Abstract
Aim:
The present study investigated the possible regulatory mechanisms of redox agents and hypoxia on the KATP current (IKATP) in acutely isolated rat ventricular myocytes.
Methods:
Single-channel and whole-cell patch-clamp techniques were used to record the KATP current (IKATP) in acutely isolated rat ventricular myocytes.
Results:
Oxidized glutathione (GSSG, 1 mmol/L) increased the IKATP, while reduced glutathione (GSH, 1 mmol/L) could reverse the increased IKATP during normoxia. To further corroborate the effect of the redox agent on the KATP channel, we employed the redox couple DTT (1 mmol/L)/H2O2 (0.3, 0.6, and 1 mmol/L) and repeated the previous processes, which produced results similar to the previous redox couple GSH/GSSG during normoxia. H2O2 increased the IKATP in a concentration dependent manner, which was reversed by DTT (1 mmol/L). In addition, our results have shown that 15 min of hypoxia increased the IKATP, while GSH (1 mmol/L) could reverse the increased IKATP. Furthermore, in order to study the signaling pathways of the IKATP augmented by hypoxia and the redox agent, we applied a protein kinase C(PKC) inhibitor bisindolylmaleimide VI (BIM), a protein kinase G(PKG) inhibitor KT5823, a protein kinase A (PKA) inhibitor H-89, and Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) inhibitors KN-62 and KN-93. The results indicated that BIM, KT5823, KN-62, and KN-93, but not H-89, inhibited the IKATP augmented by hypoxia and GSSG; in addition, these results suggest that the effects of both GSSG and hypoxia on KATP channels involve the activation of the PKC, PKG, and CaMK II pathways, but not the PKA pathway.
Conclusion:
The present study provides electrophysiological evidence that hypoxia and the oxidizing reaction are closely related to the modulation of IKATP.
Keywords: patch-clamp techniques, ATP-sensitive potassium channels, protein kinase C, protein kinase G, protein kinase A, Ca(2+)/calmodulin-dependent protein kinase II
Introduction
ATP-sensitive potassium (KATP) channels are found at a high density in the heart1, 2. These channels are thought to play a major role in protecting the heart from ischemia-induced damage. The activation of KATP channels reduces the action potential duration, thus decreasing contractility and conserving energy during periods of ischemia. The first K+channel that was found to be modulated by hypoxia in the heart was the KATP channel3. Moreover, hypoxia, presumably at a level able to cause metabolic inhibition, was shown to activate these channels4. Several studies have demonstrated that cells exposed to hypoxic conditions produce increased levels of reactive O2 species (ROS) derived from the mitochondrial electron transport chain (mtROS)5, 6, 7. The generation of mtROS under hypoxia would likely alter the intracellular redox status8. Other researchers have confirmed that oxidizing agents such as hydrogen peroxide (H2O2) can enhance IKATP9. It has been reported that glutathione levels are decreased by hypoxia in certain cell types10, 11, 12. Glutathione (GSH) acts as an intracellular redox buffer. For example, intracellular ROS formed under oxidative stress are reduced by glutathione peroxidase with the concomitant conversion of GSH to oxidized glutathione (GSSG)13. This process effectively protects the cell from oxidative damage. Typically, GSSG is then converted to GSH via glutathione reductase or effluxed from the cell and degraded by extracellular-glutamyl transpeptidase14, 15. The oxidation state and intracellular concentration of glutathione are therefore useful indicators of oxidative stress in cells. The redox modulation of ionic channels has been reported for various channels and various tissues16, 17. Many redox agents have been applied to test the link between redox regulation and hypoxic potassium channels18. However, the effects of GSH/GSSG, a major intracellular redox couple, on KATP channels are seldom reported. Furthermore, many reports indicate that IKATP are subject to modulations due to many signaling pathways such as protein kinase C(PKC), protein kinase A(PKA) and protein kinase G (PKG)19, 20, 21. However, little attention has been paid to the relationship between the effects of the redox reaction and hypoxia on IKATP about the signaling pathways.
As hypoxia appears to act as an oxidizing agent22, 23 and it is likely to alter the intracellular redox status, to probe into the relevant regulatory mechanisms underlying the relationship between hypoxia and oxidizing agents, we used GSH/hypoxia and GSH/GSSG as different “redox systems” to observe their effects on IKATP. These systems were used to further explore the possible correlation between the impacts of the redox reaction and hypoxia on IKATP about the signaling pathways by employing whole-cell and single channel patch clamp recordings in cardiomyocytes.
Materials and methods
Cell isolation
Adult rats (250−300 g, of either sex, Experiment Animal Center of Wuhan University of Science and Technology, Wuhan, China) were anesthetized with pentobarbital sodium (25 mg/kg, ip) 20 min after an intraperitoneal injection of 2000 U heparin. The hearts were rapidly excised and retrograde perfused on a Langendorff apparatus with Ca2+-free Tyrode's solution for 5 min; subsequently, the perfusate was switched to an enzyme-containing solution [0.1 g/L collagenase type I, 0.5 g/L bovine serum albumin (BSA) in the same solution] for 5 min. The perfusate was finally changed to a KB solution containing the following (mmol/L): KOH 70, taurine 20, glutamic acid 50, KCl 40, KH2PO4 20, MgCl2 3, ethylene glycol bis-(2 aminoethylether) tetraacetic acid (EGTA) 0.5, Triisopropylphenylsulfonyl (Tris) 10, and glucose 10, pH 7.4, for 5 min. These perfusates were bubbled with 95% O2+5% CO2 and maintained at 37 °C. The ventricles were cut into small chunks and gently agitated in the KB solution. The cells were filtered through nylon mesh and stored in the KB solution at 4 °C. All procedures met the Guide for the Care and Use of Laboratory Animals regulated by the Administrative Regulation of Laboratory Animals of Hubei Province.
Induction of hypoxia
The cell being examined was continuously perfused with extracellular solution that was flowed through a small plastic tube from a test tube. Hypoxia was achieved by bubbling the perfused solution in this test tube with 100% N2 and saturating for at least 50 min. The cells were perfused at a constant flow rate (2 mL/min). Meanwhile, the perfusing bath was covered by a relatively tight covering and was bubbled with 100% N2 to prevent the oxygen in the air from diffusing into the perfused solution. The oxygen tension in the bath could be reduced to about 2.66 kPa (20 mmHg) in 3–5 min and was monitored with an ISO2 isolated dissolved oxygen meter (WPI, USA).
Recording of cardiac KATP channels
Electrophysiological recordings were performed in ventricular myocytes dissociated from the rat hearts24. The pipettes (7–10 MΩ) were filled with (in mmol/L) KCl 140, CaCl2 1, MgCl2 1, HEPES-KOH 5 (pH 7.4). The bath solution contained (in mmol/L): NaCl 144, NaH2PO4 0.33, KCl 4.0, CaCl2 1.8, MgCl2 0.53, glucose 5.5 (pH 7.4) with NaOH. For the inside-out configuration, the cells were superfused with an 'internal solution' containing (in mmol/L): KCl 140, MgCl2 1.0, EGTA 10, CaCl2 2.0 and HEPES 5 (pH 7.4). An ATP-containing solution (intracellular solution, supplemented with 1.0 mmol/L MgATP and 0.8 mmol/L MgCl2) was used to close the KATP channels completely and to inhibit channel run-down. For whole-cell recording, the pipettes (2−3 MΩ) were filled with the 'internal solution' plus 1 mmol/L ATP, and the cardiomyocytes were superfused with the Tyrode solution (in mmol/L): NaCl 136.5, KCl 5.4, CaCl2 1.8, MgCl2 0.53, glucose 5.5, HEPES-NaOH 5.5 (pH 7.4). The single-channel currents and whole-cell currents were recorded alternately using two pulse protocols. The first protocol was a single pulse with 500 ms voltage steps from a holding potential of −40 to +80 mV, and the second protocol involved several depolarizing pulses with a duration of 15 ms applied at 0.25 Hz from a holding potential of −40 mV in 10 mV steps between −80 and +80 mV. All experiments were carried out at room temperature (22–24 °C). The capacitance and series resistances were adjusted to obtain the minimal contribution of the capacitive transients. A 60%−80% compensation of the series resistance was usually achieved without ringing. The currents were obtained with a patch-clamp amplifier (EPC-9, Heka Electronic, Lambrecht, Pfalz, Germany), filtered at 2 kHz, digitized at 10 kHz, and stored on a computer hard disk for further analysis.
Drugs and reagents
Collagenase type I was obtained from Gibco (GIBCO TM, Invitrogen, Paisley, UK). Bisindolylmaleimide VI BIM, KN-93, KN-62, KT5823, H-89, GSH, GSSG, Pinacidil, and Glibenclamide were purchased from Sigma Chemical (Saint Louis, MO, USA). Bovine serum albumin (BSA), HEPES, and DTT were obtained from Roche (Basel, Switzerland). H2O2 was a production of Wuhan Zhongnan Chemical Reagent Co (Wuhan, China). All other chemicals were purchased from Sigma Chemical (Saint Louis, MO, USA). BIM, KN-93, KN-62, KT5823, H-89, pinacidil, and glibenclamide were dissolved in DMSO and diluted into the test solution appropriately prior to the study. The final concentration of DMSO in the test solution did not exceed 0.01%. We confirmed that DMSO at this concentration had no effect on the KATP channel activity. After the drugs were added to the test solution, the pH was readjusted to 7.4 with KOH. BIM, KN-93, KN-62, H-89, pinacidil and glibenclamide were freshly prepared before the experiments and were diluted into the test solution to obtain the final concentrations indicated in the text.
Data and statistical analysis
When the IKATP was recorded from the single rat ventricular myocytes by the single-channel patch clamp method, a “50% threshold” criterion was used to detect the events with the help of manual confirmation. The unitary current amplitude of the IKATP was measured by two different histogram methods. In order to discriminate the distribution of the unitary amplitude of the IKATP from that of Ik1 (inward rectifier channel current), a histogram was formed using the averaged amplitudes of the openings. Openings shorter than 0.3 ms were not included. Thereafter, the histogram was expressed by a sum of several gaussian distributions with mean and variance25. The open and closed times were measured from the records where only a single channel was activated. Each apparent distribution histogram of open or closed time was formed from a length of continuous recordings lasting more than 120 s. The distribution of the open time was obtained by measuring the lifetime of the open events and bursts separately. To measure the lifetime of the open events, the cut-off frequency of the filter (fc) was set at 10 kHz to increase the resolution; meanwhile, to measure the lifetime of the bursts, the fc was set at 0.1 kHz to decrease the errors in detection caused by flickering26. The distribution of the closed time was obtained in a similar way, but measurements of the shut time within bursts were performed instead of the lifetime of open events (any shut time longer than 20 ms was discarded); similarly, the closed time between bursts, instead of the lifetime of bursts, was measured (any closed time longer than 600 ms was discarded). A simple method of least-squares analysis27 was applied to fit a probability density function to the open or closed times with a form of single or double exponentials. The number of openings was also analyzed to estimate the kinetics of the channel activity. Generally, the openings of a KATP channel are grouped in clusters of bursts, with each burst often divided into several short openings by some brief closures. In order to analyze the number of bursts per cluster, the fc was also set at 0.1 kHz to eliminate those very brief closures within the bursts. A critical closure time of 600 ms was chosen in order to separate one cluster from another. Channel activity was expressed as NP0, where N represents the number of channels and P0 represents the open channel probability. Single-channel analysis was performed as described28, 29.
