Abstract
Our objective was to explore alteration of the epidermal growth factor receptor signaling pathway in ampullary carcinoma. Immunohistochemical studies were employed to evaluate expression of amphiregulin as well as expression and activation of epidermal growth factor receptor. A lab developed assay was used to identify mutations in the epidermal growth factor receptor pathway genes, including KRAS, BRAF, PIK3CA, PTEN and AKT1. Fifty two ampullary carcinomas were identified, including 25 intestinal-type and 24 pancreatobiliary-type tumors with the intestinal type being associated with a younger age at diagnosis (p=0.03) and a better prognosis (p<0.01). Expression of amphiregulin correlated the better differentiation (p<0.01), but no difference was observed between two major histologic types. Expression and activation of epidermal growth factor receptor was more commonly seen in the pancreatobiliary type (p<0.01). Mutations were detected in 50% of the pancreatobiliary type and 60% of the intestinal type. KRAS was the most common gene mutated in the pancreatobiliary type (42%) as well as the intestinal type (52%). Other mutations detected included PIK3CA, and SMAD4 and BRAF. KRAS mutations at codons 12 and 13 did not impact adversely on overall survival. In conclusion, epidermal growth factor receptor expression and activation were different between intestinal- and pancreatobiliary-type ampullary carcinoma. KRAS mutation was common in both histologic types; however, the incidence appeared to be lower in the pancreatobiliary type compared to its pancreatic counterpart, pancreatic ductal adenocarcinoma. Mutational analysis of the epidermal growth factor receptor pathway genes may provide important insights into personalized treatment for patients with ampullary carcinoma.
Keywords: Epidermal growth factor receptor pathway, Ampullary carcinoma
INTRODUCTION
Ampullary carcinoma arises from the ampulla of Vater, a complex region composed of a flask-like structure formed by the confluence of the distal common bile duct and the main pancreatic duct, papilla of Vater (junction of duodenal and ampullary mucosa), and the duodenal surface of the ampulla[1]. The incidence of ampullary carcinoma is low, accounting for less than 1% of all digestive cancer[2]. Although ampullary carcinomas are often discovered earlier than other periampullary carcinomas due to their unique location, approximately 50% of patients are diagnosed at an advanced, unresectable stage[2]. Surgery remains the only curative treatment for these patients. However, local recurrence and/or distal metastasis continue to be a significant problem resulting in a five-year survival rate of less than 50%. Currently, there are no effective therapies for those with an unresectable cancer or for those with recurrence or distal metastasis, and the overall prognosis for patients with ampullary carcinoma remains poor.
The ampulla of Vater contains two types of epithelia: pancreatobiliary and intestinal epithelia. Kimura et al. first classified carcinomas arising from the ampulla of Vater into pancreatobiliary and intestinal histologic types[3]. It has been shown that these two histologic types are associated with different premalignant lesions, cell type specific markers and oncogene expression. The intestinal type frequently begins with a tubular/villous adenoma, has a morphology identical to its colonic counterpart, and is predominantly cytokeratin (CK)7−/CK20+/MUC2+/MUC5AC−/CDX2+, whereas most pancreatobiliary type starts with flat or micropapillary dysplasia, has a morphology similar to pancreatic ductal adenocarcinoma, and is CK7+/CK20−/MUC2−/MUC5AC+/CDX2−[4-10]. This histologic differentiation is thought to be one of the most important prognostic factors for ampullary carcinoma, with the intestinal type showing a much better prognosis than the pancreatobiliary type[4, 11, 12]. However, the molecular genetic basis for the histologic difference of ampullary carcinoma remains unclear.
Activation of epidermal growth factor receptor (EGFR) has been implicated in tumorigenesis of many solid tumors including colonic adenocarcinoma and pancreatic ductal adenocarcinoma[13-18]. In colonic adenocarcinoma and pancreatic ductal adenocarcinoma, amphiregulin appears to be a major ligand for EGFR mediated activation of downstream oncogenic signaling including the KRAS/BRAF/MARP kinase, the PI3K/Akt/mTOR and the JAK/STAT signaling pathways, which contribute to tumor growth and progression [18-22]. Genes in the EGFR downstream signaling pathways are frequently mutated in colon cancer and pancreatic ductal adenocarcinoma[23-28]. Additionally, mutations of SMAD4, also called deletion in pancreatic cancer 4 (DPC4), are detected in both diseases [29-32].
In the present study, we examined the expression of amphiregulin, EGFR and phosphor-EGFR (pEGFR) in ampullary carcinoma by immunohistochemical studies. Mutation status of several genes involved in the EGFR downstream signaling pathways and SMAD4 was also investigated using a lab developed multiplex PCR, multiplex single base extension assay and capillary electrophoresis. The expressions and gene mutations were analyzed and compared between the two major ampullary cancer histologic types.
MATERIALS AND METHODS
Patient Selection
Between January 1, 1994 and January 31, 2011, 84 patients who underwent biopsy and/or radical resection for ampullary adenocarcinoma were identified from our pathology databases. Histological blocks were available and sufficient for immunohistochemical studies and mutational analyses in 54 subjects, including 51 with resected specimen and 3 with biopsy specimen only. One patient did not undergo resection due to multiple comorbidities. Of the 53 patients who underwent pancreatoduodenectomy (Whipple procedure), one died perioperatively. Both cases were excluded from the study. Patient demographics and clinical data were collected from the electronic medical record. Pathology reports and histological slides were reviewed for tumor location, size, differentiation, extension, margin status, presence or absence of perineural and lymphovascular invasion, and presence or absence of lymph node metastasis by two pathologists (KM and CS). Some of the pathologic features were not assessed in the two cases with no resection specimen available. The pathologic slides were also reviewed for pathologic types including: the intestinal type composed of intestinal type cancer cells, the pancreatobiliary type composed of pancreatobiliary type cancer cells, adenosquamous carcinoma, signet ring cell carcinoma, and undifferentiated carcinoma. Long-term survival status was determined by review of the medical records and through use of the social security death index. This study was approved by our Institutional Review Board.
