Abstract
Objective
Although there is much evidence of hypothalamic-pituitary-adrenal (HPA) axis dysfunction among individuals who have experienced child maltreatment, dysregulation of the autonomic nervous system (ANS) has received less attention. Understanding the role of the ANS in maltreated children may help clarify how these children respond to subsequent life stress.
Method
We explored ANS reactivity among 111 youth (ages 9 to 14), 34 of whom had experienced verified child maltreatment. ANS activity was assessed via blood pressure-- a convenient, non-invasive physiological index-- while youth underwent a social stress task. Blood pressure and subjective mood ratings were obtained prior to and following the task.
Results
Non-maltreated youth experienced an increase in systolic blood pressure following the stressor while maltreated youth did not. Self-reported subjective mood worsened for both groups.
Conclusions
The current data suggest that children who experienced early stress exposure demonstrate blunted ANS reactivity. Results are discussed in terms of children’s healthy adaptations to transient social stressors. In addition, we discuss the cost-effectiveness and benefits of physiological measures such as blood pressure for understanding risk for psychopathology.
The experience of early adversity-- including child neglect, physical, sexual, or emotional maltreatment-- is associated with a vast array of negative health outcomes. Recent evidence suggests that one effect of such experiences involves dysregulation of the human stress response (Alink, Cicchetti, & Kim, 2011). This response operates primarily through two systems. The hypothalamic-pituitary-adrenal (HPA) axis involves a cascade of neural events resulting in the output of cortisol, while the autonomic nervous system (ANS) reflects the individual’s moment-by-moment response to environmental stimuli (Chrousos & Gold, 1992). The ANS is often indexed via cardiovascular activity or salivary alpha amylase (sAA).
Research with individuals who have a history of child maltreatment has primarily focused on cortisol reactivity. Although there have been mixed findings, converging evidence suggests that children who have suffered from adverse childhood experiences show a blunted HPA axis response, relative to typically developing children, as measured by diminished cortisol in response to an acute stressor (Gunnar, Frenn, Wewerka, & Van Ryzin, 2009). This attenuated stress response may be adaptive for stress-exposed children, promoting resilience by reducing fearfulness and psychophysiological activity to subsequent stressors and thereby reducing the heavy burden of chronic activation (Gunnar & Quevedo, 2007; Parker, Buckmaster, Sundlass, Schatzberg, & Lyons, 2006). There has been relatively less evidence of ANS differences in maltreated children. Yet, utilization of non-hormonal measures, such as change in blood pressure, is a direct measure of ANS reactivity. The present study provides an illustrative example of the utility of blood pressure monitoring during a laboratory stress paradigm with a sample of maltreated and non-maltreated youth.
Past research on the stress response associated with child maltreatment has focused heavily on measuring the release of cortisol, a steroid hormone, to index HPA axis activity among adult samples with a history of maltreatment (Campbell & Ehlert, 2012). Environmental stimuli perceived as stressful to an organism triggers a biological process. This process activates corticotrophin releasing factor neurons in the paraventricular nucleus of the hypothalamus, stimulating the release of adrenocorticotropic hormone from the pituitary and the glucocorticoid cortisol from the adrenals (see Hellhammer, Wüst, & Kudielka, 2009, for a review). Cortisol, found in varying concentrations in blood, urine, and saliva, serves as a biomarker of stress and stress-related disorders and is seen as a reliable measure of HPA axis reactivity to stress. Each method of measurement confers its own costs and benefits. Urinary analysis of cortisol concentration provides the ability to test over longer periods of time (e.g., 24 hour follow-up); however, urinary concentration of cortisol and compliance for follow-ups are particularly low. Blood or serum cortisol levels can provide a higher concentration of cortisol; however, blood draws are seldom used in research with children because they are invasive, stressful, and difficult to administer during an experiment. Salivary cortisol is collected by having participants spit or drool into a collection tube or by placing an absorbent pad known as a salivette in an individual’s mouth. While providing lower concentrations than blood cortisol, salivary cortisol collection has the advantage of being less invasive and stressful. However, collecting saliva requires individuals to dissociate from a given task to generate an adequate amount of saliva for the assay (Hellhammer, Wüst, & Kudielka, 2009). In addition, these methods tend to be relatively expensive. Hormone assays cost around $10 per sample and are often measured repeatedly in an experiment (e.g., every 15 minutes) to track changes over time. Additionally, each sample is often assayed in duplicate for reliability purposes (with the mean of the two values reported). For a study with 50 children who provided baseline cortisol and samples every 15 minutes for an hour that are assayed in duplicate, this would result in a cost of approximately $5000 in addition to the cost of collection equipment and shipping.