When the IKATP was recorded from single rat ventricular myocytes by the whole-cell patch clamp method, the increased times of KATP by GSSG or hypoxia was calculated according to the following equation: Increased times=(IGSSG-Ibas)/Ibas, where IGSSG and Ibas were the currents recorded with GSSG and the basal (background) current, respectively. The amplitude of the currents was calculated from the values of IKATP at the end of ten consecutive pulses. Figures were plotted by Origin (V7.5, Origin Lab Co, MA, USA). Statistical significance between two groups and multiple groups were evaluated by Student's t-test and one-way analysis of variance (ANOVA), respectively. All values were expressed as means±SD, and the number of cells (n) in each group was given. P<0.05 was considered statistically significant.
Results
Confirmation of KATP currents
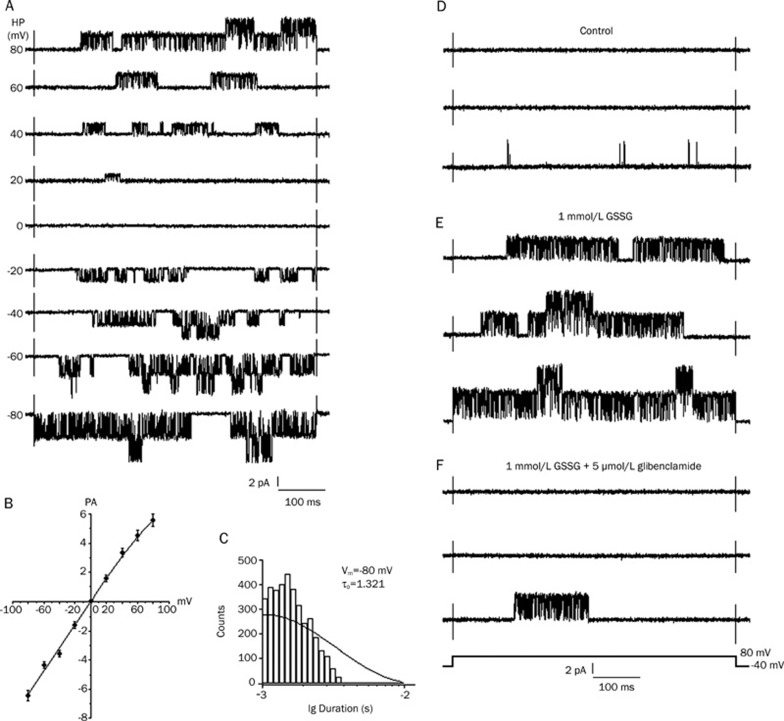
After 15 min of perfusion with 1 mmol/L GSSG to obtain stable KATP channel activity, the single-channel K+currents were recorded, and glibenclamide was subsequently applied to confirm these results in the cell-attached patch mode (symmetrical 140 mmol/L K+) from the rat ventricular cells at various membrane potentials (Figure 1A). The current-voltage relation for this channel is shown in figure 1B, with the conductance of the unitary inward current being 80±3.6 pS (n=6); in addition, a slight inward rectification was observed at the positive membrane potentials. The open-time histograms (at −80 mV) were fitted by a single exponential curve with a time constant of 1.321±0.078 ms (n=7, Figure 1C). The conductance and kinetic properties of this K+ channel were similar to those previously reported for the KATP channel for cardiac cells30, 31. The effects of GSSG on these KATP channels were examined under various conditions. The individual current traces were evoked by a 500 ms voltage pulse to +80 mV from a holding potential of −40 mV in a cell-attached patch. The bath application of 1 mmol/L GSSG stimulated the KATP channels, which were previously abolished by 5 μmol/L glibenclamide (Figure 1D–1F). After perfusion with 1 mmol/L GSSG, the IKATP increased markedly and reached its maximum at about 15 min, while the current amplitudes remained unchanged. The application of 5 μmol/L glibenclamide (applied after 15 min perfusion with 1 mmol/L GSSG) abolished the GSSG-increased IKATP. On the other hand, 1 mmol/L GSSG increased the mean open probability of single KATP channels from the control value of 0.006±0.0007 to 0.496±0.044 (n=6, P<0.01 vs control), which was reduced to 0.018±0.0012 after exposure to 1 mmol/L GSH (n=6, P<0.01 vs 1mmol/L GSSG).
Figure 1.
Conductance, kinetic properties and effect of the KATP channel currents, stimulation of KATP current by GSSG in cell-attached patch. (A) KATP channel currents recorded in cell-attached patch (symmetrical 140 mmol/L K+) from rat ventricular cells at various membrane potentials. After 15 min perfusion with 1 mmol/L GSSG, KATP channel activity increased remarkably. (B) Current-voltage relationship of the KATP channel. The slope conductance of unitary inward current was 80±3.6 pS (n=6). (C) Open-time histograms of the unitary KATP channel currents at −80 mV. The histogram at −80 mV was fitted by a single exponential curve with a time constant of 1.321±0.078 ms (n=7). (D–F) Effect of Glibenclamide on KATP channel activities induced by GSSG. The individual current trace was evoked by a voltage step to +80 mV from a holding potential of −40 mV in a cell-attached patch. (D) Control. (E) 15 min after perfusion with 1 mmol/L GSSG. (F) 1 mmol/L GSSG+5 μmol/L glibenclamide. Data were filtered at 2 kHz and sampled at 10 kHz. Note the scale.
Modulation of channel kinetics by oxidized reductant and hypoxia
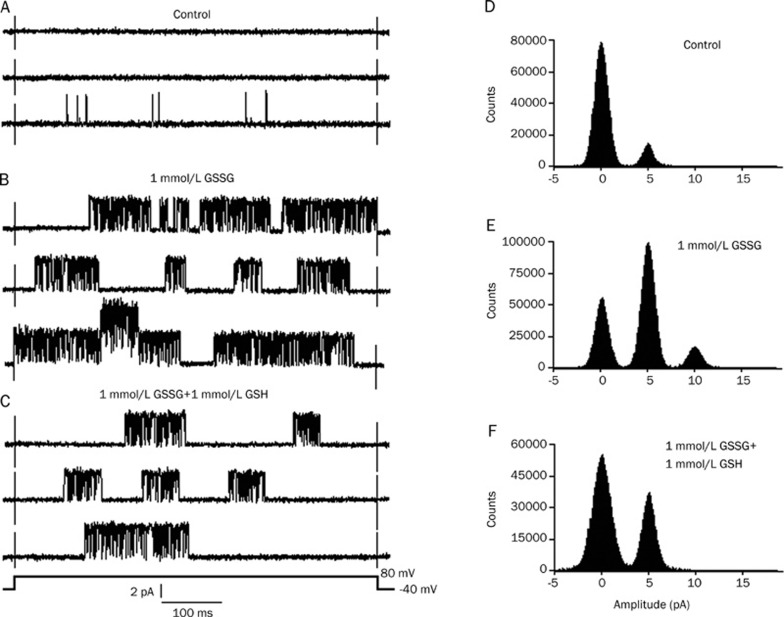
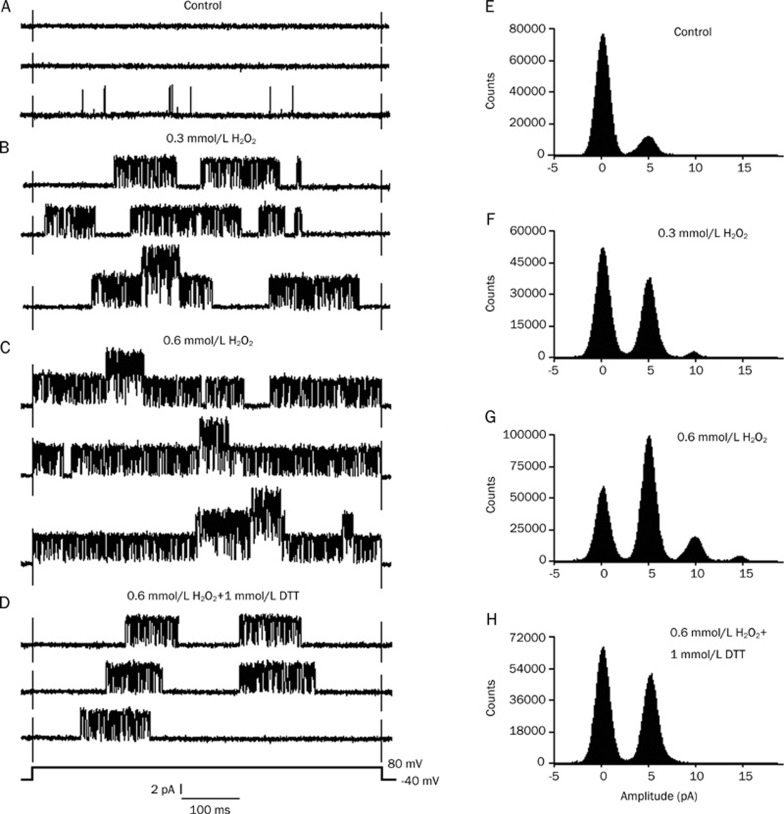
The individual current traces were evoked by a 500 ms voltage pulse to +80 mV from a holding potential of −40 mV in a cell attached patch. In six cell-attached patches, the IKATP was very poor or even invisible before the application of GSSG (Figure 2A). After perfusion with 1 mmol/L GSSG, the IKATP increased markedly and reached a maximum at about 15 min, but the current amplitudes remained unchanged. Figure 2B shows a burst of IKATP after 15 min of perfusion with 1 mmol/L GSSG. Application of 1 mmol/L GSH (applied after 15 min of perfusion with 1 mmol/L GSSG) depressed the GSSG-increased IKATP (Figure 2C). Figure 2 illustrates the typical currents recorded from one patch. The application of 1 mmol/L GSSG increased the mean open probability of single KATP channels from the control value of 0.008±0.0007 to 0.463±0.038 (n=6, P<0.01 vs control). It was reduced to 0.154±0.009 after exposure to 1 mmol/L GSH (n=6, P<0.01 vs 1 mmol/L GSSG). Figure 2D–2F presents an example of the corresponding all-point histograms from another cell-attached patch. To further corroborate the effect of the redox agent on the KATP channel, we employed the redox couple DTT/H2O2. Similarly, in another six cell-attached patches, after perfusion with 0.3 mmol/L H2O2, the IKATP increased remarkably, and the current type gradually changed from the original background currents to burst currents (Figure 3B). After perfusion with 0.6 mmol/L H2O2, the KATP channel activity increased more significantly, but the current amplitudes remained unchanged (Figure 3C). After the application of 1 mmol/L DTT, the KATP channel activities were reversed (Figure 3D). Figure 3 illustrates the typical currents recorded from one patch. H2O2 increased the mean open probability of single KATP channels from the control value of 0.007±0.0006 to 0.364±0.032 (0.03 mmol/L H2O2, n=6, P<0.01 vs control) and to 0.649±0.060 (0.06 mmol/L H2O2, n=6, P<0.01 vs 0.03 mmol/L H2O2). The mean open probability was reduced to 0.215±0.017 after exposure to 1 mmol/L DTT (0.06 mmol/L H2O2+1 mmol/L DTT, n=6, P<0.01 vs 0.06 mmol/L H2O2). Figure 3E-H shows a representative example of the corresponding all-point histograms from another cell-attached patch. Consequently, we can conclude from the results that the action of the redox couples was bidirectional: the reducing agents decreased the KATP channel activity, whereas the oxidizing agents increased the activity.