Immunohistochemistry
Four-μm unstained sections from formalin fixed paraffin embedded tissue were first deparaffinized by routine methods. For antigen retrieval, the sections were heated to 105°C for 20 minutes in a pH-6.0 citrate buffer for MUC2 (Abcam, Cambridge, Massachusetts; dilution: 1:300), CDX2 (Cell signaling, Boston, Massachusetts; dilution: 1:400), and amphiregulin (Lab Vision, Kalamazoo, Michigan; dilution: 1:100), or to 98°C for 20 minutes in a pH-9.0 EDTA buffer for pEGFR (Cell Signaling; dilution: 1:150), and then allowed to cool to room temperature. Antigen retrieval was not performed for EGFR (Dako, Carpinteria, California; dilution: 1:120) labeling, but the sections were pretreated with proteinase for 5 minutes. After the retrievals or the pretreatment, the tissue sections were quenched with 3% H2O2 in sodium azide for 5 minutes at room temperature. Primary antibodies including anti-amphiregulin, anti-EGFR, and anti-pEGFR were then incubated with the tissue sections, followed by antibody localization using the Dako Envision+ HRP-labeled polymer (DAKO). Staining was visualized by 5 minute incubation with diaminobenzidine.
Immunohistochemical stains for MUC2 (membrane labeling), CDX2 (nuclear labeling), EGFR (membrane labeling) and pEGFR (membrane and cytoplasmic labeling) were considered positive when >5% of the cancer cells are labeled. For amphiregulin (cytoplasmic labeling), the immunohistochemical results were scored by multiplying the staining intensity by the proportion of positive cancer cells. The intensity of stain was scaled as: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong), while the proportion of positive cells was graded as: 0 (<5% positive cells), 1 (5-25%), 2 (25-50%), 3 (50-75%) and 4 (75-100%). The maximal immunohistochemical score was 12. Immunohistochemical stains were reviewed by two pathologists (KM and CS).
DNA Extraction
DNA was extracted from 3-5 10-micron sections of formalin fixed paraffin embedded tissue. In all cases, tissue sections were mounted on slides for macrodissection of tumor enriched areas to increase the percentage of tumor burden in the extracted DNA specimen to at least 20%. Following cell lysis and proteinase K treatment, the DNA was eluted using QIAquick spin columns (Qiagen, Valencia, California).
Gene Mutational Analysis
Tumor tissue-derived DNA from 52 ampullary adenocarcinomas was screened for 62 mutations in 7 cancer genes, including AKT1 (codon: E17), BRAF (codons: G466, G469, D594, G596, V600), KRAS (codons: G12, G13, Q61, A146, K117), NRAS (codons: G12, Q61), PIK3CA (codons: H1047, E542, E545, Q546, D549), PTEN (codons: R233, R159, R267) and SMAD4 (codons: E330, D351, D355, R361), using a lab developed assay. In general, the assay includes a multiplex PCR with 100-200 ng of template DNA, a multiplex base extension assay including the SNaPshot reaction mixture ((Life Technologies Corporation, Carlsbad, California) and mutation detection by capillary electrophoresis on an ABI Genetic Analyzer 3130Xl[33]. The assay has a limit of detection at approximately 10%.
Statistical analysis
The primary endpoint, overall survival, was defined as the time from surgery to the date of all-cause death or last follow-up. For patients’ demographic and clinical variables, continuous variables were summarized using the median with the 25th and 75th percentiles (interquartile range), and frequency with percentages for categorical variables. The Wilcoxon rank sum test was used for continuous variables, and Fisher’s exact test was used for categorical variables to compare the differences between any two groups. The Kaplan-Meier method and Log-rank test were used in univariate analysis on survival outcome. Cox proportional hazard models were used in multivariable analyses to investigate the associations between the risk factors and overall survival while adjusting a priori selected covariates age and tumor stage. All statistical inferences were assessed at a two-sided 5% significant level and all summary statistics, graphics, and survival models were generated using R version 2.15 statistical software [34].
RESULTS
Overall Clinicopathological Features
The 52 patients included 19 females (37%) and 33 males (63%) with a median age of 63 years (interquartile range: 52 to 72 years). Resection specimens were available for analysis in 50 cases. The median size of the resected invasive carcinomas was 2.0 cm, ranging from 0.2 cm to 7.0 cm. The invasive carcinomas in all cases (n=52) were graded using a four grading scale of well differentiated (n=7, 13%), moderately differentiated (n=29, 56%), poorly differentiated (n=15, 29%), and undifferentiated carcinoma (n=1, 2%). In the case with undifferentiated carcinoma, very focal glandular differentiation was also observed. Lymphovascular and perineural invasion were observed in 19 (38%) and 14 (28%) of the 50 resected specimens, respectively, with 7 cases (14%) showing both lymphovascular and perineural invasion. Based on the American Joint Committee on Cancer 7th edition staging system for ampullary adenocarcinoma, the 50 resected cases were comprised of 5 stage IA, 8 stage IB, 8 stage IIA, 28 stage IIB, and 1 stage III. Margin status was able to be assessed in 47 cases with 44 (94%) showing negative resection margins (R0 resection), and 3 (6%) having a microscopically positive superior mesenteric artery margin (R1 resection) (Table 1).
Table 1. Overall Patient Demographics and Clinicopathologic Features.
| All cases (N=52)* | ||
|---|---|---|
| Age (years) | 63 (52 - 72) | |
| Sex | ||
| Male | 33 (63) | |
| Female | 19 (37) | |
| Differentiation | ||
| Well | 7(13) | |
| Moderately | 29 (56) | |
| Poorly | 15 (29) | |
| Undifferentiated | 1 (2) | |
| Invasive tumor Size (cm, n=50) | 2.0 (1.2 – 3.2) | |
| Tumor stage (n=50) | ||
| Stage IA | 5 (10) | |
| Stage IB | 8 (16) | |
| Stage IIA | 8 (16) | |
| Stage IIB | 28 (56) | |
| Stage III | 1 (2) | |
| LVI and PNI (n=50) | ||
| LVI only | 12 (24) | |
| PNI only | 7 (14) | |
| LVI+PNI | 7 (14) | |
| Neither LVI nor PNI | 24 (48) | |
| Resection margin status (n=47) | ||
| Positive | 3 (6) | |
| Negative | 44 (94) | |
Median (interquartile range) for continuous variables and N (%) for categorical variables
Abbreviations: LVI-lymphovascular invasion; PNI-perineural invasion.