While normal amounts of circulating cortisol released via the HPA axis in response to stress triggers the activation of appropriate responses to environmental stimuli, excessive exposure to stress creates an excess of cortisol. This has been found to damage specific regions of the brain implicated in the regulation of the HPA axis stress response (Goldstein & McEwen, 2002). Such damage may then modulate normal operations of the stress response pathway resulting in maladaptive emotional and behavioral responses. Several studies have reported a blunted cortisol response to acute laboratory stressors among individuals with a history of child maltreatment. One recent study, for example, found a blunted cortisol response to an experimental laboratory stressor, the Trier Social Stress Test (TSST), among adults with a history of physical abuse but not among adults with no such history (Carpenter, Shattuck, Tyrka, Geracioti, & Price, 2010). A study from our lab found blunted cortisol levels among maltreated girls post-stressor, but not among maltreated boys or controls (citation omitted for blind review). Corroborating this evidence, a longitudinal study analyzed adolescents’ physiological response to a conflict discussion with their parents in a laboratory setting. The authors found that those children who came from families with high levels of aggression were more reactive to such conflict and displayed an attenuated cortisol output during and after the exposure (Saxbe, Margolin, Spies Shapiro, & Baucom, 2012). Additionally, a study analyzing physiological reactivity to an adapted TSST among non-, early-, and post-institutionalized children revealed that early adopted children displayed a blunted cortisol response to a laboratory stressor but not post-institutionalized or non-adopted children (Gunnar, Frenn, Wewerka, & Van Ryzin, 2009). The authors proposed that moderate levels of early life stress as opposed to severe levels might be associated with a blunted cortisol response.
Despite this converging evidence, there is some inconsistency within the literature. A recent study analyzing cortisol response among individuals suffering from abuse-related post-traumatic stress disorder (PTSD) found no differences between those with and without PTSD in their cortisol response to a laboratory stressor (Bremner et al., 2003). However, researchers found that PTSD patients had higher levels of cortisol in anticipation of the stressor. The directionality of HPA axis activity among victims of maltreatment may possibly be moderated by certain individual differences (e.g., severity of abuse; Gunnar, Frenn, Wewerka, & Van Ryzin, 2009), and while further research is warranted to tease apart inconsistences in the literature, the link between maltreatment and dysregulated HPA axis activity is generally accepted.
Similar to HPA axis activation, ANS activation is commonly indexed by measuring hormone secretion, i.e., the release of salivary alpha amylase (sAA), an enzyme secreted by salivary glands in response to autonomic stimulation (Rohleder & Nater, 2009). However, the ANS has other indexes including changes in heart rate (HR), systolic blood pressure (SBP), or diastolic blood pressure (DBP), as well as more expensive and cumbersome data processing techniques such as impedance cardiography and electrocardiograms. Salivary alpha amylase sampling is completed via salivary collection, performed in a manner similar to that described above for cortisol. This method has been seen as problematic by some researchers due to issues regarding sampling methodology and variations in salivary flow, as stimulating salivary flow has been known to change salivary concentrations (see Bosch, Veerman, de Geus, & Proctor, 2011; Rohleder & Nater, 2009). While debate continues regarding the utility of sAA measurement as a surrogate for ANS activity, caution seems warranted as there are a large number of factors besides stress which contribute to whole mouth sAA concentration (see Allwood, Handwerger, Kivlighan, Granger, & Stroud, 2011; Bosch, Veerman, de Geus, & Proctor, 2011). Research investigating ANS reactivity to stress has primarily focused on adult samples using sAA as a surrogate marker (e.g., Bosch, Veerman, de Geus, & Proctor, 2011; van Stegeren, Wolf, & Kindt, 2008) with a few recent studies extending this evidence to youth (Davis & Granger, 2009; Gordis, Granger, Susman, & Trickett, 2006; Yim, Granger, & Quas, 2010). In a sample of healthy youth no relationship was found between cortisol and sAA response to a psychological laboratory stressor, but both were found to be positively correlated with SBP (Allwood, Handwerger, Kivlighan, Granger, & Stroud, 2011). The authors concluded that sAA provided a reliable surrogate marker of ANS activity and regulation. In a recent study of healthy adults, participants who underwent an acute physical laboratory stressor showed elevations in both sAA and cortisol. However, participants who underwent an acute psychological laboratory stressor only showed elevations in sAA, indicating that the ANS may be particularly sensitive to emotional and psychological content (van Stegeren, Wolf, & Kindt, 2008). While the reactivity of the ANS to various stressors has demonstrated the need for superior indexes, the utility of using sAA as a marker of stress reactivity is debatable (see Bosch, Veerman, de Geus, & Proctor, 2011).
As an alternative to sAA, cardiovascular measures such as heart rate, SBP and DBP have been used as a marker of ANS reactivity and confer key advantages relative to hormonal markers. These measures can be attained in a research setting using an ambulatory blood pressure monitor, which provides maximum arterial pressure during heart contraction (SBP), and minimum arterial pressure during heart relaxation (DBP) in addition to heart rate. Additionally, changes in cardiovascular activity are a direct result of ANS activation in response to environmental stimuli (Sapolsky, Romero, & Munck, 2000). This makes cardiovascular reactivity ideal for sensitive, moment-to-moment monitoring of stress response via autonomic activation. Further, this measure is non-invasive and can be implemented during an experiment without drastic dissociation to directly measure online stress reactivity. While much recent research in the area of physiological stress response has utilized cortisol and sAA as markers of reactivity, accumulating evidence has demonstrated correlations between HPA axis and ANS reactivity as indexed by cardiovascular measures. For example, heart rate recovery was comparable to the cortisol response to stress among girls of depressed mothers (Waugh, Muhtadie, Thompson, Joormann, & Gotlib, 2012). Additionally, cortisol and sAA response were demonstrated to be positively correlated with SBP in a sample of healthy children, ages 7-16 (Allwood, Handwerger, Kivlighan, Granger, & Stroud, 2011). In another study, adolescents displayed increases in cortisol and cardiovascular activity in response to a performance stressor and increases in cardiovascular activity along with sAA in response to a peer rejection stressor (Stroud et al., 2009). These data corroborated evidence demonstrating the sensitivity of the ANS and cardiovascular reactivity in particular to psychosocial stressors.