Figure 2.
Effect of GSH on KATP channel activities induced by GSSG. The individual current trace was evoked by a voltage step to +80 mV from a holding potential of −40 mV in a cell-attached patch. (A–C) show the original current records, and (D–F) show the all-point histograms. (A, D) Control; (B, E) 15 min after perfusion with 1 mmol/L GSSG; (C, F) 1 mmol/L GSSG+1 mmol/L GSH. Note the scale.
Figure 3.
Effect of DTT on KATP channel activities induced by H2O2. The individual current trace was evoked by a voltage step to +80 mV from a holding potential of −40 mV in a cell-attached patch. (A–D) show the original current records, and (E–H) show the all-point histograms. (A, E) Control; (B, F) 10 min after perfusion with 0.3 mmol/L H2O2; (C, G) 20 min after perfusion with 0.6 mmol/L H2O2; (D, H) 0.6 mmol/L H2O2+1 mmol/L DTT. Note the scale.
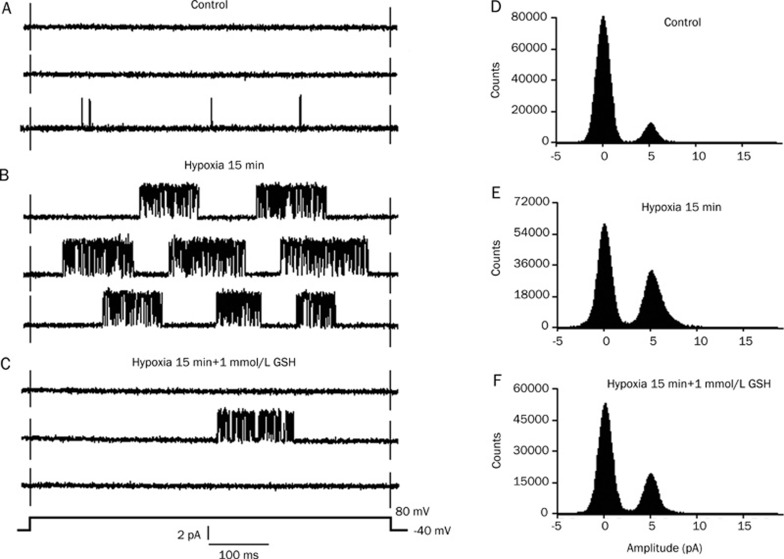
To study the possible correlation between the redox reaction and hypoxia on the IKATP, we used hypoxia/GSH as a “redox system” to observe its effects on IKATP. Similarly, six cell-attached patches were perfused with modified Tyrode's solution saturated with 95% O2+5% CO2 (control) and then exposed to a hypoxia solution for 15 min (hypoxia). The IKATP were very poor or even invisible before hypoxia (Figure 4A). After hypoxia for 15 min, the IKATP increased markedly, and stable KATP channel activity was obtained; however, the current amplitudes remained unchanged. Figure 4B shows a burst of IKATP after hypoxia, which reached a maximum at about 15 min. The application of 1 mmol/L GSH (after hypoxia for 15 min) depressed the hypoxia-increased IKATP (Figure 4C). Figure 4 illustrates the typical currents recorded from one patch. Hypoxia for 15 min increased the mean open probability of single KATP channels from the control value of 0.005±0.0007 to 0.320±0.027 (n=6, P<0.01 vs control). The mean open probability was reduced to 0.127±0.014 after exposure to 1 mmol/L GSH (n=6, P<0.01 vs hypoxia). Figure 4D–4F presents an example of the corresponding all-point histograms from another cell-attached patch.
Figure 4.
Effect of GSH on KATP channel activity induced by hypoxia. The individual current traces were evoked by a voltage step to +80 mV from a holding potential of −40 mV in a cell attached patch. (A–C) show the original current records, and (D–F) show the all-point histograms. (A, D) Control; (B, E) hypoxia for 15 min; (C, F) application of 1 mmol/L GSH in the presence of hypoxia for 15 min. (B) shows hypoxia induced a type of IKATP from few to more with increasing open probability; however in C, 1 mmol/L GSH obviously restored hypoxia-induced IKATP.
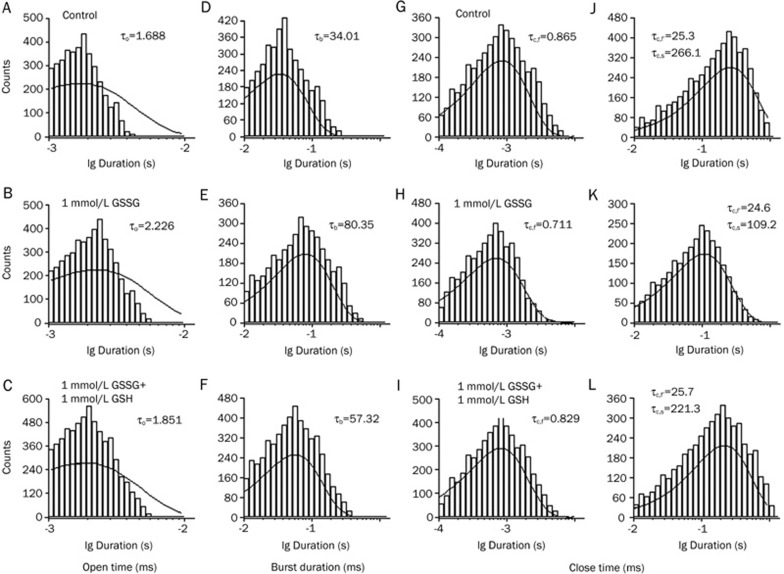
The open and closed time distributions were analyzed to estimate the actions of the oxidized reductant and hypoxia on the kinetic properties of the IKATP. The experimental protocol was the same in principle as those shown in Figure 2, 3, and 4. To examine the effect of the oxidized reductant on the gating kinetics of the KATP channels, the open time histogram, which was analyzed from the current record filtered at a cut-off frequency of 10 kHz, revealed a single exponential distribution with a time constant (τo) of 1.688 ms for the control condition (Figure 5A). In the presence of 1 mmol/L GSSG (Figure 5B), the open time constant did not differ from that in the control (to 2.226 ms). After exposure to 1 mmol/L GSH (1 mmol/L GSSG+1 mmol/L GSH), it still did not differ from that in the presence of 1 mmol/L GSSG (to 1.851 ms). The lifetime of a burst was defined as the open period observed in the data filtered at a cut-off frequency of 0.1 kHz. The histogram of the burst duration consisted of a single exponential distribution (Figure 5D–5F). Its time constant, designated τb, was markedly prolonged by GSSG (from 34.01 to 80.35 ms). The subsequent exposure to 1 mmol/L GSH (1 mmol/L GSSG+1 mmol/L GSH) explicitly decreased (from 80.35 to 57.32 ms). The histogram of the closed time within bursts was best fit to a single exponential function (Figure 5G–5I). This analysis was performed after closed times longer than 20 ms were discarded, and the data were filtered at a cut-off frequency of 10 kHz. The time constant of the closed time within the bursts was designated as τc.f. The value of τc.f was not changed markedly by 1 mmol/L GSSG (from 0.865 to 0.711 ms) or by subsequent exposure to 1 mmol/L GSH (1 mmol/L GSSG+1 mmol/L GSH, from 0.711 to 0.829 ms). The closed time between bursts was analyzed using data filtered at a cut-off frequency of 0.1 kHz (Figure 5J–5L). The histogram was fitted using a biexponential function, with time constants of a fast (τc.f') and a slow component (τc.s). The time constant τc.f' was equivalent to τc.f filtered at a cut-off frequency of 10 kHz, which was distorted by heavy filtering. The value of τc.f' was not influenced by 1 mmol/L GSSG (from 25.3 to 24.6 ms) or by subsequent exposure to 1 mmol/L GSH (1 mmol/L GSSG+1 mmol/L GSH, from 24.6 to 25.7 ms). The value of τc.s was 266.1 ms for the control condition (Figure 5J). This value was markedly decreased to 109.2 ms by GSSG (Figure 5K). However, after exposure to 1 mmol/L GSH (1 mmol/L GSSG+1 mmol/L GSH), it was markedly increased to 221.3 ms. Thus, it seems that GSSG and GSH do not influence the rapid open and closed times within the burst. Rather, GSSG may produce an increase in the burst durations and a decrease in the interburst intervals, as shown in Figure 5, resulting in an increase in the channel activity (Figure 2). On the other hand, GSH may produce a decrease in burst durations and an increase in the interburst intervals, as shown in Figure 5, resulting in an increase in the channel activity (Figure 2). To further corroborate the effect of the redox agent on the KATP channel, we employed the redox couple, DTT/H2O2 and GSH/Hypoxia (Table 1). The experimental protocol was in principle the same as those shown in Figure 5. Our data from this study demonstrate that τb was largely increased by hypoxia or oxidant (GSSG and H2O2), whereas it was decreased by reductant (GSH and DTT). Conversely, τc.s was largely decreased by hypoxia or oxidant (GSSG and H2O2), whereas it was increased by reductant (GSH and DTT). However, it is worth noting that the open time (τo) and short closed time (τc.f and τc.f') were not affected by GSH/GSSG, DTT/H2O2 or hypoxia, because these values were not statistically significant under those conditions.