Histologic Types
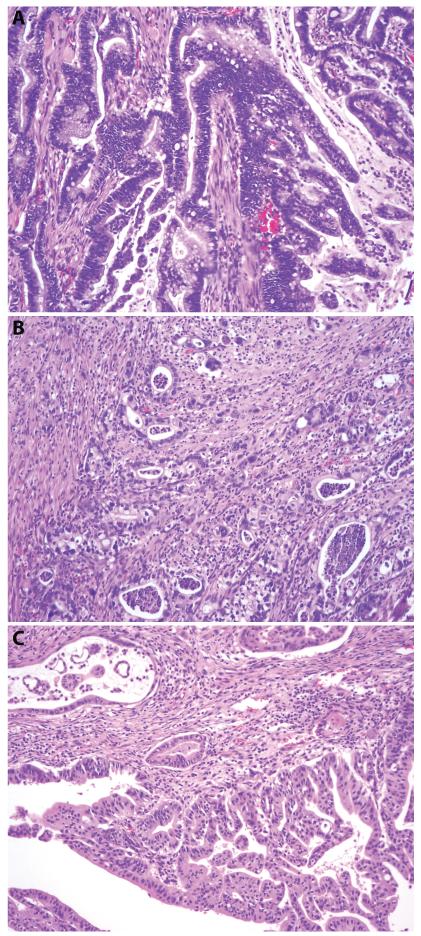
Several histologic types were identified, with intestinal (25/52, 48%, Figure 1A) and pancreatobiliary types (24/52, 46%, Figure 1B) comprising the vast majority of the cases. All intestinal-type adenocarcinomas were positive for CDX2 expression with (n=19) or without (n=6) MUC2 expression. Three mucinous adenocarcinomas expressed both MUC2 and CDX2, and were included in the intestinal group. Two intestinal-type carcinomas harbored focal signet ring cell feature. Eighteen pancreatobiliary-type adenocarcinomas were MUC2−/CDX2−, 5 MUC2−/CDX2+(weak and focal), and 1 MUC2+(focal)/CDX2−. The 6 cases with either CDX2 or MUC2 expression had a typical morphology of pancreatic ductal adenocarcinoma. While most of the pancreatobiliary-type tumors had a morphology of tubular (conventional) pancreatic ducal adenocarcinoma, a subset (7/25, 28%) were featured by complex branching papillae and secondary micropapillae, composed of cuboidal pancreatobiliary-type cells (Figure 1C). According to the 2010 WHO classification of tumors of the digestive system, the latter was classified as invasive papillary adenocarcinoma, pancreatobiliary-type. Other minor subtypes included 1 signet ring cell carcinoma, 1 adenosquamous carcinoma, and 1 undifferentiated carcinoma.
Figure 1.
Major histologic types of ampullary carcinoma. A. Intestinal type (hematoxylin and eosin, original magnification 200×); B. Pancreatobiliary type with conventional pancreatic ductal adenocarcinoma morphology (hematoxylin and eosin, original magnification 100X); C. Invasive papillary adenocarcinoma, pancreatobiliary type (hematoxylin and eosin, original magnification 100×).
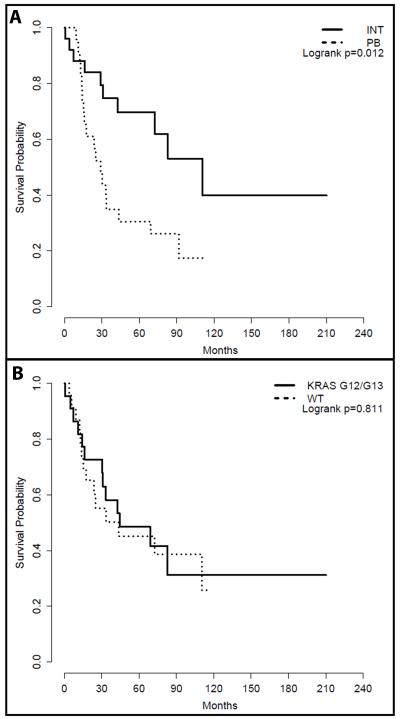
Patients with a pancreatobiliary-type carcinoma were older than those with an intestinal-type carcinoma (median 60, interquartile range 50-65, n=25 versus 69, 62-74, n=24; p=0.028). However, there was no significant gender difference (p=0.377), with males outnumbering females in both groups (Table 2). Pancreatobiliary-type carcinomas tended to be diagnosed at a more advanced stage than intestinal-type carcinomas; 46% (11/24) of the patients with an intestinal-type cancer were diagnosed with a stage I tumor, whereas majority of the patients (21/23, 91%) with a pancreatobiliary-type cancer had either a stage II or stage III tumor (p=0.008, Table 2). Median overall survival from date of diagnosis was 110 months (73 to NA) and 29 months (16 to NA) for patients with an intestinal-type and a pancreatobiliary-type carcinoma, respectively. Univariate analysis showed that the patients with a pancreatobiliary-type cancer had a worse overall survival than those with an intestinal-type cancer (p=0.013, Figure 2A). When the patients’ age and tumor stage were adjusted, compared to those with an intestinal-type cancer, patients with a pancreatobiliary-type cancer had 2.15 times higher hazard (95% CI: 0.75 to 6.16), though this difference did not reach statistical significance (p=0.153).