Because of the divergent profiles of the various indexes of HPA axis and ANS reactivity, some researchers have argued for measurement across multiple systems (HPA axis and ANS) as the most appropriate and sensitive means of assessing individual differences in stress reactivity (Bauer, Quas, & Boyce, 2002; Gordis, Granger, Susman, & Trickett,, 2006). However, inconsistencies have been presented regarding the markers of both HPA axis and ANS reactivity. In addition, assaying for hormones can be cost-prohibitive, time consuming, and difficult to obtain among particular populations. Cardiovascular reactivity, however, can be measured quickly, less invasively, and is more convenient and affordable than the collection and measurement of hormones. Heart rate, SBP, and DBP can all be measured simultaneously using an ambulatory blood pressure monitor utilizing an arm cuff and a waist-band mounted device. Because one of the goals of this special issue is to highlight relatively low-cost physiological methodologies, it is worth noting that this type of monitor incurs a one-time cost of typically less than $3,000 with no additional fees per participant, unlike hormone assays.
The present study set out to demonstrate a convenient and cost-effective means of measuring ANS reactivity to a laboratory-based performance/peer rejection stressor by examining the impact of acute stress on the physiological reactivity of non-maltreated and maltreated children. As indicated above, the ANS is particularly sensitive to psychosocial stressors. The current study capitalizes on these prior findings by utilizing an ambulatory blood pressure monitor to measure change in cardiovascular reactivity in response to an acute interpersonal stressor. In addition, discordance in self-report and objective ratings have led to the conclusion that a multi-factor approach is often necessary to accurately capture physiological reactivity (Thomas, Aldao, & De Los Reyes, 2012). To address this, the current study measured subjective mood ratings in response to the stressor concurrently with objective cardiovascular measures to better clarify the nature of stress reactivity among non-maltreated and maltreated children. Children ages 9-14 were tested as this is a key time for social development prior to the sharp increase in symptoms of psychopathology seen in adolescence (Hankin et al., 1998; Stroud et al., 2009), and these symptoms are often exacerbated by the presence of adverse childhood experiences (Alink, Cicchetti, & Kim, 2011). We hypothesized that while non-maltreated children would display an increase in cardiovascular activity in response to an acute stressor, maltreated children would display an attenuated or blunted response. These findings may serve to elucidate the impact of childhood maltreatment on individuals’ physiological capacity to respond to subsequent life stressors.
Method
Participants and procedure
One hundred-eleven youth, ranging in age from 9 years, 1 month to 14 years, 3 months (64% girls) participated (M age = 11.60, SD = 1.52). Twenty-four percent of the participants had a history of maltreatment. There was a similar distribution of boys and girls in the maltreated group compared to the control group, χ2 = 2.27, ns. The maltreated group was slightly older (M age = 12.22) than the control group (M age = 11.40), t(109) = -2.43, p = .019, and age emerged as significant covariate; thus, we controlled for age in all analyses. Participants were recruited from the community (via advertisements and fliers regarding research on child and adolescent emotion) and youth classified as maltreated were either identified after participation or recruited from our lab database as having previously qualified as maltreated based on the requirements described below. Participants identified their racial group as 68% Caucasian, 17% African American, 10% Asian, 1% Native American, and 4% other; 5% identified as having Hispanic ethnicity. Median family income was $70,000 (lowest income bracket reported was “less than $5,000” and highest was “$250,000 – 275,000”). The study was approved by the Institutional Review Board at the University of Wisconsin - Madison. Parents gave informed consent, youth over the age of 12 provided written assent while children under the age of 12 provided verbal assent and all children were debriefed at the completion of the study. Youth participants received a small prize and $10 for their participation. Parents received $20.
Following the consent procedure, participants completed two brief cognitive tasks unrelated to the present study. These tasks were designed to measure attention biases toward emotional faces and were not intended to be stressful. After taking a short break, participants returned to the testing room and were set up with a blood pressure cuff on their non-dominant arm, with an ambulatory monitor attached to their waist on the opposite side of the body. The experimenter stayed with the participant to do a practice blood pressure rating and adjusted equipment if necessary. After the experimenter showed the participant how to complete self-report ratings on the computer, she left the room and participants reported on their subjective mood. Following this, participants underwent the stressor and again reported on their subjective mood. Additionally, a blood pressure rating was obtained immediately before and after the stressor. Parents completed questionnaires in the waiting room while their child completed the study.
History of child maltreatment
Parents completed the Parent–Child Conflict Tactics Scale (CTS-PC; Straus, Hamby, Finkelhor, Moore, & Runyan, 1998), a questionnaire consisting of 20 items that measure parental discipline, corporal punishment, and the frequency with which a parent has performed specific acts of physical aggression toward the child. In the present study, five youth were classified as maltreated based upon CTS-PC greater than 20 (in addition, three of these five families also had open CPS cases). Twenty-two youth were classified as maltreated due to reports of abuse on record with the Dane County Department of Human Services.