Figure 5.
Effect of GSH on the mean open time and the mean close time of KATP channel activated by GSSG. The individual current was evoked by a voltage step to +80 mV from a holding potential of −40 mV in a cell-attached patch. (A, D, G, J) Control; (B, E, H, K) 15 min after perfusion with 1 mmol/L GSSG; (C, F, I, L) 1 mmol/L GSSG and 1 mmol/L GSH. (A–F) shows the effect of GSH on the mean open time of KATP channel activated by GSSG. (A–C) histograms of open time, analysed with high fc (fc=10 kHz); (D–F) histogram of life-time of bursts analysed with low fc (fc=0.1 kHz). Open time and that of bursts (τb) obtained under three different conditions is indicated. (G–L) shows the effect of GSH on the mean close time of KATP channel activated by GSSG. (G–I) shows analysis of closed times shorter than 20 ms at an fc of 10 kHz; (J–L) shows the analysis of those up to 600 ms at an fc of 0.1 kHz. Perfusions with 1 mmol/L GSSG and 1 mmol/L GSH do not alter the fast component time constant (τc.f) of distribution of short (G–I) or long (J–L) closed times. The slow component time constant (τc.s) of distribution of long closed times (J–L) is shortened by 1 mmol/L GSSG, and prolonged by 1 mmol/L GSH. Data was obtained from the same patch as (A–F), showing only a single level of the channel activity throughout the three conditions. The analysis of the open and the closed time distributions was made using long recordings from the one patch of such examples.
Table 1. Effect of DTT/H2O2, GSH/Hypoxia on the kinetic properties of KATP channel. The individual current was evoked by a voltage step to +80 mV from a holding potential of −40 mV in a cell-attached patch. The experimental protocol was in principle the same as those shown in Figure 5. The analysis of the open and the closed time distributions was made using long recordings from the one patch of such examples.
| Parameters | Open time (ms) | Burst duration (ms) | Short closed time (ms) | Long closed time (ms) | |
|---|---|---|---|---|---|
| Control | 1.715 | 32.99 | 0.895 | τc.f′=23.2 | τc.s=258.0 |
| 0.3 mmol/L H2O2 | 2.192 | 69.45 | 0.689 | τc.f′=25.1 | τc.s=201.8 |
| 0.6 mmol/L H2O2 | 2.468 | 88.10 | 0.723 | τc.f′=24.5 | τc.s=133.1 |
| 0.6 mmol/L H2O2+1 mmol/L DTT | 1.880 | 50.70 | 0.781 | τc.f′=25.6 | τc.s=203.5 |
| Control | 1.663 | 30.08 | 0.881 | τc.f′=24.7 | τc.s=242.7 |
| Hypoxia 15 min | 1.939 | 52.28 | 0.756 | τc.f′=25.8 | τc.s=167.9 |
| Hypoxia 15 min+1 mmol/L GSH | 1.741 | 40.89 | 0.829 | τc.f′=24.7 | τc.s=214.6 |
Therefore, we can conclude from the results that from the kinetic perspective, the changing tendency by which hypoxia and oxidant (GSSG and H2O2) modulate the KATP channel kinetics are similar, whereas the varying trend by which oxidant (GSSG and H2O2) and reductant (GSH and DTT) modulate the KATP channel kinetics are opposite to each other. Hence, we maintain that the redox reaction definitely plays a role in the modulation of IKATP. Furthermore, we assume that hypoxia and the oxidizing reaction are closely involved in the modulation of IKATP.
Consequently, we further confirmed our previous results from the kinetic perspective, in that the changing tendency by which hypoxia and oxidant GSSG/H2O2 modulate the KATP channel kinetics are similar, whereas the varying trend by which oxidant GSSG/H2O2 and reductant GSH/DTT modulate the KATP channel kinetics are opposite to each other. As a result, we reiterate that the redox reaction definitely plays a role in the modulation of IKATP. Furthermore, we assume that hypoxia and the oxidizing reaction have a close relationship with the modulation of IKATP.
Both hypoxia and GSSG failed to increase the KATP current in the inside-out experiment
The excision of the patch membrane into an ATP-free solution produced a robust opening of those single-channel currents that were sensitive to glibenclamide (5 μmol/L). In the absence of internal ATP, the channels ran down gradually, indicating that the single-channel events were openings of the KATP channels. In contrast, either hypoxia for 15 min (only 5.2% increase of control, n=6) or 1 mmol/L GSSG applied to the internal side of the patch membrane failed to produce a similar effect (only 4.9% increase of control, n=5). Therefore, these findings suggest that both hypoxia and GSSG do not act upon the KATP channels directly but affect them indirectly by activation of the signaling pathway.
GSSG or hypoxia increases the KATP current through the PKC, PKG, and CaMK II pathways without being mediated by the PKA pathway
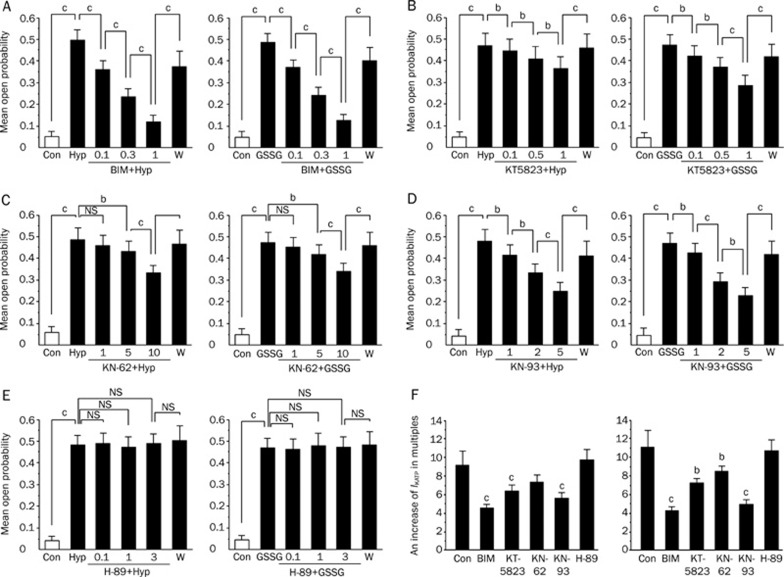
To further explore the signaling pathway through which GSSG or hypoxia increased IKATP, we applied a PKC inhibitor BIM, a PKG inhibitor KT5823, Calcium/calmodulin-dependent protein kinase II (CaMK II) inhibitors KN-62 and KN-93 and a PKA inhibitor H-89 to investigate their impacts on the mean open probability of KATP channels induced by hypoxia or GSSG (Figure 6A–6E). The open probability (Po) was calculated as a fraction of the total length of time, so that each channel was in an open state over the total recording duration. Figure 6A–6E illustrates the external application of inhibitors to the rat ventricular myocytes. After hypoxia for 15 min or the application of 1 mmol/L GSSG to obtain a stable KATP channel activity, the recorded cells were then exposed to an increasing dose of inhibitors (PKC inhibitor BIM, PKG inhibitor KT5823, CaMK II inhibitors KN-62 and KN-93 and PKA inhibitor H-89). Most of the inhibitors decreased the mean open probability of the KATP channels. The blocks were reversed after washout of the inhibitors. Our data showed that the PKC inhibitor BIM, the PKG inhibitor KT5823, and the CaMKII inhibitors KN-62 and KN-93 produced a concentration-dependent suppressive impact on the KATP channel activities induced by hypoxia and GSSG (Figure 6A–6D). However, H-89 had no effect on the mean open probability during hypoxia or in the presence of 1 mmol/L GSSG (Figure 6E). Surprisingly, these findings suggest that the effects of both GSSG and hypoxia on KATP channels involve the activation of the PKC, PKG, and CaMK II signaling pathways, but not that of the PKA pathway.
Figure 6.
Effects of BIM, KT-5823, KN-62, KN-93, H-89 on the mean open propability and current amplitude of ATP-sensitive potassium (KATP) channels activated by Hypoxia/GSSG. (A–E) suggests the effects of BIM, KT5823, KN-62, KN-93, and H-89 on the mean open propability of ATP-sensitive potassium (KATP) channels activated by Hypoxia/GSSG with a voltage step to +80 mV from a holding potential of −40 mV in a cell-attached patch from rat ventricular myocytes. Open probability (Po) was calculated as a fraction of the total length of time that each channel was in an open state over the total recording duration. (F) suggests the effects of hypoxia and oxidized glutathione (GSSG) on KATP channels in the presence of BIM, KT5823, KN-62, KN-93 and H-89 respectively. KATP current amplitude was measured at the end of 500 ms test pulses at 0 mV from the holding potential of −40 mV. Control means KATP current increase in multiples after application of 1 mmol/L GSSG 15 min later or hypoxia for 15 min without any inhibitors inside the patch pipette. (F) (left panel) suggests comparison of effect of hypoxia on KATP channels in the presence of PKC inhibitor BIM (1 μmol/L), a PKG inhibitor KT5823 (3 μmol/L), CaMK II inhibitors KN-62 (10 μmol/L), KN-93 (5 μmol/L) and a PKA inhibitor H-89 (10 μmol/L) inside the patch pipette. (F) (right panel) suggests comparison of effect of glutathione (GSSG) on KATP channels in the presence of PKC inhibitor BIM (1 μmol/L), a PKG inhibitor KT5823 (3 μmol/L), CaMK II inhibitors KN-62 (10 μmol/L), KN-93 (5 μmol/L) and a PKA inhibitor H-89 (10 μmol/L) inside the patch pipette in whole-cell configuration. Values are mean±SD. n=10–21. cP<0.01, bP<0.05;. Abbreviations: Con, control; Hyp, Hypoxia; W, Washout; BIM, Bisindolylmaleimide VI; NS, not significant, P>0.05.