Table 2. Patient Demographics, Tumor Stage, Alterations in the EGFR Signaling Pathway and Survival in Patients with Pancreatobiliary- and Intestinal-Type Ampullary Adenocarcinoma.
| Intestinal (n=25) | Pancreatobiliary (N=24)* |
P Value | ||
|---|---|---|---|---|
| Age (years) | 60 (50 – 65) | 69 (62 – 74) | 0.028* | |
| Sex | 0.3772 | |||
| Female | 44% (11) | 29% ( 7) | ||
| Male | 56% (14) | 71% (17) | ||
| Tumor stage | 0.0082 | |||
| I | 46% (11) | 9% ( 2) | ||
| II-III | 54% (13) | 91% (21) | ||
|
AR immunohistochemstry
score |
8 (3 – 12) | 5 (2 – 12) | 0.696* | |
| EGFR | 0.0022 | |||
| Positive | 4% ( 1) | 42% (10) | ||
| Negative | 96% (24) | 58% (14) | ||
| pEGFR | 0.0022 | |||
| Positive | 0% ( 0) | 33% ( 8) | ||
| Negative | 100% (25) | 67% (16) | ||
| Gene mutation | 0.2232 | |||
| KRAS codons 12 and 13 | 52% (13) | 29% ( 7) | ||
| Other mutation | 8% ( 2) | 21% ( 5) | ||
| No mutation | 40% (10) | 50% (12) | ||
| Overall survival status | 0.0212 | |||
| Alive | 60% (15) | 25% ( 6) | ||
| Dead | 40% (10) | 75% (18) | ||
| Follow up time (months) | 52 (29 – 83) | 27 (14– 69) | 0.0931 | |
:Median (interquartile range) for continuous variables and N (%) for categorical variables
:Wilcoxon test
:Fisher’s exact test
Abbreviations: EGFR - epidermal growth factor receptor; AR - amphiregulin; pEGFR - phosphor-EGFR
Figure 2.
Effect of histologic types and KRAS mutation status on survival. A. Kaplan-Meier survival analysis of patients with intestinal- and pancreatobiliary-type ampullary carcinoma. B. Kaplan-Meier survival analysis of patients with a KRAS-wild type (WT) and a KRAS G12/13 mutant tumor.
Expression of Amphiregulin and EGFR in Ampullary Adenocarcinoma
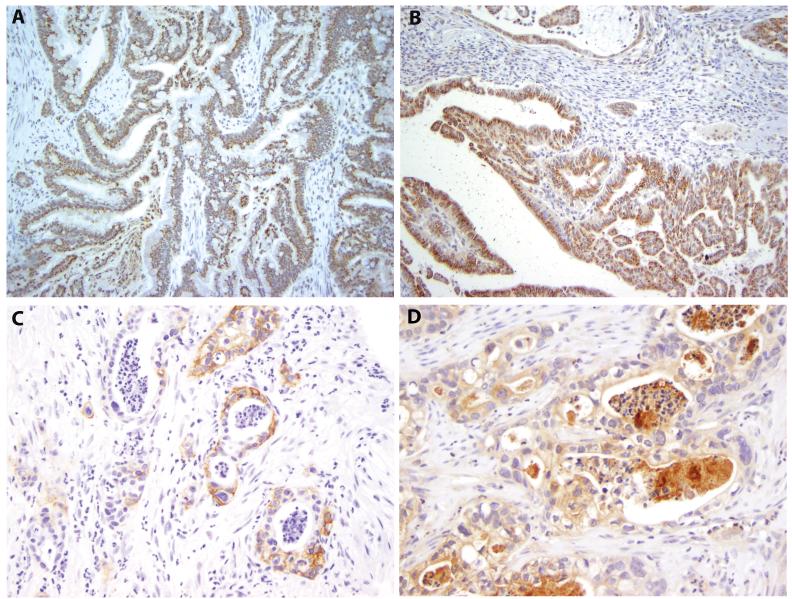
Amphiregulin, a major ligand for EGFR, was highly expressed in most (43 of 52, 82.7%) ampullary adenocarcinomas (Figures 3A and 3B). There was no difference in amphiregulin expression between the pancreatobiliary and intestinal groups (p=0.696). However, strong and diffuse amphiregulin labeling was more frequently observed in invasive papillary adenocarcinoma, pancreatobiliary type (Figures 3B) than in all other cases (6/7, 86% versus 8/45, 18%; p<0.001). In addition, well and moderately differentiated carcinomas (median 8.5, interquartile range 4.0 to 12.0, n=36) were more likely to express amphiregulin compared to those with poor differentiation and undifferentiation (median 1.0, interquartile range 0 to 4.5, n=16, p<0.001). All the 7 invasive papillary adenocarcinomas were moderately differentiated. The signet ring cell carcinoma, adenosquamous carcinoma and undifferentiated carcinoma showed minimal to no amphiregulin expression.
Figure 3.
Expression of amphiregulin, EGFR and phosphor-EGFR (pEGFR). A. Immunohistochemical labeling for amphiregulin of the tumor in Figure 1A (original magnification 200×); B. Immunohistochemical labeling for amphiregulin of the tumor in Figure 1C (original magnification 100×); C. Immunohistochemical labeling for EGFR of the tumor in Figure 1B (original magnification 200×); D. Immunohistochemical labeling for pEGFR of the tumor in Figure 1B (original magnification 200×).
EGFR expression was detected in 13 of 52 (25%) invasive carcinomas by immunohistochemistry (Figure 3C). Ten of 24 (42%) pancreatobiliary-type tumors were labeled with anti-EGFR antibody (Figure 3C), whereas only 1 of 25 (4%) intestinal-type tumors expressed EGFR. EGFR was more commonly expressed in pancreatobiliary-than in intestinal-type tumors (p=0.002). Activated EGFR (pEGFR) was detected in 10 of 52 cases (19%) by immunohistochemistry (Figure 3D). Similar to EGFR expression, the activation of EGFR was more common in pancreatobiliary-than in intestinal-type tumors (8/24, 33% versus 0/25, 0%; p=0.002). Neither EGFR labeling nor activation of EGFR was observed in the single signet ring cell carcinoma case. Interestingly, both adenosquamous and undifferentiated carcinomas displayed expression and activation of EGFR. The activation of EGFR was negatively correlated to amphiregulin expression (p=0.017).
Gene Mutations in Ampullary Adenocarcinoma
One or more mutations were detected in 29 of 52 (56%) tumors (Table 3). As expected, KRAS was the most commonly mutated gene detected in ampullary carcinoma; 25 of 52 (48%) tumors harbored one (n=24) or two KRAS mutations (n=1), including 21 (40%) with one (n=20) or two (n=1) mutations at codon 12, 1 (2%) with a codon 13 mutation (p.G13D), and 3 (6%) with a codon 61 mutation (p.Q61H). Interestingly, one case carried two different types of KRAS mutations, with p.G12R being the major component and p.G12D the minor component, suggesting tumor heterogeneity.