Stressor
We developed a novel stressor involving a surprise speech task, of which participants had no prior knowledge, followed by mildly negative peer feedback (see Hilt & Pollak, 2012). Participants were told they had the opportunity to audition for a new reality television show and would be recorded giving a 3-minute speech about what makes them special or unique. The images of four youth appeared on the computer screen (2 boys, 2 girls; mixed ethnicity), and participants were told these youth had already been chosen for the show and would be judging their speeches to decide if the videos should be sent to the television network. The experimenter instructed the participant to prepare for the speech while she set the peer judges up with microphones. Three minutes later the experimenter returned telling the participant to stand in front of the video camera, with the viewing screen turned so the participant could see his/her image. If the participant finished early, he/she was instructed to continue and talk about what makes him/her special or unique and why he/she should get picked for the show. Following the speech, participants sat down and listened to feedback from the peer judges. Although the participant was led to believe that the feedback was live and specific to his/her speech, it was pre-recorded and played back over an intercom from the control room. The feedback was neutral to slightly negative (e.g., His/Her speech was ok, He/she was average, I just don’t think he/she would be good for a reality TV show). Following the feedback, the experimenter left the room.
Subjective mood
To assess the impact of the stressor on participants’ mood as well as to provide an additional measure of emotional response, participants reported on subjective mood using visual analog scales (VAS). Questions appeared on a computer screen with a black line below the question ranging from 0% (not at all) to 100% (extremely). Participants clicked the mouse pointer on the line to indicate their response (range = 0 to 100). Three negative items (sad, anxious, irritated) and three positive items (happy, pleased, calm) were interspersed with three neutral items and presented in a randomized order during each rating period. During an instructional practice session, the experimenter made sure that participants understood the instructions and intended meanings of the words used.
Blood pressure
Blood pressure ratings were obtained with an ambulatory blood pressure monitor attached to the participant’s body (Spacelabs Healthcare; Issaquah, WA). Participants were prompted on the computer screen to take their blood pressure. Maximum arterial pressure during heart contraction (systolic reading), and minimum arterial pressure during heart relaxation (diastolic reading) were obtained for each data point in addition to heart rate. While all three measures were recorded, the present study focused on systolic stress reactivity over diastolic and heart rate. This is because systolic blood pressure is more reactive to psychosocial stress than diastolic blood pressure and heart rate (Hilmert & Kvasnicka, 2010). Additionally, as medication use may have an impact on blood pressure reactivity, we assessed medication usage from each participant and found no group differences in medication usage, X(2) = .774, p = .679.
Debriefing
At the completion of the study, participants were told about the nature of the study. We allowed adolescents to re-play back the feedback in order to demonstrate that it was pre-recorded.
Results
To examine the effect of the stressor on subjective mood, two repeated-measures ANCOVAs were conducted to predict changes in positive and negative affect, covarying age. These analyses revealed a main effect of the stressor on positive mood, F(1, 105)= 32.39, p < .001, (Fig. 1), such that all participants rated their mood as less positive after the stressor (M = 164.65, SD = 77.28) relative to baseline (M = 209.58, SD = 58.49). There was no statistically significant interaction with maltreatment status (F < 2). In addition, there was a main effect for subjective negative mood, F(1, 105) = 38.97, p < .001, (Fig. 2), such that all participants rated their negative mood as higher after the stressor (M = 85.88, SD = 70.43) relative to baseline (M = 50.48, SD = 52.68). Additionally, there was a significant between-subjects effect, F(1, 105) = 7.76, p = .006, such that maltreated participants rated their negative affect as greater at baseline (M = 69.38, SD = 55.46) and post-stressor (M = 123.08, SD = 82.65) relative to non-maltreated participants (Ms = 44.32, 74.68, SDs = 50.57, 62.65, respectively). Despite these two main effects, there was no interaction of time and maltreatment status for negative mood, F(1, 105)= 2.14, p = .147,
Figure 1.
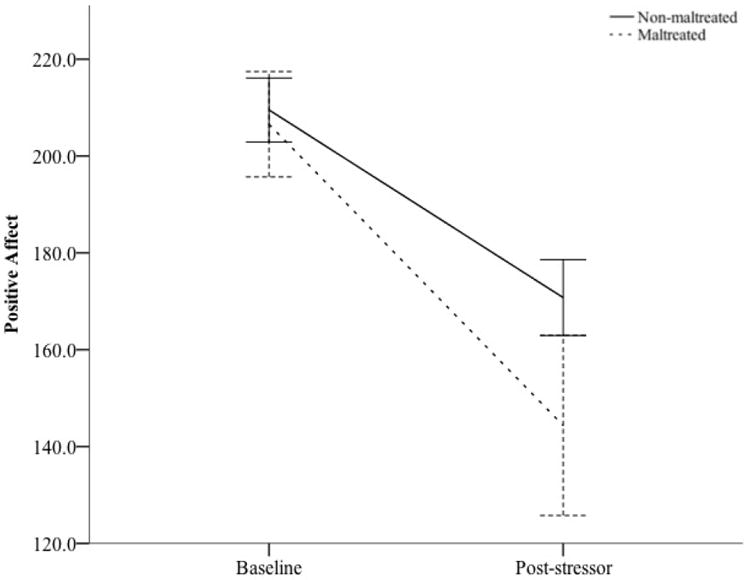
Change in Positive Affect in Response to Acute Stressor by Maltreatment Status
Figure 2.