Stimulation of the KATP current
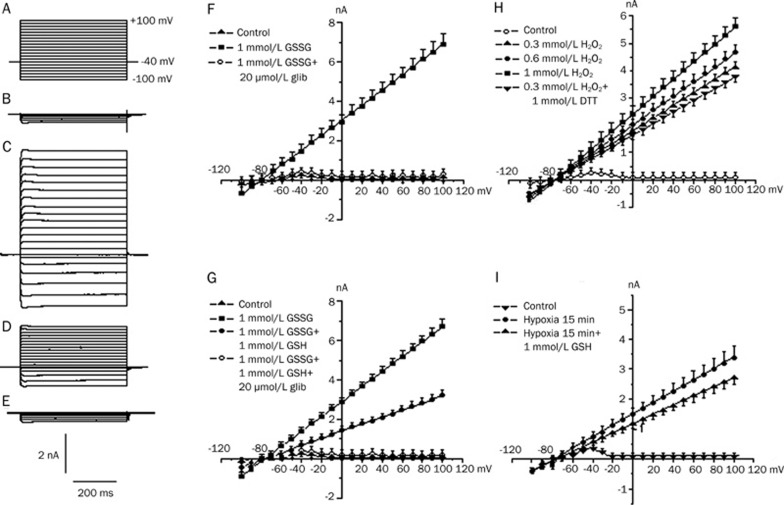
The ramp pulses were applied every 15 s from −100 to +100 mV (at 40 mV/s). The outward current was rapidly increased by the GSSG. The average reversal potential (Vrev) was −78.6±0.6 mV (n=6), which was close to the equilibrium potential for K+ (EK) (-83 mV) [EK=-59 mV×log(140/5.4)], suggesting that most of the current stimulated by GSSG was a K+ current. This effect usually began 1 to 5 min after exposure to GSSG and reached a maximum within 1 to 2 min from the onset of the response. Glibenclamide (20 μmol/L), a relatively specific inhibitor of the KATP channels, eliminated the GSSG-stimulated current (n=6), as shown in Figure 7F. Therefore, the GSSG-stimulated current is similar to IKATP in pattern.
Figure 7.
The effect of GSH/GSSG, DTT/H2O2, GSH/Hypoxia on current-voltage relationships of outward membrane currents in rat ventricular myocytes. The holding potential was held at −40 mV. Command voltage pulses of 500 ms in duration to various potentials from −100 to +100 mV were applied to the cells at 0.2 Hz. (A–E) shows voltage protocol and sample current traces. (A) Voltage protocol; (B) control; (C) 1 mmol/L GSSG; (D) 1 mmol/L GSSG and 1 mmol/L GSH; (E) 1 mmol/L GSSG+1 mmol/L GSH+20 μmol/L glibenclamide. (F) suggests the stimulation of K+ current by GSSG in rat ventricular cells. The outward current was rapidly increased by GSSG and intersected with the abscissa at ∼ −78.6 mV. The reversal potential (Vrev) was close to the EK (-83 mV), suggesting that most of the current stimulated by GSSG was a K+ current. Glibenclamide (20 μmol/L) abolished the GSSG-stimulated currents. (G–I) summarizes effects of GSH/GSSG, DTT/H2O2 and GSH/Hypoxia. Each point represents means±SD from 6 different cells.
To investigate the effects of GSH/GSSG, GSH/Hypoxia and DTT/H2O2 on the current-voltage relationship of the outward currents, we applied command voltage pulses of 500 ms in duration from a holding potential of −40 mV to various membrane potentials (from −100 to +100 mV) to the ventricular cells at 0.2 Hz. After 15 min of perfusion with 1 mmol/L GSSG, the prominent outward currents increased markedly. However, the application of 1 mmol/L GSH (applied after 15 min perfusion with 1 mmol/L GSSG) depressed the GSSG-increased IKATP (Figure 7G). Figure 7H presents the current-voltage relations for the currents produced by the different doses of H2O2 (0.3, 0.6, and 1 mmol/L), and 1 mmol/L DTT, which could reverse the increased KATP channel current induced by H2O2, was then employed after the addition of 1 mmol/L H2O2. After hypoxia for 15 min, the prominent outward currents returned (Figure 7I) when 1 mmol/L GSH, which could reverse the increased IKATP, was added into the hypoxia solution (hypoxia+GSH).
To investigate the signaling pathway involved in the hypoxia-mediated and GSSG-mediated action of IKATP, we applied the PKC inhibitor BIM (1 μmol/L), the PKG inhibitor KT5823 (3 μmol/L), and the CaMK II inhibitors KN-62 (10 μmol/L) and KN-93 (5 μmol/L) inside the patch pipette to intervene IKATP produced by hypoxia for 15 min or the application of 1 mmol/L GSSG. The increased values were 4.67±0.42 (BIM, P<0.01 vs control, n=17), 6.53±0.68 (KT5823, P<0.01 vs control, n=15), 7.47±0.83 (KN-62, P<0.05 vs control, n=16), and 5.79±0.63 (KN-93, P<0.01 vs control, n=21), respectively (Figure 6F, left panel). These values were significantly different from those obtained without these inhibitors, suggesting that the increase in the IKATP by hypoxia for 15 min might involve the activation of PKC, PKG, and CaMK II. However, in the presence of the selective PKA inhibitor H-89 (10 μmol/L), the increased times of IKATP produced by hypoxia for 15 min was 9.73±1.24 (H-89, P>0.05 vs control, n=15) (Figure 6F, left panel). These values were not significantly different from those obtained without these drugs, suggesting that the hypoxia-induced increase in the IKATP might not involve the activation of PKA. Furthermore, in the respective presence of the PKC inhibitor BIM (1 μmol/L), PKG inhibitor KT5823 (3 μmol/L), and the CaMK II inhibitors KN-62 (10 μmol/L) and KN-93 (5 μmol/L), the IKATP produced by GSSG (1 mmol/L) increased several times were 4.27±0.19 (BIM, P<0.01 vs control, n=18), 7.25±0.48 (KT5823, P<0.05 vs control, n=16), 8.53±0.59 (KN-62, P<0.05 vs control, n=19), and 4.91±0.51 (KN-93, P<0.01 vs control, n=16) (Figure 6F, right panel). These values were significantly different from those obtained without these inhibitors, indicating that the GSSG-induced increase in the IKATP might involve the activation of PKC, PKG, CaMK II. However, in the presence of the selective PKA inhibitor H-89 (10 μmol/L), the increased times of the IKATP produced by GSSG (1 mmol/L) was 10.71±1.22 (H-89, P>0.05 vs control, n=17) (Figure 6F). These values were not significantly different from those obtained without these drugs, suggesting that the GSSG-induced increase in the IKATP might not involve the activation of PKA. These results illustrate that the GSSG-induced augmentation of IKATP involves the activation of PKC, PKG, and CaMK II, but it is not mediated by the activation of PKA.
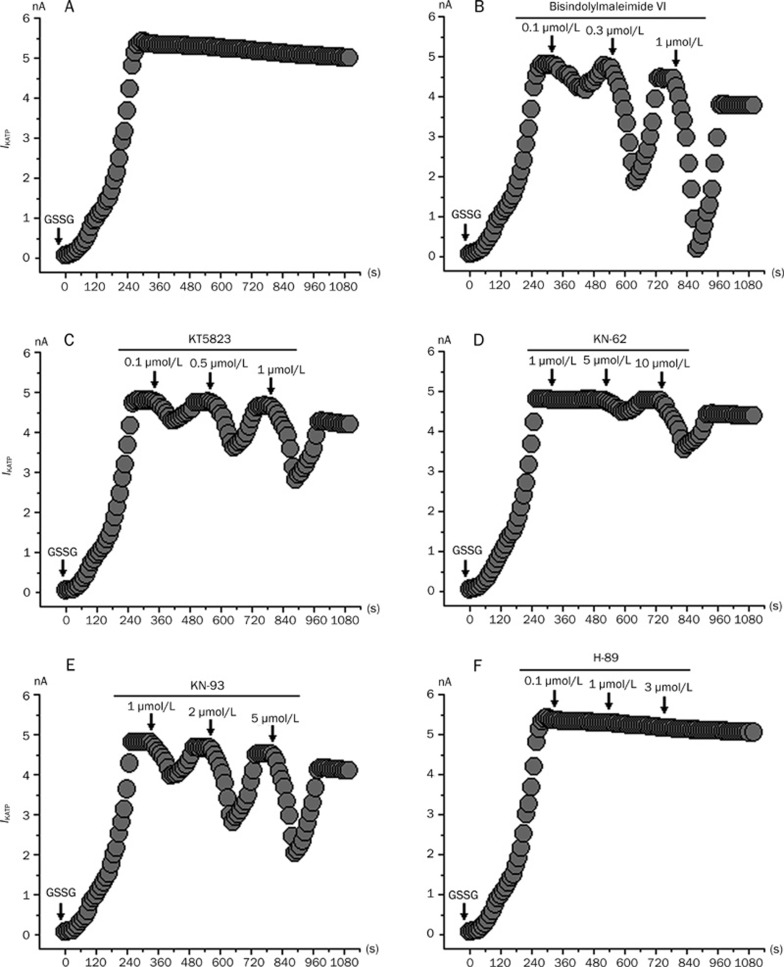
To further investigate the signaling pathway involved in the GSSG-mediated action of IKATP, we examined the effects of the PKC inhibitor BIM, the PKG inhibitor KT5823, the CaMK II inhibitors KN-62 and KN-93 and the PKA inhibitor H-89 on the IKATP induced by GSSG. Figure 8 shows that BIM (Figure 8B), KT5823 (Figure 8C), KN-62 (Figure 8D) and KN-93 (Figure 8E) suppressed IKATP in a concentration dependent pattern. In our study, the procedure for the application of these drugs was in principle the same as those shown in Figure 6A–6E, and the current amplitude of the KATP channels remained unchanged when no GSSG was externally applied to the rat ventricular myocytes in the whole cell (data not shown). After the application of 1 mmol/L GSSG to obtain a stable IKATP, the recorded cells were then exposed to an increasing dose of inhibitors (PKC inhibitor BIM, PKG inhibitor KT5823, CaMK II inhibitors KN-62 and KN-93 and PKA inhibitor H-89). Most of the inhibitors decreased IKATP. The blocks were reversed after the washout of the inhibitors. However, the blocks were also reversed partially with time, probably as a consequence of the high IKATP activity induced by GSSG. Our data suggests that the GSSG-induced increase of IKATP involves the activation of PKC, PKG, and CaMK II but is not mediated by the activation of PKA. Therefore, the effects of both GSSG and hypoxia on the KATP channels involve the activation of PKC, PKG, and CaMK II but not that of the PKA pathway. These results are consistent with the single channel patch-clamp techniques (Figure 6A–6E) and the whole-cell recording patch clamp techniques (Figure 6F).
Figure 8.
Inhibitory action of BIM, KT5823, KN-62, KN-93, and H-89 on KATP currents induced by 1 mmol/L GSSG respectively. Currents were evoked by a voltage step to +80 mV from a holding potential of −40 mV. (A) Control. (B) Inhibitory action of BIM (0.1–1 μmol/L) on KATP currents. BIM suppressed KATP currents induced by 1 mmol/L GSSG in a concentration-dependent fashion. (C) Inhibitory action of KT5823 (0.1–1 μmol/L) on KATP currents. KT5823 suppressed KATP currents induced by 1 mmol/L GSSG in a concentration-dependent fashion. (D) Inhibitory action of KN-62 (1–10 μmol/L) on KATP currents. KN-62 also suppressed KATP currents induced by 1 mmol/L GSSG in a concentration-dependent fashion. (E) Inhibitory action of KN-93 (1–5 μmol/L) on KATP currents. KN-93 also suppressed KATP currents induced by 1 mmol/L GSSG in a concentration-dependent fashion. (F) Effect of H-89 (0.1–3 μmol/L) on the KATP channel current. H-89 failed to suppress KATP currents induced by 1 mmol/L GSSG. The arrows in the figure mean continuous application for drugs.