Table 3. Mutations in the EGFR Pathway Genes and SMAD4 in Intestinal- and Pancreatobiliary-Tyoe Ampullary Carcinoma.
| KRAS codon12 |
KRAS codon13 |
KRAS codon61 |
BRAF | PIK3CA | KRAS +PIK3CA |
KRAS +SMAD4 |
KRAS +PIK3CA +SMAD4 |
Total (%) |
|
|---|---|---|---|---|---|---|---|---|---|
| Intestinal (n=25) | 8 | 0 | 0 | 0 | 2 | 2 | 2* | 1 | 15 (60) |
|
Pancreatobiliary
(n=24) |
7 | 0 | 3 | 1 | 1 | 0 | 0 | 0 | 12 (50) |
|
Signet ring cell
(n=1) |
1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100) |
|
Adenosquamous
(n=1) |
1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100) |
|
Undifferentiated
(n=1) |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 (0) |
| Total | 17 | 0 | 3 | 1 | 3 | 2 | 2 | 1 | 29 |
: One of KRAS mutation was in codon 13.
Abbreviations: EGFR – epidermal growth factor receptor
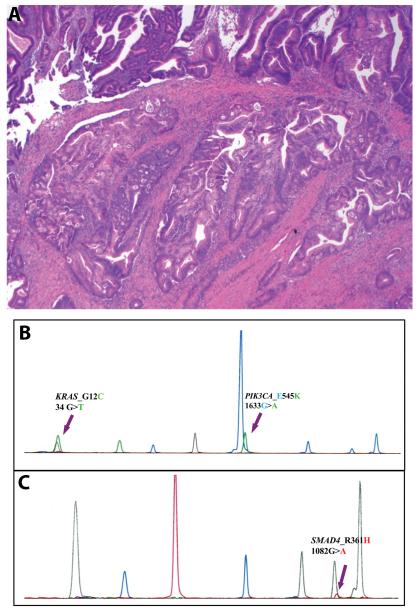
Among the 25 cases with KRAS mutation, 20 (20/52, 38%) had mutation only in the KRAS gene. The other 5 (5/52, 10%) tumors contained coexisting mutation(s) in one or two other genes: 2 with a PIK3CA exon 9 mutation, 2 with a SMAD4 mutation, 1 with both SMAD4 and PIK3CA exon 9 mutation. In the case with 3 gene mutations, KRAS p.G12C mutant cells accounted for the majority of the cells tested, while only approximately 20% of the cells harbored PIK3CA and SMAD4 mutations (Figure 4).
Figure 4.
An intestinal-type ampullary carcinoma with multiple gene mutations. A. An hematoxylin and Eosin section of the tumor showing invasive carcinoma arising in a tubular adenoma (Hemotoxylin and eosin stain, original magnification 100×); B. A panel showing mutations in KRAS and PIK3CA; C. Another panel showing a mutation in SMAD4.
Six of 52 cases (12%) had a mutation in PIK3CA, all in exon 9 (5 p.E545K and 1 p.Q546R). Three of them had coexisting mutations in other genes: 2 KRAS, and 1 KRAS+SMAD4. While PIK3CA can be the only EGFR pathway gene mutated in ampullary carcinoma, SMAD4 mutation seemed to be only detected in the tumors with one or more mutations in other genes. Three of 52 cases (6%) harbored a SMAD4 mutation, all of which had coexisting mutations in KRAS and/or PIK3CA.
Multiple BRAF mutations were analyzed; however, p.V600E is the only mutation detected, and only one case contained the mutation. Curiously, this mutation was detected in one invasive papillary adenocarcinoma, pancreatobiliary-type. Unexpectedly, no intestinal-type tumors (0/25, 0%) had BRAF mutation identified. No mutation was detected in NRAS, PTEN and AKT1.
Twelve of 24 (50%) pancreatobiliary-type tumors harbored one mutation in the genes tested, including 7 (7/24, 29%) in KRAS codon 12, 3 (3/24, 13%) in KRAS codon 61, 1 (1/24, 4%) in PIK3CA codon 545, and 1 (1/24, 4%) in BRAF codon 600. Among the 7 pancreatobiliary-type invasive papillary adenocarcinomas, only two (29%) contained either a KRAS or a BRAF mutation. On the other hand, 10 of 17 (59%) pancreatobiliary-type tumors with conventional ductal adenocarcinoma morphology had either KRAS or PIK3CA mutation. However, statistically there was no difference in the mutation frequency between the two morphologies (p=0.37).
Fifteen of 25 (60%) intestinal-type tumors had one or more mutations. These included 8 (32%) with one (n=7) or two mutations (n=1) in KRAS codon 12 only, 5 (20%) with a mutation in KRAS codon 12 or 13 plus a mutation in PIK3CA and/or SMAD4, and 2 (8%) with a mutation in PIK3CA codon 545 only. KRAS mutation was seen in 13 of 25 (52%) intestinal-type versus 10 of 24 (42%) pancreatobiliary-type tumors. There was no significant difference in KRAS mutation status between the two groups (p=0.57).
Mutations in KRAS codons 12 and 13 have been reported to be a prognostic factor for colonic adenocarcinoma with the mutations being associated with a poor prognosis. The effect of KRAS codon 12 and 13 mutations on overall survival of the patients with ampullary carcinoma was assessed using a Kaplan-Meier analysis, which demonstrated no association with overall survival (Figure 2B). Median (95% CI) for overall survival from date of diagnosis was 45 months (31 to NA) and 44 months (18 to NA) for patients with a tumor with the mutations and for those with a tumor without the mutations (p=0.811).
DISCUSSION
Ampullary carcinomas with intestinal and pancreatobiliary differentiation comprised of the vast majority of carcinomas arising in the ampulla of Vater. While some studies demonstrated that intestinal-type ampullary carcinomas were associated with a better prognosis compared to the pancreatobiliary type[11, 12], others reported that stage by stage, they tended to have the same prognosis[7, 25, 35]. Our results were largely consistent with both findings; univariate analysis showed that patients with a pancreatobiliary-type cancer had a worse overall survival compared to those with an intestinal-type carcinoma, whereas the difference in overall survival became not so significant when the patients’ age and tumor stage were adjusted. Interestingly, our study showed that patients diagnosed with a pancreatobiliary-type carcinoma were older than those with an intestinal-type carcinoma. Older age may partly confer a poorer survival in patients with a pancreatobiliary-type tumor.