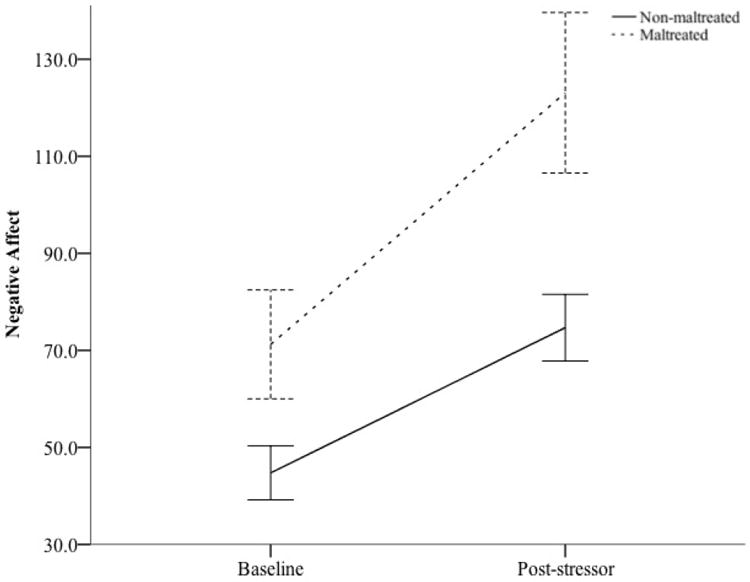
Change in Negative Affect in Response to Acute Stressor by Maltreatment Status
To examine the effect of the stressor on autonomic reactivity, a repeated-measures ANCOVA was conducted to predict changes in systolic and diastolic blood pressure, covarying age. There was a main effect of time for systolic blood pressure, F(1, 108) = 7.15, p < .01, , indicating that participants had higher systolic blood pressure post-stressor (M = 122.86, SD = 14.34) than they did at baseline (M = 117.16, SD = 10.77). A significant interaction between maltreatment status and time was revealed for systolic blood pressure, F(1, 108) = 8.122, p = .005, (see Fig. 2). Post-hoc analyses revealed that systolic blood pressure did not differ between non-maltreated youth (M = 117.61, SD = 10.95) and maltreated youth (M = 115.78, SD = 10.24) at baseline, t(109) = .767, p = ns; however, systolic blood pressure was higher for non-maltreated youth (M = 124.94, SD = 12.77) than maltreated youth (M = 116.37, SD = 17.07) after the stressor, t(109) = 2.783, p = .0061,2. We also tested the effects of the stressor on diastolic blood pressure and heart rate. Results revealed a main effect of time for diastolic blood pressure, F(1, 104) = 27.79, p < .001, , indicating that participants’ diastolic blood pressure was higher post-stressor (M = 78.06, SD = 8.13) than it was at baseline (M = 73.04, SD = 7.34). A significant between-subject effect was found showing that maltreated youth had lower diastolic blood pressure, F(1, 104) = 4.59, p < .05, , at baseline (M = 71.28, SD = 6.21) and post-stressor (M = 75.28, SD = 7.42) as compared to non-maltreated youth (Ms = 73.57, 78.90, SDs = 7.61, 8.20, respectively). The maltreatment status × time interaction for diastolic blood pressure was not significant (F < 1) in addition to there being no significant main effects or interactions for heart rate (Fs < 1). Examining the association between dependent measures, DBP and SBP measures demonstrated strong association at baseline, r(109) = .467, p < .001, and post-stressor, r(109) = .422, p < .001, while HR demonstrated no correlation with SBP or DBP at baseline (r(109) = .106, p = .303; r(109) = -.056, p = .585, respectively) or post-stressor (r(109) = -.022, p = .836; r(109) = .034, p = .743, respectively).
Discussion
Here, we utilized an ambulatory blood pressure monitor to measure change in systolic blood pressure among youth with and without a history of maltreatment. Maltreated youth showed an attenuated cardiovascular response to an acute interpersonal stressor relative to their non-maltreated peers. The current results demonstrate the role of adverse childhood experiences as a potential moderator of ANS reactivity to subsequent life stressors.
The present findings highlight one way in which aberrant early life experiences result in behavioral adaptations to stress. An attenuated stress response may be an adaptive feature present in individuals who have experienced either extreme or chronic early life stress, which happens in greater frequency among maltreated children. This has been suggested as a means of regulating one’s emotion and alleviating feelings of fearfulness (Parker, Buckmaster, Sundlass, Schatzberg, & Lyons, 2006). This may be particularly salient in response to stressors which may not present imminent threat of harm or physical danger but may rather elicit strong emotional reactions. Reducing the intensity and chronicity of frequently activated stress response systems may not only mitigate the negative consequences of excessive activation, but may even promote healthy functioning (Gunnar, Frenn, Wewerka, & Van Ryzin, 2009). However, research on the directionality of the stress response among maltreated children remains inconsistent.
The HPA axis and the ANS have been shown to share overlap in particular brain activation patterns and display a certain level of interconnectivity (Dunn & Berridge, 1990). However, the effects of chronic stress on cortisol response (Gunnar & Quevedo, 2007) and the validity of sAA as a reliable biomarker of the stress response (Bosch, Veerman, de Geus, & Proctor, 2011) continue to be debated. One potential discrepancy between systems may be divergent levels of sensitivity, with greater sensitivity possibly leading to a resistance to adaptive attenuation. Gordis, Granger, Susman, and Trickett (2008) hypothesized that ANS reactivity may be more sensitive to certain types of arousal and thus, may be resistant to attenuation. The results from the current study, however, would contradict this notion as we found that ANS reactivity was sensitive to arousal. Differing from the stressor in the current study, Gordis and colleagues (2008) implemented a modified TSST requiring participants to give a brief speech in front of live judges with no feedback, peer or otherwise, and then measured change in sAA. The difference in the task or measures of ANS reactivity could possibly account for differences found in ANS reactivity between the two studies. However, the present findings are consistent with previous studies finding an attenuated cortisol response to acute stress among maltreated children (e.g., Gunnar & Quevedo, 2007; Seltzer, Ziegler, Connolly, Prososki, & Pollak, in press).