Discussion
In the present study, we have demonstrated the possible regulatory mechanisms of a redox agent and hypoxia on the IKATP in rat ventricular myocytes. Oxidants and reductants have been used as various redox systems to investigate the link between the redox state and the KATP channels. In our study, we employed the redox couple GSH/GSSG, DTT/H2O2 directly. Under normal conditions, cellular defenses against hypoxia include a high intracellular content of GSH and a low content of GSSG, which remains protein thiol groups in a reduced state. During hypoxia, the content of GSH is decreased, while GSSG is increased. Glutathione, the major cytosolic redox buffer in cardiomyocytes, is mainly reduced under normal physiological conditions, and the ratio of GSH to GSSG is generally >1032. However, under pathological conditions, the cytosolic GSH/GSSG ratio, whose maintenance is a critical factor in antioxidant defense, can decrease significantly33. The changes in the redox state of proteins play an important role in many cellular functions. Typically, the SH groups of cysteine residues are potential targets for the redox modification of ion channel proteins. The alteration in the redox state of the SH groups of two neighboring cysteine residues can lead to the formation or breaking of disulfide bonds. This redox modification of disulfide bonds affects the structure and function of ion regulatory proteins. Moreover, it is well established that the activity of various ion regulatory proteins can be modulated by redox dependent mechanisms34, 35, 36. Since hypoxia is one of the general problems that any tissue can encounter, many investigators have studied the sensing mechanism of the hypoxic response in various tissues. However, little attention has been paid to the relationship between the effects of redox reactions and hypoxia on IKATP. To examine the possible regulatory mechanisms of redox agents and hypoxia on the KATP current (IKATP) and the underlying relationship between them, we used GSH/Hypoxia, GSH/GSSG, and DTT/H2O2 as the different “redox systems” to observe their effects on IKATP and to explore further the possible correlation between the impacts of the redox reaction and hypoxia on IKATP. Our results suggest that hypoxia, quite unexpectedly, acts as an 'oxidizing agent', probably indirectly via the modulation of or a conformational change in a KATP channel regulatory protein, thereby triggering KATP channel activity. It is perhaps surprising that lower O2 levels translate into an oxidizing reaction. However, other experiments also support this idea. From the kinetic perspective, it can be seen that the changing tendency by which hypoxia and the oxidants GSSG and H2O2 modulate the KATP channel kinetics are similar. Currently, several studies on the increase in IKATP during hypoxia involve O2 sensing, whose possible mechanisms implicate redox modulation and/or oxygen sensing by membrane-bound and iron-containing structures37. The mechanism by which the KATP channel senses O2 levels still remains unclear, but it is clear that the O2 sensor is attached to the plasma membrane, and a redox reaction is involved22. Hypoxia may prevent electron transfer through the electron transport systems38. Consequently, electron donors may accumulate in the chain, which may affect the redox state of the KATP channels. In addition to its direct effect on the cellular membrane, hypoxia can potentially influence the cellular redox state indirectly. It has become increasingly evident that ROS and reactive nitrogen species (RNS) are overproduced from metabolic processes during hypoxia39, 40. However, the regulation of the channel appears to be more complex than that and may involve the modulation by PKC, G proteins and changes in the redox potential of the cell41, 42. In order to determine whether the increase in the KATP channel activity triggered by hypoxia or oxidants involved cytosolic second messenger(s), we exposed excised inside-out patches to hypoxia or the representative GSSG, but both of them failed to activate the KATP channels to increase IKATP. Therefore, this suggests that neither hypoxia nor GSSG acts upon the KATP channels directly but rather affect them indirectly by activating the signaling pathway. However, to our knowledge, it has been reported that IKATP are subjected to dual modulation by PKC and PKA43, 44. Further, the activities of the KATP channels have been shown to be modulated by PKA- and PKC-mediated phosphorylation19, 45, 46. In addition, it has also been reported that other protein kinases, such as tyrosine protein kinase and AMP-activated kinases, are involved in the modulation of KATP channels47, 48, 49, 50. However, it still remains unclear whether the signaling pathway is activated in the process when the redox agent acts upon IKATP. To investigate the signaling pathway involved in the regulatory mechanisms of the redox agent on IKATP, we employed an oxidizing agent, the representative GSSG, to mediate the action of IKATP. We employed the PKC inhibitor BIM, the PKG inhibitor KT5823, the CaMK II inhibitors KN-62 and KN-93 and the PKA inhibitor H-89 to intervene IKATP in whole-cell experiments (the drugs in the patch pipette, Figure 6F) and cell-attached experiments (external application of inhibitors to rat ventricular myocytes, Figure 6A–6E), which were induced by GSSG. The results suggest that the GSSG-induced increase in IKATP involves the activation of PKC, PKG and CaMK II but is not mediated by the activation of PKA. Therefore, several signaling mechanisms are related to the KATP channel modulation, with fluctuations in the oxidant/antioxidant balance influencing the activation of protein kinases and phosphatases51, 52, 53. In our experiment, GSSG increased the open probability of the KATP channel, which can be increased by PKC activation in cardiomyocyte membrane patches24. PKC, which is sensitive to redox modifications54, 55, can be activated by oxidative stress54, 56, 57 and inhibited by antioxidants54. Certainly, there is evidence that the KATP channels have potential phosphorylation sites, including serine/threonine residues58, 59, 60, 61 and that the channels are activated by the phosphorylation of these residues62. Since PKG is a serine/threonine protein kinase, it is likely that PKG leads to the phosphorylation of the KATP channels. In this study, we examined the signal transduction pathways involved in the PKG-dependent phosphorylation. Han and coworkers63 investigated the surface KATP channel activity in adult rabbit ventricular myocytes using the patch-clamp technique. Their study showed that the KATP channel activation in these cells can occur through a signal transduction pathway that involves guanylyl cyclase activation, increased production and accumulation of cGMP, and activation of PKG; moreover, they proposed that the KATP channel phosphorylation and activation was the result. However, since PKG and PKA share some similarities in protein substrate sequence specificity, it is possible that PKG can phosphorylate PKA-selective sites. To determine which are the more potent kinase in activating the KATP channels, we applied the PKA inhibitor H-89 to intervene in the KATP currents, which were induced by GSSG. The results suggest that H-89 had an effect on the KATP channels at concentrations up to 10 μmol/L (Figure 8). These findings strongly suggest that the KATP channels are stimulated by PKG but not by PKA. As previously described, PKA is inhibited by the oxidation via either glutathionylation of Cys199 in the activation loop or the formation of an internal disulfide bond between Cys199 and Cys34364. CaMK II can phosphorylate and alter the function of many substrates65, 66. CaMK II, the predominant isoform in the heart67, 68 and initially identified in the nervous system, is found in most tissues67, 69. Our study demonstrated that GSSG could activate the CaM kinase II in rat ventricular myocytes. Furthermore, previous work suggested that Ca2+/calmodulin kinase (CaMK) can be activated by H2O2 and is sensitive to redox70.
Surprisingly, our findings show that the effects of hypoxia on the KATP channels involve activation of the PKC, PKG, and CaMK II signaling pathways, but not that of the PKA pathway; similar results were also found for the oxidant GSSG. Judging from these results, we speculate that hypoxia and the redox reaction are closely related to the modulation of IKATP. In general, hypoxia-increased IKATP may result from the direct effect of hypoxia on the KATP channel protein71, 72. However, in our present study, the results suggest that the impact of hypoxia on the KATP channels involve the activation of PKC, PKG, and CaMK II, but it is not mediated by the activation of PKA (Figure 8). During hypoxia, the generation of mtROS is likely to alter the intracellular redox status, thus activating PKC, PKG, and CaMKII indirectly and finally resulting in the phosphorylation of the KATP channels and an increase in IKATP.
KATP channels are thought to play a key role in cardioprotection. When the concentration of ATP decreases and the Ca2+ concentration increases by metabolic stress such as hypoxia, the KATP channels are activated in the cardiac myocytes73, 74. The activation of the KATP channels protects the myocardium from Ca2+ overloading and mediates preconditioning75. In early preconditioning research, KATP channels were proposed to play an important role in preconditioning-mediated cardioprotection76. Intracellular ROS formed under oxidative stress has also been presumed to be involved in preconditioning77, 78, 79, 80. Although it is well established that high levels of ROS are detrimental81 moderate levels of H2O2 have been shown to elicit a cardioprotective effect similar to that observed with ischemic preconditioning77, 78. Cellular antioxidant capacity has been observed to fall during preconditioning, as the total tissue glutathione levels drop to below 70% of the initial preischemic values80, suggesting thiol oxidation in the course of ischemic preconditioning. Furthermore, previous studies77, 78, 80 suggest that a pro-oxidant environment before ischemia such as ischemic preconditioning is important for cardioprotection. Thus, we believe that oxidizing agents or oxidants should be considered in the future as new therapeutic regimens or targets for clinical ischemic heart diseases. For example, diazoxide, a selective opener of the mitochondrial KATP channel, has been shown to elicit tolerance to ischemia in cardiac myocytes and in perfused hearts.
Although we are curious about how GSH, GSSG, DTT, and H2O2 permeate the cell membranes and take effect, their extracellular applications do affect the intracellular redox state. The underlying mechanisms remain to be studied further.
In our study, single KATP channels were recorded in 320–340 of 430−470 patches under our experimental conditions. Furthermore, this channel was sensitive to glibenclamide (a relatively specific inhibitor of IKATP). It seems that the KATP channel was the dominant type of channel. Although there are several kinds of potassium channels in ventricular myocytes, such as transient outward potassium channels, delayed rectifier potassium channels and calcium activated potassium channels, in addition to the KATP channel, the current due to these potassium channels can be ignored because it is comparable to the significant IKATP (Figure 7).
However, in our experiment, there were no specific potassium channel blockers in the bath solution or the pipette solution because these blockers may affect other ion channels. Further, in many previous studies that recorded IKATP, there was nothing special in the formula for the extra/intracellular solution or for the voltage protocols used in whole-cell patch clamp experiments50, 75, 82, 83, 84, 85.