Based on the location, Adsay et al. recently classified ampullary carcinomas into 4 different subtypes 1) intra-ampullary carcinoma arising in intra-ampullary papillary-tubular neoplasms; 2) ampullary-ductal infiltrative carcinoma that are presumably due to the pancreatobiliary histology/origin; 3) Peri-ampullary-duodenal carcinoma with significant adenoma component; and 4) ampullary carcinoma-not otherwise specified arising at the papilla of Vater[1]. According to the above classification, the majority of ampullary adenocarcinomas in this series were either ampullary-ductal infiltrative carcinomas or ampullary carcinomas-not otherwise specified. The 7 invasive papillary adenocarcinomas may be classified as intra-ampullary carcinomas. Intra-ampullary carcinomas were associated with a lower TMN stage, and were more likely to be intestinal type in Adsay’s study[1]; however, 6 of the 7 cases in our series were T3N1M0, and all of them were pancreatobiliary type.
EGFR ligands are overexpressed in majority of colonic adenocarcinomas, with amphiregulin being the EGFR ligand with the most enhanced expression[36]. Amphiregulin has been shown to stimulate EGFR activation and tumor cell growth in colon cancer cell lines [20]. In addition, amphiregulin expression may be related to disease-free survival and liver metastasis[36-40]. In pancreatic ductal adenocarcinoma, amphiregulin has been demonstrated to be involved in the tumor progression through the activation of EGFR[19, 41]. Amphiregulin is also a potential target for pancreatic cancer therapy[19, 42]. Additionally, negative expression of amphiregulin was reported to be associated with a unfavorable prognosis in pancreatic cancer[43]. The present study showed that amphiregulin was frequently expressed by both pancreatobiliary- and intestinal- type ampullary carcinoma. Expression of amphiregulin was associated with better differentiation. However, no difference in amphiregulin expression was observed in two major histologic types. Other variants of ampullary carcinoma including adenosquamous carcinoma, signet ring cell carcinoma and undifferentiated carcinoma displayed no to little amphiregulin expression.
Enhanced EGFR expression is also observed in both colorectal cancer and pancreatic ductal adenocarcinoma[13-18]. EGFR overexpression and activation were detected in up to 50% of colonic adenocarcinomas[44, 45]. However, the expression and activation of EGFR may be infrequent in intestinal-type ampullary carcinomas, as only one intestinal-type tumor expressed EGFR and none of them had activation of EGFR detected by immunohistochemistry. On the other hand, a significant portion of pancreatobiliary-type ampullary carcinomas had expression and/or activation of EGFR detected by immunohistochemistry. These results suggest that EGFR may play different roles in carcinogenesis of the two histologic types. In addition, our results demonstrated that positive pEGFR expression was associated with lower amphiregulin expression, indicating that amphiregulin may not be a major contributor for activation of EGFR in ampullary adenocarcinoma.
Frequencies of KRAS, BRAF, and PIK3CA mutations have been described in carcinomas of pancreatobiliary origin. The reported prevalence of KRAS mutation in extrahepatic cholangiocarcinomas ranges from 0-100%, whereas BRAF and PI3KCA mutations have been consistently rare in all studies[46-49]. One study specifically looked into mutational spectrum of 13 carcinomas of the distal common bile duct and found no mutations in these three genes[48]. Like extrahepatic cholangiocarcinoma, mutations in BRAF and PIK3CA are also infrequent in pancreatic ductal adenocarcinoma; however, KRAS is almost universally mutated in this cancer[50-52]. A few studies have investigated mutations of KRAS and BRAF in ampullary carcinoma. While 20-67% of ampullary carcinomas harbor KRAS mutation, only up to 10% have a mutation in BRAF[4, 53, 54]. Only one study examined PIK3CA mutation in ampullary carcinomas and reported no PIK3CA mutations in 21 cases[54]. Consistent to these studies we observed approximately 50% of ampullary carcinomas with one or two KRAS mutations and only one case (2%) with a BRAF mutation. In addition, we also detected PIK3CA mutation in 10% of the cases.
As described above, activating mutations in the KRAS proto-oncogene occur almost ubiquitously in pancreatic ductal adenocarcinoma[51], with the vast majority of the mutations occurring at codon 12. On the contrary, we only observed KRAS mutations in 10 of 24 (42%) pancreatobiliary-type carcinomas: 7 (29%) at codon 12 and 3 (13%) at codon 61. Mutation at KRAS codon 61 is a rare event in pancreatic ductal adenocarcinoma. These data suggest that KRAS may play different roles in carcinogenesis of pancreatobiliary-type ampullary carcinoma and pancreatic ductal adenocarcinoma. On the other hand, our data showed that pancreatobiliary-type ampullary carcinoma frequently expressed EGFR and in some cases activated EGFR was detected by immunohistochemistry. Therefore, it is rational to postulate that anti-EGFR therapy may be effective at least for some pancreatobiliary-type ampullary carcinomas, in contrast to pancreatic ductal adenocarcinoma, where essentially no response is observed due to an almost universal KRAS mutation. Therefore, KRAS mutational analysis of ampullary carcinoma could guide treatment of patients with advanced stage ampullary carcinoma.
In the pancreatobiliary histologic type, invasive papillary adenocarcinoma variant showed a unique histologic morphology[55]. The tumors were composed of cuboidal pancreatobiliary type cancer cells, and therefore were classified as pancreatobiliary type. Rather than forming tubular structures seen in conventional pancreatic ductal adenocarcinoma, the cancer cells formed a complex architecture with prominent secondary micropapillae. The subgroup was strongly associated with amphiregulin overexpression, with 6 of 7 cases being intensely and diffusely labeled with anti-amphiregulin antibody. KRAS mutation was only detected in 1 of 7 (15%) of these cases, and one tumor harbored a BRAF V600E mutation. It appeared that the molecular mechanism underlying this subgroup is different from that of ampullary adenocarcinoma with a morphology similar to pancreatic ductal adenocarcinoma; however, the sample size is small, and therefore a definitive difference cannot be determined.