The current study has several limitations. As the first study to report blunted cardiovascular response to an acute stressor among maltreated children, subsequent replications with larger samples are needed to bolster confidence in the presently reported findings. In addition, the current study employed a psychological stressor consisting of interpersonal- and performance-based stress to elicit ANS reactivity. Past research has found differential physiological responses between physical and psychological stressors (van Stegeren, Wolf, & Kindt, 2008). Future research could test whether cardiovascular response to acute stress exhibits specificity to a particular type of stressor.
The blunted stress response we found was reflected only in systolic blood pressure, not in diastolic blood pressure or heart rate. As these measures were not correlated in the current study and systolic blood pressure tends to be more responsive to psychological stressors relative to diastolic blood pressure and heart rate (Hilmert & Kvasnicka, 2010), discrepancies among cardiovascular measures was not entirely unexpected. It is worth noting, however, that blood pressure, both at rest and in response to stress, can be influenced by multiple factors including body mass index, amount of physical activity, and nutrition (Schuster-Decker, Foster, Porcarl, & Maher, 2002). While the current study did not collect this type of information, future research would benefit from analyzing these factors as possible contributors toward moderating BP reactivity. Finally, the current study collected cardiovascular activity before and after the stressor, but not continuously throughout the stressor. It is possible that maltreated children, as opposed to exhibiting a blunted response to stress, merely demonstrated a rapid recovery to an initial, sharper spike in cardiovascular reactivity to the stressor relative to non-maltreated children. Further research utilizing continuous measurement of blood pressure and heart rate and exploring the full time-course of the stress response would aid in further delineating individual differences in the physiological stress response.
The downregulation of physiological reactivity to acute stressors after exposure to prior chronic stress may act as a buffer to the harmful effects of excessive levels of circulating neurotransmitters or cardiac activity. The experience of maltreatment is associated with a vast array of negative outcomes including risk for the development of various forms of psychopathology (Alink et al., 2011). It is possible that adverse childhood experiences differentially confer vulnerability or resilience to the development of psychopathology, and stress reactivity may moderate this effect (Carpenter et al., 2007; Saxbe, Margolin, Spies Shapiro, & Baucom, 2012). Downstream consequences of the stress response have been linked to brain functioning and general cognitive functioning. For example, excessive levels of circulating cortisol have been associated with a reduction of both neurogenesis and overall cognitive functioning (Goldstein & McEwen, 2002; McEwen & Gianaros, 2011). Alternatively, lower levels of circulating cortisol have been associated with increased neurogenesis and enhanced levels of cognitive functioning in addition to lower levels of depressive symptoms (Jacobs, Van Praag, & Gage, 2000). The role of the physiological stress response and in particular its ability to adapt to environmental stressors may be a key moderator in the developmental path from early life stress to future psychopathology.
Finally, the documentation of a blunted stress response as measured by cardiovascular reactivity via ambulatory blood pressure monitoring demonstrates a means of measuring participants’ stress response with a convenient and cost-effective tool. While many laboratories and researchers measure hormones as a marker of stress response systems, this procedure is often costly. However, an inexpensive method such as ambulatory blood pressure monitoring has only an initial purchase cost with minimal subsequent or recurrent fees. This provides a means of conducting quality research without the high price of assaying for hormones. Alternative low-cost options worth further exploration would be measuring skin temperature or using pulse plethysmograph monitors. Pulse plethysmography measures the both the saturation of oxygen and the volume of blood in the skin, essentially measuring pulse pressure from the periphery, while skin temperature has been found to change in response to stress as well (Lin, Lin, Lin, & Huang, 2011).
A low-cost means of identifying stress reactivity could provide clinical utility in identifying high-risk individuals to target for intervention. Children who have suffered aberrant early life experiences like maltreatment are at greater risk to experience negative outcomes including substance abuse (Lo & Cheng, 2007), incarceration (Thornberry, Henry, Ireland, & Smith, 2010), and psychopathology (Fani, Bradley-Davino, Ressler, & McClure-Tone, 2011; Kim & Cicchetti, 2006) among others. Identifying surrogate markers of dysregulated physiology has the potential to lead to earlier identification of individuals in need of intervention. While implementing a stress test in a clinical setting may not be feasible, results form the current study suggest that blood pressure reactivity from such a test could prove useful in identifying those with atypical physiological response patterns.
In conclusion, the present study found evidence for a blunted response to acute stress among maltreated youth but not among non-maltreated youth. Stress response was indexed by change in systolic blood pressure and measured with an ambulatory blood pressure monitor, an inexpensive and convenient method of measuring ANS reactivity which has the potential for practical use in research settings dedicated to investigating the physiological stress response. Adverse childhood experiences are associated with numerous negative health outcomes, both physiological and psychological, in addition to alterations of the physiological stress response systems. Understanding the effects of adverse childhood experiences on human physiology and responses to subsequent life stressors will aid in targeting intervention for youth at risk for deleterious outcomes as a result of maladaptive responses to stress and also in identifying adaptive features which may confer resilience to some sufferers of maltreatment. While chronic stress has been found to result in predominantly negative outcomes, how one handles subsequent stressors may play an imperative role in the development and impairment of future psychopathology. The ability to measure stress reactivity in large and at-risk populations with cost-effective and practical methodology can provide necessary screening for identification, intervention, and prevention for these individuals.
