environ Toxicol Chem. 2002;21:1804–1810. [PubMed] [Google Scholar]
- 18.Wania F, Dugani CB. Assessing the long-range transport potential of polybrominated diphenyl ethers: a comparison of four multimedia models. Environ Toxicol Chem. 2003;22:1252–1261. [PubMed] [Google Scholar]
- 19.Braekevelt E, Tittlemier SA, Tomy GT. Direct measure of octanol-water partition coefficients of some environmentally relevant brominated diphenyl ether congeners. Chemosphere. 2003;51:563–567. doi: 10.1016/S0045-6535(02)00841-X. [DOI] [PubMed] [Google Scholar]
- 20.Bao L, You J, Zeng EY. Sorption of PBDE in low-density polyethylene film: implications for bioavailability of BDE-209. Environ Toxicol Chem. 2011;30:1731–1738. doi: 10.1002/etc.564. [DOI] [PubMed] [Google Scholar]
- 21.Reiss R, Mackay N, Habig C, Griffin J. Ecological risk assessment for triclosan in lotic systems following discharge from wastewater treatment plants in the United States. Environ Toxicol Chem. 2002;21:24843–2492. [PubMed] [Google Scholar]
- 22.Di Toro DM, Zarba CS, Hansen DJ, Berry WJ, Swartz RC, Cowan CE, Pavlou SP, Allen HE, Thomas NA, Paquin PR. Technical basis for establishing sediment quality criteria for nonionic organic chemicals using equilibrium partitioning. Environ Toxicol Chem. 1991;10:1541–1583. [Google Scholar]
- 23.Huckins JN, Petty JD, Booij K. Monitors of Organic Chemicals in the Environment. Springer; New York, NY, USA: 2006. [Google Scholar]
- 24.Hawthorne SB, Grabanski CB, Miller DJ, Kreitinger JP. Solid-phase microextraction measurement of parent and alkyl polycyclic aromatic hydrocarbons in milliliter sediment pore water samples and determination of KDOC values. Environ Sci Technol. 2005;39:2795–2803. doi: 10.1021/es0405171. [DOI] [PubMed] [Google Scholar]
- 25.Maruya KA, Zeng EY, Tsukada D, Bay SM. A passive sampler based on solid-phase microextraction for quantifying hydrophobic organic contaminants in sediment pore water. Environ Toxicol Chem. 2009;28:733–740. doi: 10.1897/08-322R.1. [DOI] [PubMed] [Google Scholar]
- 26.Gschwend PM, MacFarlane JK, Reible DD, Lu X, Hawthorne SB, Nakles DV, Thompson T. Comparison of polymeric samplers for accurately assessing PCBs in pore waters. Environ Toxicol Chem. 2011;30:1288–1296. doi: 10.1002/etc.510. [DOI] [PubMed] [Google Scholar]
- 27.Endo S, Hale SE, Goss K, Arp HPH. Equilibrium partition coefficients of diverse polar and nonpolar organic compounds to polyoxymethylene (POM) passive sampling devices. Environ Sci Technol. 2011;45:10124–10132. doi: 10.1021/es202894k. [DOI] [PubMed] [Google Scholar]
- 28.Sacks VP, Lohmann R. Development and use of polyethylene passive samplers to detect triclosans and alkylphenols in an urban estuary. Environ Sci Technol. 2011;45:2270–2277. doi: 10.1021/es1040865. [DOI] [PubMed] [Google Scholar]
- 29.Sacks VP, Lohmann R. Freely dissolved PBDEs in water and porewater of an urban estuary. Environ Pollut. 2012;162:287–293. doi: 10.1016/j.envpol.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 30.Hartmann PC, Quinn JG, Cairns RW, King JW. Depositional history of organic contaminants in Narragansett Bay, Rhode Island, USA. Mar Poll Bull. 2005;50:388–395. doi: 10.1016/j.marpolbul.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 31.Latimer JS, Quinn JG. Historical trends and current inputs of hydrophobic organic compounds in an urban estuary: the sedimentary record. Environ Sci Technol. 1996;30:623–633. [Google Scholar]
- 32.Perron MM, Burgess RM, Suuberg EM, Cantwell MG, Pennell KG. Performance of passive samplers for monitoring estuarine water column concentrations of contaminants of concern. doi: 10.1002/etc.2321. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarzenbach RP, Gschwend PM, Imboden DM. Environmental Organic Chemistry. 2. John Wiley; Hoboken, NJ, USA: 2003. [Google Scholar]
- 34.Yang Z, Zhao Y, Tao F, Ran Y, Mai B, Zeng EY. Physical origin for the nonlinear sorption of very hydrophobic organic chemicals in a membrane-like polymer film. Chemosphere. 2007;69:1518–1524. doi: 10.1016/j.chemosphere.2007.05.080. [DOI] [PubMed] [Google Scholar]
- 35.ter Laak TL, Busser FJM, Hermens JLM. Poly(dimethylsiloxane) as passive sampler material for hydrophobic chemicals: effect of chemical properties and sampler characteristics on partitioning and equilibration times. Anal Chem. 2008;80:3859–3866. doi: 10.1021/ac800258j. [DOI] [PubMed] [Google Scholar]
- 36.Canosa P, Rodriguez I, Rubi E, Cela R. Optimization of solid-phase microextraction conditions for the determination of triclosan and possible related compounds in water samples. J Chromatogr A. 2005;1072:107–115. doi: 10.1016/j.chroma.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 37.Pietrogrande MC, Basaglia G. GC-MS analytical methods for the determination of personal-care products in water matrices. Trends in Anal Chem. 2007;26:1086–1094. doi: 10.1007/s00216-010-4609-4. [DOI] [PubMed] [Google Scholar]
- 38.Yu Y, Wu L. Analysis of endocrine disrupting compounds, pharmaceuticals and personal care products in sewage sludge by chromatography-mass spectrometry. Talanta. 2012;89:258–263. doi: 10.1016/j.talanta.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 39.Sabaliunas D, Webb SF, Hauk A, Jacob M, Eckhoff WS. Environmental fate of triclosan in the river Aire basin, UK. Water Res. 2003;37:3145–3154. doi: 10.1016/S0043-1354(03)00164-7. [DOI] [PubMed] [Google Scholar]
- 40.Katz D, Cantwell MG, Sullivan J, Perron MM, Burgess RM, Ho KT, Charpentier MA. Factors regulating the accumulation and spatial distribution of the emerging contaminant triclosan in the sediments of an urbanized estuary: Greenwich Bay, Rhode Island, USA. Sci Total Environ. 2013;443:123–133. doi: 10.1016/j.scitotenv.2012.10.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.