The effects of GSSG or hypoxia on the IKATP are similar both under whole-cell and single-channel recording models due to the changing tendency between them, the channel kinetics and the signaling pathways that modulate IKATP. Furthermore, our experimental results demonstrated that the mechanisms by which hypoxia and the oxidants GSSG or H2O2 modulate the KATP channel kinetics are similar, whereas the mechanisms by which the oxidants GSSG or H2O2 and the reductants GSH or DTT modulate the KATP kinetics are opposite to each other. Therefore, we assume that the KATP channel activities may exist during hypoxia and even during a redox reaction, depending on the different cellular redox environments. Furthermore, we also suggest that the hypoxia-induced KATP channel activities are closely associated with the redox reactions and that the cellular redox state regulates the properties of the KATP channels. At present, we can adequately prove that the increased IKATP induced by hypoxia is intimately related to oxidization, but we can only interpret the underlying mechanism by proposing the assumption that hypoxia and the oxidizing reaction are closely related to the modulation of IKATP based on our current findings. To discern the concrete mechanisms, we need to study the properties of the KATP channels further.
Author contribution
Ji-hua MA and Xi-sheng YAN designed the research; Xi-sheng YAN and Pei-hua ZHANG performed the research; Xi-sheng YAN analyzed the data; Xi-sheng YAN and Ji-hua MA wrote the paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 30670764).
References
- Nichols CG, Lederer WJ. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991;261:H1675–86. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- Babenko A, Vassort G. Enhancement of the ATP-sensitive K+ current by extracellular ATP in rat ventricular myocytes. Involvement of adenylyl cyclase-induced subsarcolemmal ATP depletion. Circ Res. 1997;80:589–600. doi: 10.1161/01.res.80.4.589. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP–regulated K+ channels in cardiac muscle. Nature. 1983;305:147–8. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Hool LC. Acute hypoxia differentially regulates K+ channels. Implications with respect to cardiac arrhythmia. Eur Biophys J. 2005;34:369–76. doi: 10.1007/s00249-005-0462-3. [DOI] [PubMed] [Google Scholar]
- Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–8. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- Dirmeier R, O′Brien KM, Engle M, Dodd A, Spears E, Poyton RO. Exposure of yeast cells to anoxia induces transient oxidative stress. Implications for the induction of hypoxic. genes. J Biol Chem. 2002;277:34773–84. doi: 10.1074/jbc.M203902200. [DOI] [PubMed] [Google Scholar]
- Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem. 1998;273:11619–24. doi: 10.1074/jbc.273.19.11619. [DOI] [PubMed] [Google Scholar]
- Wang W, Ma J, Zhang P, Luo A. Redox reaction modulates transient and persistent sodium current during hypoxia in guinea pig ventricular myocytes. Pfluegers Arch Eur J Physiol. 2007;454:461–75. doi: 10.1007/s00424-007-0219-1. [DOI] [PubMed] [Google Scholar]
- Ichinari K, Kakei M, Matsuoka T, Nakashima H, Tanake H. Direct activation of the ATP–sensitive potassium channel by oxygen free radicals in guinea-pig ventricular cells: Its potential by MgADP. J Mol Cell Cardiol. 1996;28:1867–77. doi: 10.1006/jmcc.1996.0179. [DOI] [PubMed] [Google Scholar]
- Cargnoni A, Ceconi C, Gaia G, Agnoletti L, Ferrari R. Cellular thiols redox status: A switch for NF–kappaB activation during myocardial post-ischaemic reperfusion. J Mol Cell Cardiol. 2002;34:997–1005. doi: 10.1006/jmcc.2002.2046. [DOI] [PubMed] [Google Scholar]
- Murata Y, Ohteki T, Koyasu S, Hamuro J. IFN-gamma and pro-inflammatory cytokine production by antigen-presenting cells is dictated by intracellular thiol redox status regulated by oxygen tension. Eur J Immunol. 2002;32:2866–73. doi: 10.1002/1521-4141(2002010)32:10<2866::AID-IMMU2866>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Rajpurohit R, Koch CJ, Tao Z, Teixeira CM, Shapiro IM. Adaptation of chondrocytes to low oxygen tension: Relationship between hypoxia and cellular metabolism. J Cell Physiol. 1996;168:424–32. doi: 10.1002/(SICI)1097-4652(199608)168:2<424::AID-JCP21>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Forman HJ.Glutathione in defense and signaling: Lessons from a small thiolIn: Diederich M editor. Cell signaling, transcription, and translation as therapeutic targets 2002. p488–504. [DOI] [PubMed]
- Dickinson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem Pharmacol. 2002;64:1019–26. doi: 10.1016/s0006-2952(02)01172-3. [DOI] [PubMed] [Google Scholar]
- Islam MS, Berggren PO, Larsson O. Sulfhydryl oxidation induces rapid and reversible closure of the ATP-regulated K+ channel in the pancreatic beta-cell. FEBS Lett. 1993;319:128–32. doi: 10.1016/0014-5793(93)80051-u. [DOI] [PubMed] [Google Scholar]
- Chiamvimonvat N, O'Rourke B, Kamp TJ, Kallen RG, Hofmann F, Flockerzi V, et al. Functional consequences of sulfhydryl modification in the pore-forming subunits of cardiovascular Ca2+ and Na+ channels. Circ Res 1995; 76: pp. 325–34. [DOI] [PubMed]
- Lipton SA. Prospects for clinically tolerated NMDA antagonists: open–channel blockers and alternative redox states of nitric oxide. Trends Neurosci. 1993;16:527–32. doi: 10.1016/0166-2236(93)90198-u. [DOI] [PubMed] [Google Scholar]
- Park MK, Lee SH, Ho WK, Earm YE. Redox agents as a link between hypoxia and the responses of ionic channels in rabbit pulmonary vascular smooth muscle. Exp Physiol. 1995;80:835–42. doi: 10.1113/expphysiol.1995.sp003891. [DOI] [PubMed] [Google Scholar]
- Hu K, Duan D, Li GR, Nattel S. Protein kinase C activates ATP-sensitive K+ current in human and rabbit ventricular myocytes. Circ Res. 1996;78:492–8. doi: 10.1161/01.res.78.3.492. [DOI] [PubMed] [Google Scholar]
- Quinn KV, Giblin JP, Tinker A. Multisite phosphorylation mechanism for protein kinase A activation of the smooth muscle ATP-sensitive K+ channel. Circ Res. 2004;94:1359–66. doi: 10.1161/01.RES.0000128513.34817.c4. [DOI] [PubMed] [Google Scholar]
- Costa ADT, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, et al. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res. 2005;97:329–36. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- Hammarstrom AKM, Gage PW. Oxygen-sensing persistent sodium channels in rat hippocampus. J Physiol (Cambridge) 2000;529:107–18. doi: 10.1111/j.1469-7793.2000.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarstrom AK, Gage PW. Hypoxia and persistent sodium current. Eur Biophys J. 2002;31:323–30. doi: 10.1007/s00249-002-0218-2. [DOI] [PubMed] [Google Scholar]
- Light PE, Sabir AA, Allen BG, Walsh MP, French RJ. Protein kinase C-induced changes in the stoichiometry of ATP binding activate cardiac ATP-sensitive K+ channels. A possible mechanistic link to ischemic preconditioning. Circ Res. 1996;79:399–406. doi: 10.1161/01.res.79.3.399. [DOI] [PubMed] [Google Scholar]
- Bhattacharya CG. A simple method of resolution of a distribution into gaussian components. Biometrics. 1967;23:115–35. [PubMed] [Google Scholar]
- Sakmann B, Trube G. Voltage-dependent inactivation of inward–rectifying single-channel currents in the guinea-pig heart cell membrane. J Physiol. 1984;347:659–83. doi: 10.1113/jphysiol.1984.sp015089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelder JA, Mead R. A simplex method for function minimization. Computer J. 1965;7:308–13. [Google Scholar]
- Alekseev AE, Brady PA, Terzic A. Ligand-insensitive state of cardiac ATP-sensitive K+ channels: Basis for channel opening. J Gen Physiol. 1998;111:381–94. doi: 10.1085/jgp.111.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseev AE, Kennedy ME, Navarro B, Terzic A. Burst kinetics of co-expressed kir6.2/SUR1 clones: Comparison of recombinant with native ATP-sensitive K+ channel behavior. J Membrane Biol. 1997;159:161–8. doi: 10.1007/s002329900279. [DOI] [PubMed] [Google Scholar]
- Kakei M, Noma A, Shibasaki T. Properties of adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985;363:441–62. doi: 10.1113/jphysiol.1985.sp015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung RT, Kurachi Y. On the mechanism of nucleotide diphosphate activation of the ATP-sensitive K+ channel in ventricular cell of guinea-pig. J Physiol. 1991;437:239–56. doi: 10.1113/jphysiol.1991.sp018593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radical Biol Med. 1999;27:922–35. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- Palace V, Kumar D, Hill MF, Khaper N, Singal PK. Regional differences in non-enzymatic antioxidants in the heart under control and oxidative stress conditions. J Mol Cell Cardiol. 1999;31:193–202. doi: 10.1006/jmcc.1998.0859. [DOI] [PubMed] [Google Scholar]
- Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol. 1998;275:C1–C24. doi: 10.1152/ajpcell.1998.275.1.C1. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Kim KH, Feng W. Redox sensing properties of the ryanodine receptor complex. Frontiers Biosci. 2002;7:a72–a79. doi: 10.2741/A741. [DOI] [PubMed] [Google Scholar]
- Waring P. Redox active calcium ion channels and cell death. Arch Biochem Biophys. 2005;434:33–42. doi: 10.1016/j.abb.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J, Pardal R, Ortega-Saenz P. Cellular mechanisms of oxygen sensing. Annu Rev Physiol. 2001;63:259–87. doi: 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- Cross AR, Henderson L, Jones OT, Delpiano MA, Hentschel J, Acker H. Involvement of an NAD(P)H oxidase as a pO2 sensor protein in the rat carotid body. Biochem J. 1990;272:743–7. doi: 10.1042/bj2720743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–73. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- Lefer DJ, Granger DN. Oxidative stress and cardiac disease. Am J Med. 2000;109:315–23. doi: 10.1016/s0002-9343(00)00467-8. [DOI] [PubMed] [Google Scholar]
- Wollheim CB, Biden TJ. Signal transduction in insulin secretion: comparison between fuel stimuli and receptor agonists. Ann N Y Acad Sci. 1986;488:317–33. doi: 10.1111/j.1749-6632.1986.tb46568.x. [DOI] [PubMed] [Google Scholar]