Figure 3.
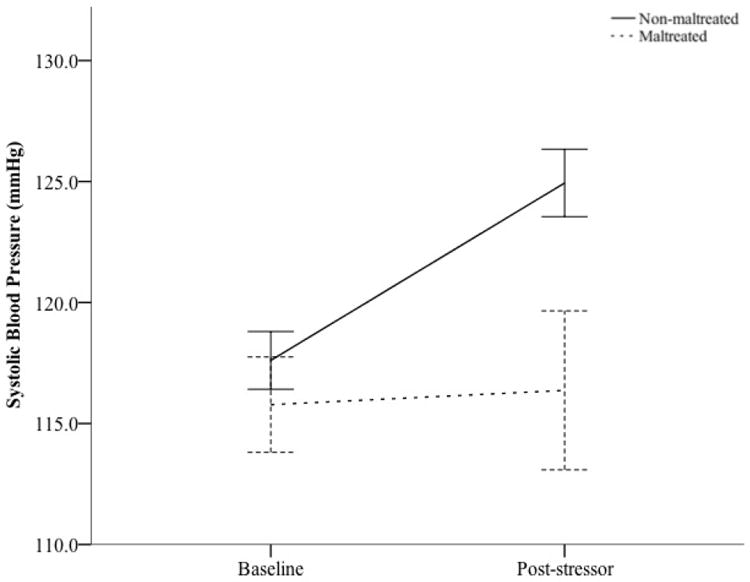
Change in Systolic Blood Pressure in Response to Acute Stressor by Maltreatment Status
Table 1.
Descriptives (Baseline - Poststressor by Maltreatment Status)
| Maltreateda
|
Non-Maltreatedb
|
t(109) | d | |||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| Negative Affect | ||||||
| Baseline | 69.63 | 55.46 | 44.32 | 50.57 | -2.21* | .48 |
| Post-stressor | 123.08 | 82.65 | 74.68 | 62.65 | -3.13* | .66 |
| Positive Affect | ||||||
| Baseline | 211.07 | 54.98 | 209.10 | 59.89 | -.15 | .03 |
| Post-stressor | 144.36 | 93.01 | 170.76 | 71.40 | 1.51 | .32 |
| Systolic Blood Pressure | ||||||
| Baseline | 115.78 | 10.24 | 117.61 | 10.95 | .77 | .17 |
| Post-stressor | 116.37 | 17.07 | 124.94 | 12.77 | 2.78** | .57 |
Note.
p<.05;
p<.01;
n = 27;
n = 84
Acknowledgments
Funding was provided by National Institute of Mental Health grant R01-MH061285 to SDP; BTL was supported by T32-MH018931-23; LMH was supported by T32-MH018931-21.
Footnotes
We also conducted the analysis after removing three participants who withdrew from the study following the stressor, as they perceived the performance task to be too stressful to continue; these participants included two maltreated participants and one non-maltreated participant. The pattern of results for positive and negative affect held, as did systolic blood pressure although the interaction effect was only marginally significant, F(1, 105) = 3.396, p = .068. Post-hoc analyses revealed findings consistent with that of the entire sample such that systolic blood pressure was also higher for non-maltreated youth (M = 125.55, SD = 11.54) than maltreated youth (M = 119.32, SD = 13.90) after the stressor, t(106) = 2.255, p = .026. And also consistent with the entire sample, systolic blood pressure still did not differ between non-maltreated youth (M = 117.83, SD = 10.82) and maltreated youth (M = 115.12, SD = 10.04) at baseline, t(106) = 1.116, p = ns.
To test for the impact of depression, we entered this into our analyses as a covariate; results for time by maltreatment status interaction on systolic blood pressure was comparable, F(1, 108) = 11.25, p = .001, .