Approximately 50% intestinal-type ampullary carcinomas had a mutation in the KRAS gene, which is compatible with colonic adenocarcinoma. Similar to colonic adenocarcinoma, a PI3KCA mutation was detected in about 20% of intestinal-type ampullary carcinoma, with some of the mutations coexisting with a KRAS and/or a SMAD4 mutation[23, 24, 27]. BRAF mutation is detected in 10-15% of colonic adenocarcinomas [23, 56-58]. In our series, none of 25 intestinal-type cancers harbored a mutation in the BRAF gene. It is well known that BRAF gene mutation is more frequently seen in colon cancers that follow a serrated pathway. Our data suggests that the serrated pathway is rarely involved in carcinogenesis of ampullary carcinoma.
In conclusion, alterations in the EGFR signaling pathway are frequently present in ampullary carcinoma. KRAS mutation is common; however, compared with conventional pancreatic ductal adenocarcinoma, the incidence of KRAS mutation is lower in pancreatobiliary-type ampullary carcinoma. Therefore, anti-EGFR therapy might be effective in some ampullary carcinomas, including both histologic types. Mutational analysis of the EGFR pathway genes in ampullary carcinoma may provide valuable information for cancer treatment.
ACKNOWLEDGEMENT
This project is funded by NIH/NCI P50CA095103 (KW and CS)
Footnotes
Disclosure of Potential Conflicts of Interest:
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Adsay V, Ohike N, Tajiri T, Kim GE, Krasinskas A, Balci S, et al. Ampullary region carcinomas: definition and site specific classification with delineation of four clinicopathologically and prognostically distinct subsets in an analysis of 249 cases. Am J Surg Pathol. 2012;36:592–1608. doi: 10.1097/PAS.0b013e31826399d8. [DOI] [PubMed] [Google Scholar]
- 2.Benhamiche AM, Jouve JL, Manfredi S, Prost P, Isambert N, Faivre J. Cancer of the ampulla of Vater: results of a 20-year population-based study. Eur J Gastroenterol Hepatol. 2000;12:75–9. doi: 10.1097/00042737-200012010-00014. [DOI] [PubMed] [Google Scholar]
- 3.Kimura W, Futakawa N, Yamagata S, Wada Y, Kuroda A, Muto T, et al. Different clinicopathologic findings in two histologic types of carcinoma of papilla of Vater. Jpn J Cancer Res. 1994;85:161–6. doi: 10.1111/j.1349-7006.1994.tb02077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohler I, Jacob D, Budzies J, Lehmann A, Weichert W, Schulz S, et al. Phenotypic and genotypic characterization of carcinomas of the papilla of Vater has prognostic and putative therapeutic implications. Am J Clin Pathol. 2011;135:202–11. doi: 10.1309/AJCPCTCUQSYI89YT. [DOI] [PubMed] [Google Scholar]
- 5.Chu PG, Schwarz RE, Lau SK, Yen Y, Weiss LM. Immunohistochemical staining in the diagnosis of pancreatobiliary and ampulla of Vater adenocarcinoma: application of CDX2, CK17, MUC1, and MUC2. Am J Surg Pathol. 2005;29:359–67. doi: 10.1097/01.pas.0000149708.12335.6a. [DOI] [PubMed] [Google Scholar]
- 6.Baumhoer D, Zlobec I, Tornillo L, Dietmaier W, Wuensch PH, Hartmann A, et al. Immunophenotyping and oncogene amplifications in tumors of the papilla of Vater. Virchows Arch. 2008;453:579–88. doi: 10.1007/s00428-008-0669-7. [DOI] [PubMed] [Google Scholar]
- 7.Zhou H, Schaefer N, Wolff M, Fischer HP. Carcinoma of the ampulla of Vater: comparative histologic/immunohistochemical classification and follow-up. Am J Surg Pathol. 2004;28:875–82. doi: 10.1097/00000478-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Matsubayashi H, Watanabe H, Ajioka Y, Nishikura K, Yamano M, Seki T, et al. Different amounts of K-ras mutant epithelial cells in pancreatic carcinoma and mass-forming pancreatitis. Pancreas. 2000;21:77–85. doi: 10.1097/00006676-200007000-00055. [DOI] [PubMed] [Google Scholar]
- 9.Sessa F, Furlan D, Zampatti C, Carnevali I, Franzi F, Capella C. Prognostic factors for ampullary adenocarcinomas: tumor stage, tumor histology, tumor location, immunohistochemistry and microsatellite instability. Virchows Arch. 2007;451:649–57. doi: 10.1007/s00428-007-0444-1. [DOI] [PubMed] [Google Scholar]
- 10.Fischer HP, Zhou H. Pathogenesis of carcinoma of the papilla of Vater. J Hepatobiliary Pancreat Surg. 2004;11:301–9. doi: 10.1007/s00534-004-0898-3. [DOI] [PubMed] [Google Scholar]
- 11.Kim WS, Choi DW, Choi SH, Heo JS, You DD, Lee HG. Clinical significance of pathologic subtype in curatively resected ampulla of vater cancer. J Surg Oncol. 2012;105:266–72. doi: 10.1002/jso.22090. [DOI] [PubMed] [Google Scholar]
- 12.Carter JT, Grenert JP, Rubenstein L, Stewart L, Way LW. Tumors of the ampulla of vater: histopathologic classification and predictors of survival. J Am Coll Surg. 2008;207:210–8. doi: 10.1016/j.jamcollsurg.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 13.Kim ES. Epidermal growth factor receptor as a target in cancer therapy. J Natl Compr Canc Netw. 2003;1(Suppl 1):S87–95. [PubMed] [Google Scholar]
- 14.O’Dwyer PJ, Benson AB., 3rd Epidermal growth factor receptor-targeted therapy in colorectal cancer. Semin Oncol. 2002;29:10–17. doi: 10.1053/sonc.2002.35643. [DOI] [PubMed] [Google Scholar]
- 15.Cohen RB. Epidermal growth factor receptor as a therapeutic target in colorectal cancer. Clin Colorectal Cancer. 2003;2:246–51. doi: 10.3816/CCC.2003.n.006. [DOI] [PubMed] [Google Scholar]