- Dunne MJ, Findlay I, Petersen OH. Effects of pyridine nucleotides on the gating of ATP-sensitive potassium channels in insulin-secreting cells. J Membr Biol. 1988;102:205–16. doi: 10.1007/BF01925714. [DOI] [PubMed] [Google Scholar]
- Bonev AD, Nelson MT. Vasoconstrictors inhibit ATP-sensitive K+ channels in arterial smooth muscle through protein kinase C. J Gen Physiol. 1996;108:315–23. doi: 10.1085/jgp.108.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayabuchi Y, Davies NW, Standen NB. Angiotensin II inhibits rat arterial KATP channels by inhibiting steady-state protein kinase A activity and activating protein kinase Ce. J Physiol. 2001;530:193–205. doi: 10.1111/j.1469-7793.2001.0193l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light PE, Bladen C, Winkfein RJ, Walsh MP, French RJ. Molecular basis of protein kinase C-induced activation of ATP-sensitive potassium channels. Proc Natl Acad Sci USA. 2000;97:9058–63. doi: 10.1073/pnas.160068997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoshiki H, Sunagawa M, Seki T, Sperelakis N. ATP-sensitive K+ channels in pancreatic, cardiac, and vascular smooth muscle cells. Am J Physiol Cell Physiol. 1998;274:C25–C37. doi: 10.1152/ajpcell.1998.274.1.C25. [DOI] [PubMed] [Google Scholar]
- Hatakeyama N, Wang Q, Goyal RK, Akbarali HI. Muscarinic suppression of ATP-sensitive K+ channel in rabbit esophageal smooth muscle. Am J Physiol. 1995;268:C877–85. doi: 10.1152/ajpcell.1995.268.4.C877. [DOI] [PubMed] [Google Scholar]
- Maulik N, Watanabe M, Zu YL, Huang CK, Cordis GA, Schley JA, et al. Ischemic preconditioning triggers the activation of MAP kinases and MAPKAP kinase 2 in rat hearts. FEBS Lett. 1996;396:233–7. doi: 10.1016/0014-5793(96)01109-x. [DOI] [PubMed] [Google Scholar]
- Stadnicka A, Kwok WM, Warltier DC, Bosnjak ZJ. Protein tyrosine kinase-dependent modulation of isoflurane effects on cardiac sarcolemmal KATP channel. Anesthesiology. 2002;97:1198–208. doi: 10.1097/00000542-200211000-00025. [DOI] [PubMed] [Google Scholar]
- Sukhodub A, Jovanovic S, Du Q, Budas G, Clelland AK, Shen M, et al. AMP-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K+ channels. J Cell Physiol. 2007;210:224–36. doi: 10.1002/jcp.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum DR, Dinarello CA, Cleveland JC, Jr, Cain BS, Shames BD, Meng X, et al. Hydrogen peroxide induces tumor necrosis factor alpha-mediated cardiac injury by a P38 mitogen-activated protein kinase-dependent mechanism. Surgery (St Louis) 1998;124:291–7. doi: 10.1067/msy.1998.90570. [DOI] [PubMed] [Google Scholar]
- Takeishi Y, Jalili T, Ball NA, Walsh RA. Responses of cardiac protein kinase C isoforms to distinct pathological stimuli are differentially regulated. Circ Res. 1999;85:264–71. doi: 10.1161/01.res.85.3.264. [DOI] [PubMed] [Google Scholar]
- Whisler RL, Goyette MA, Grants IS, Newhouse YG. Sublethal levels of oxidant stress stimulate multiple serine/threonine kinases and suppress protein phosphatases in Jurkat T cells. Arch Biochem Biophys. 1995;319:23–35. doi: 10.1006/abbi.1995.1263. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R, Chen ZH, Gundimeda U. Modifications of cysteine–rich regions in protein kinase C induced by oxidant tumor promoters and enzyme-specific inhibitors. Methods Enzymol. 1995;252:132–46. doi: 10.1016/0076-6879(95)52016-3. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radical Biol Med. 2000;28:1349–61. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- Boscoboinik D, Szewczyk A, Hensey C, Azzi A. Inhibition of cell proliferation by alpha-tocopherol. Role of protein kinase C. J Biol Chem. 1991;266:6188–94. [PubMed] [Google Scholar]
- Gopalakrishna R, Gundimeda U, Chen ZH. Cancer-preventive selenocompounds induce a specific redox modification of cysteine-rich regions in Ca2+-dependent isoenzymes of protein kinase C. Arch Biochem Biophys. 1997;348:25–36. doi: 10.1006/abbi.1997.0334. [DOI] [PubMed] [Google Scholar]
- Ashford ML, Bond CT, Blair TA, Adelman JP. Cloning and functional expression of a rat heart KATP channel. Nature. 1994;370:456–9. doi: 10.1038/370456a0. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature. 1995;374:135–41. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Krapivinsky L, Velimirovic B, Wickman K, Navarro B, Clapham DE. The cardiac inward rectifier K+ channel subunit, CIR, does not comprise the ATP-sensitive K+ channel, IKATP. J Biol Chem. 1995;270:28777–9. doi: 10.1074/jbc.270.48.28777. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Tsuura Y, Namba N, Masuda K, Gonoi T, Horie M, et al. Cloning and functional-characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat-tissues, including pancreatic-islets, pituitary, skeletal-muscle, and heart. J Biol Chem. 1995;270:5691–4. doi: 10.1074/jbc.270.11.5691. [DOI] [PubMed] [Google Scholar]
- Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, et al. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci U S A. 1997;94:11233–7. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Kim N, Joo H, Kim E, Earm YE. ATP-sensitive K+ channel activation by nitric oxide and protein kinase G in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol. 2002;283:H1545–H1554. doi: 10.1152/ajpheart.01052.2001. [DOI] [PubMed] [Google Scholar]
- Humphries KM, Pennypacker JK, Taylor SS. Redox regulation of cAMP-dependent protein kinase signaling – Kinase versus phosphatase inactivation. J Biol Chem. 2007;282:22072–9. doi: 10.1074/jbc.M702582200. [DOI] [PubMed] [Google Scholar]
- Braun AP, Schulman H. The multifunctional calcium/calmodulin–dependent protein kinase: from form to function. Annu Rev Physiol. 1995;57:417–45. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- Maier LS, Bers DM. Calcium, calmodulin, and calcium-calmodulin kinase II: Heartbeat to heartbeat and beyond. J Mol Cell Cardiol. 2002;34:919–39. doi: 10.1006/jmcc.2002.2038. [DOI] [PubMed] [Google Scholar]
- Edman CF, Schulman H. Identification and characterization of delta B-CaM kinase and delta C–CaM kinase from rat heart, two new multifunctional Ca2+/calmodulin–dependent protein kinase isoforms. Biochim Biophys Acta. 1994;1221:89–101. doi: 10.1016/0167-4889(94)90221-6. [DOI] [PubMed] [Google Scholar]
- Tobimatsu T, Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J Biol Chem. 1989;264:17907–12. [PubMed] [Google Scholar]
- Uemura A, Okazaki K, Takesue H, Matsubara T, Hidaka H. A novel Ca2+/calmodulin-dependent protein kinase lacking autophosphorylation activity in the rabbit heart. Biochem Biophys Res Commun. 1995;211:562–9. doi: 10.1006/bbrc.1995.1850. [DOI] [PubMed] [Google Scholar]
- Howe CJ, Lahair MM, McCubrey JA, Franklin RA. Redox regulation of the calcium/calmodulin-dependent protein kinases. J Biol Chem. 2004;279:44573–81. doi: 10.1074/jbc.M404175200. [DOI] [PubMed] [Google Scholar]
- Rodrigo GC, Standen NB. ATP–sensitive potassium channels. Current Pharmaceutical Design. 2005;11:1915–40. doi: 10.2174/1381612054021015. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiological Reviews. 1997;77:1165–232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- Knopp A, Thierfelder S, Koopmann R, Biskup C, Bohle T, Benndorf K. Anoxia generates rapid and massive opening of KATP channels in ventricular cardiac myocytes. Cardiovasc Res. 1999;41:629–40. doi: 10.1016/s0008-6363(98)00238-7. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Turner MR, Burrell HE, Kamishima T. Effects of hypoxia, anoxia, and metabolic inhibitors on KATP channels in rat femoral artery myocytes. Am J Physiol Heart Circ Physiol. 2006;291:H71–80. doi: 10.1152/ajpheart.01107.2005. [DOI] [PubMed] [Google Scholar]
- Budas GR, Jovanovic S, Crawford RM, Jovanovic A. Hypoxia-induced preconditioning in adult stimulated cardiomyocytes is mediated by the opening and trafficking of sarcolemmal KATP channels. FASEB J. 2004;18:1046–8. doi: 10.1096/fj.04-1602fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross GJ, Auchampach JA. Blockade of ATP–sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res. 1992;70:223–33. doi: 10.1161/01.res.70.2.223. [DOI] [PubMed] [Google Scholar]
- Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol. 1997;29:207–16. doi: 10.1006/jmcc.1996.0265. [DOI] [PubMed] [Google Scholar]
- Tritto I, D'Andrea D, Eramo N, Scognamiglio A, De Simone C, Violante A, et al. Oxygen radicals can induce preconditioning in rabbit hearts. Circ Res. 1997;80:743–8. doi: 10.1161/01.res.80.5.743. [DOI] [PubMed] [Google Scholar]
- Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem. 1998;273:18092–8. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- Chen W, Gabel S, Steenbergen C, Murphy E. A redox-based mechanism for cardioprotection induced by ischemic preconditioning in perfused rat heart. Circ Res. 1995;77:424–9. doi: 10.1161/01.res.77.2.424. [DOI] [PubMed] [Google Scholar]
- Jeroudi MO, Hartley CJ, Bolli R. Myocardial reperfusion injury: role of oxygen radicals and potential therapy with antioxidants. Am J Cardiol. 1994;73:2B–7B. doi: 10.1016/0002-9149(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Yokoshiki H, Katsube Y, Sunagawa M, Sperelakis N. The novel calcium sensitizer levosimendan activates the ATP-sensitive K+ channel in rat ventricular cells. J Pharmacol Exp Ther. 1997;283:375–83. [PubMed] [Google Scholar]
- Han J, Kim N, Joo H, Kim E. Ketamine abolishes ischemic preconditioning through inhibition of K(ATP) channels in rabbit hearts. Am J Physiol Heart Circ Physiol. 2002;283:H13–21. doi: 10.1152/ajpheart.01064.2001. [DOI] [PubMed] [Google Scholar]
- Jovanovic S, Jovanovic N, Jovanovic A. High glucose protects single beating adult cardiomyocytes against hypoxia. Biochem Biophys Res Commun. 2006;341:57–66. doi: 10.1016/j.bbrc.2005.12.147. [DOI] [PubMed] [Google Scholar]
- Wang JW, Yazawa K, Hao LY, Onoue Y, Kameyama M. Verrucotoxin inhibits KATP channels in cardiac myocytes through a muscarinic M3 receptor-PKC pathway. Eur J Pharmacol. 2007;563:172–9. doi: 10.1016/j.ejphar.2007.02.004. [DOI] [PubMed] [Google Scholar]