Contributor Information
Brian T. Leitzke, Department of Psychology, Waisman Center, University of Wisconsin - Madison, 1500 Highland Avenue, Madison, WI, 53705
Lori M. Hilt, Department of Psychology, Lawrence University and University of Wisconsin – Madison 711 E Boldt Way SPC 24, Appleton, WI 54913
Seth D. Pollak, Department of Psychology, University of Wisconsin—Madison, Madison, 1500 Highland Avenue, Madison, WI, 53705
References
- Alink L, Cicchetti D, Kim J. Longitudinal associations among child maltreatment, social functioning, and cortisol regulation. Developmental Psychology. 2011 doi: 10.1037/a0024892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allwood MA, Handwerger K, Kivlighan KT, Granger DA, Stroud LR. Direct and moderating links of salivary alpha-amylase and cortisol stress-reactivity to youth behavioral and emotional adjustment. Biological Psychology. 2011;88(1):57–64. doi: 10.1016/j.biopsycho.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Journal of Developmental & Behavioral Pediatrics. 2002;23(2):102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Bosch JA, Veerman ECI, de Geus EJ, Proctor GB. α-Amylase as a reliable and convenient measure of sympathetic activity: don’t start salivating just yet! Psychoneuroendocrinology. 2011;36(4):449–453. doi: 10.1016/j.psyneuen.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, et al. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003;28(6):733–750. doi: 10.1016/S0306-4530(02)00067-7. [DOI] [PubMed] [Google Scholar]
- Campbell J, Ehlert U. Acute psychosocial stress: Does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology. 2012;37(8):1111–1134. doi: 10.1016/j.psyneuen.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Bps. 2007;62(10):1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology. 2010;214(1):367–375. doi: 10.1007/s00213-010-2007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Davis EP, Granger DA. Developmental differences in infant salivary alpha-amylase and cortisol responses to stress. Psychoneuroendocrinology. 2009;34(6):795–804. doi: 10.1016/j.psyneuen.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses. Brain Res Rev. 1990;15(71):100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Fani N, Bradley-Davino B, Ressler KJ, McClure-Tone EB. Attention bias in adult survivors of childhood maltreatment with and without posttraumatic stress disorder. Cognitive Therapy and Research. 2011;35(1):57–67. [Google Scholar]
- Goldstein DS, McEwen B. Allostasis, homeostats, and the nature of stress. Stress: the International Journal on the Biology of Stress. 2002;5(1):55–58. doi: 10.1080/10253890290012345. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Salivary alpha amylase–cortisol asymmetry in maltreated youth. Hormones and Behavior. 2008;53(1):96–103. doi: 10.1016/j.yhbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and [alpha]-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31(8):976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10–12-year-old children. Psychoneuroendocrinology. 2009;34(1):62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The Neurobiology of Stress and Development. Annual Review of Psychology. 2007;58(1):145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34(2):163–171. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Hilmert C, Kvasnicka L. Blood pressure and emotional responses to stress: Perspectives on cardiovascular reactivity. Social and Personality Psychology Compass. 2010;4:470–483. [Google Scholar]
- Hilt LM, Pollak SD. Getting Out of Rumination: Comparison of Three Brief Interventions in a Sample of Youth. Journal of Abnormal Child Psychology. 2012;40(7):1157–1165. doi: 10.1007/s10802-012-9638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Van Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Molecular Psychiatry. 2000;5(3):262. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Kim J, Cicchetti D. Longitudinal trajectories of self-system processes and depressive symptoms among maltreated and nonmaltreated children. Child Development. 2006;77(3):624–639. doi: 10.1111/j.1467-8624.2006.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H-P, Lin H-Y, Lin W-L, Huang AC-W. Effects of stress, depression, and their interaction on heart rate, skin conductance, finger temperature, and respiratory rate: sympathetic-parasympathetic hypothesis of stress and depression. Journal of clinical psychology. 2011;67(10):1080–1091. doi: 10.1002/jclp.20833. [DOI] [PubMed] [Google Scholar]
- Lo CC, Cheng TC. The Impact of Childhood Maltreatment on Young Adults’ Substance Abuse. The American Journal of Drug and Alcohol Abuse. 2007;33(1):139–146. doi: 10.1080/00952990601091119. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and Allostasis-Induced Brain Plasticity. Annual Review of Medicine. 2011;62(1):431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(8):3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N, Nater UM. Determinants of salivary α-amylase in humans and methodological considerations. Psychoneuroendocrinology. 2009;34(4):469–485. doi: 10.1016/j.psyneuen.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Saxbe DE, Margolin G, Spies Shapiro LA, Baucom BR. Does Dampened Physiological Reactivity Protect Youth in Aggressive Family Environments? Child Development. 2012;83(3):821–830. doi: 10.1111/j.1467-8624.2012.01752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler TE, Connolly MJ, Prososki AR, Pollak SD. Stress induced elevation of oxytocin and early life stress in children: Evolution, neurodevelopment, and social behavior. Child Development. doi: 10.1111/cdev.12136. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster-Decker R, Foster C, Porcarl JP, Maher MA. Acute effects of dynamic exercises and nutritional supplementation on blood pressure in mildly hypertensive patients. Clinical Exercise Physiology. 2002;4:17–21. [Google Scholar]
- Straus MA, Hamby SL, Finkelhor D, Moore DW, Runyan D. Identification of child maltreatment with the Parent-Child Conflict Tactics Scales: Development and psychometric data for a national sample of American parents. Child Abuse & Neglect. 1998;22(4):249–270. doi: 10.1016/s0145-2134(97)00174-9. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21(01):47. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Aldao A, De Los Reyes A. Implementing clinically feasible psychophysiological measures in evidence-based assessments of adolescent social anxiety. Professional Psychology: Research and Practice. 2012;43(5):510–519. doi: 10.1037/a0029183. [DOI] [Google Scholar]
- Thornberry TP, Henry KL, Ireland TO, Smith CA. The causal impact of childhood-limited maltreatment and adolescent maltreatment on early adult adjustment. Journal of Adolescent Health. 2010;46(4):359–365. doi: 10.1016/j.jadohealth.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Stegeren AH, Wolf OT, Kindt M. Salivary alpha amylase and cortisol responses to different stress tasks: Impact of sex. International Journal of Psychophysiology. 2008;69(1):33–40. doi: 10.1016/j.ijpsycho.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Waugh CE, Muhtadie L, Thompson RJ, Joormann J, Gotlib IH. Affective and physiological responses to stress in girls at elevated risk for depression. Development and Psychopathology. 2012;24(02):661–675. doi: 10.1017/S0954579412000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim IS, Granger DA, Quas JA. Children“s and adults” salivary alpha-amylase responses to a laboratory stressor and to verbal recall of the stressor. Developmental Psychobiology. 2010;52(6):598–602. doi: 10.1002/dev.20453. [DOI] [PubMed] [Google Scholar]