- 16.Valsecchi ME, McDonald M, Brody JR, Hyslop T, Freydin B, Yeo CJ, et al. Epidermal growth factor receptor and insulinlike growth factor 1 receptor expression predict poor survival in pancreatic ductal adenocarcinoma. Cancer. 2012;118:3484–93. doi: 10.1002/cncr.26661. [DOI] [PubMed] [Google Scholar]
- 17.Tzeng CW, Frolov A, Frolova N, Jhala NC, Howard JH, Vickers SM, et al. EGFR genomic gain and aberrant pathway signaling in pancreatic cancer patients. J Surg Res. 2007;143:20–6. doi: 10.1016/j.jss.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 18.Higginbotham JN, Demory Beckler M, Gephart JD, Franklin JL, Bogatcheva G, Kremers GJ, et al. Amphiregulin exosomes increase cancer cell invasion. Curr Biol. 2011;21:779–86. doi: 10.1016/j.cub.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yotsumoto F, Fukami T, Yagi H, Funakoshi A, Yoshizato T, Kuroki M, et al. Amphiregulin regulates the activation of ERK and Akt through epidermal growth factor receptor and HER3 signals involved in the progression of pancreatic cancer. Cancer Sci. 2010;101:2351–60. doi: 10.1111/j.1349-7006.2010.01671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merchant NB, Rogers CM, Trivedi B, Morrow J, Coffey RJ. Ligand-dependent activation of the epidermal growth factor receptor by secondary bile acids in polarizing colon cancer cells. Surgery. 2005;138:415–421. doi: 10.1016/j.surg.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 21.Bardelli A, Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol. 2010;28:1254–61. doi: 10.1200/JCO.2009.24.6116. [DOI] [PubMed] [Google Scholar]
- 22.Silva CM. Role of STATs as downstream signal transducers in Src family kinase-mediated tumorigenesis. Oncogene. 2004;23:8017–23. doi: 10.1038/sj.onc.1208159. [DOI] [PubMed] [Google Scholar]
- 23.Sun T, Simon I, Moreno V, Roepman P, Tabernero J, Snel M, et al. A combined oncogenic pathway signature of BRAF, KRAS and PI3KCA mutation improves colorectal cancer classification and cetuximab treatment prediction. GUT. 2013;62:540–9. doi: 10.1136/gutjnl-2012-302423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–62. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 25.Tol J, Dijkstra JR, Klomp M, Teerenstra S, Dommerholt M, Vink-Borger ME, et al. Markers for EGFR pathway activation as predictor of outcome in metastatic colorectal cancer patients treated with or without cetuximab. Eur J Cancer. 2010;46:1997–2009. doi: 10.1016/j.ejca.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 26.Lievre A, Blons H, Laurent-Puig P. Oncogenic mutations as predictive factors in colorectal cancer. Oncogene. 2010;29:3033–43. doi: 10.1038/onc.2010.89. [DOI] [PubMed] [Google Scholar]
- 27.Baldus SE, Schaefer KL, Engers R, Hartleb D, Stoecklein NH, Gabbert HE. Prevalence and heterogeneity of KRAS, BRAF, and PIK3CA mutations in primary colorectal adenocarcinomas and their corresponding metastases. Clin Cancer Res. 2010;16:790–9. doi: 10.1158/1078-0432.CCR-09-2446. [DOI] [PubMed] [Google Scholar]
- 28.Immervoll H, Hoem D, Kugarajh K, Steine SJ, Molven A. Molecular analysis of the EGFR-RAS-RAF pathway in pancreatic ductal adenocarcinomas: lack of mutations in the BRAF and EGFR genes. Virchows Arch. 2006;448:788–96. doi: 10.1007/s00428-006-0191-8. [DOI] [PubMed] [Google Scholar]
- 29.Moore PS, Sipos B, Orlandini S, Sorio C, Real FX, Lemoine NR, et al. Genetic profile of 22 pancreatic carcinoma cell lines. Analysis of K-ras, p53, p16 and DPC4/Smad4. Virchows Arch. 2001;439:798–802. doi: 10.1007/s004280100474. [DOI] [PubMed] [Google Scholar]
- 30.Koyama M, Ito M, Nagai H, Emi M, Moriyama Y. Inactivation of both alleles of the DPC4/SMAD4 gene in advanced colorectal cancers: identification of seven novel somatic mutations in tumors from Japanese patients. Mutat Res. 1999;406:71–7. doi: 10.1016/s1383-5726(99)00003-5. [DOI] [PubMed] [Google Scholar]
- 31.Schutte M. DPC4/SMAD4 gene alterations in human cancer, and their functional implications. Ann Oncol. 1999;10(Suppl 4):56–9. [PubMed] [Google Scholar]
- 32.Woodford-Richens KL, Rowan AJ, Gorman P, Halford S, Bicknell DC, Wasan HS, et al. SMAD4 mutations in colorectal cancer probably occur before chromosomal instability, but after divergence of the microsatellite instability pathway. Proc Natl Acad Sci U S A. 2001;98:9719–23. doi: 10.1073/pnas.171321498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su Z, Dias-Santagata D, Duke M, Hutchinson K, Lin YL, Borger DR, et al. A platform for rapid detection of multiple oncogenic mutations with relevance to targeted therapy in non-small-cell lung cancer. J Mol Diagn. 2011;13:74–84. doi: 10.1016/j.jmoldx.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Team RDC . A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. [Google Scholar]
- 35.de Paiva Haddad LB, Patzina RA, Penteado S, Montagnini AL, da Cunha JE, Machado MC, et al. Lymph node involvement and not the histophatologic subtype is correlated with outcome after resection of adenocarcinoma of the ampulla of vater. J Gastrointest Surg. 2010;14:719–28. doi: 10.1007/s11605-010-1156-4. [DOI] [PubMed] [Google Scholar]
- 36.Ohchi T, Akagi Y, Kinugasa T, Kakuma T, Kawahara A, Sasatomi T, et al. Amphiregulin is a prognostic factor in colorectal cancer. Anticancer Res. 2012;32:2315–21. [PubMed] [Google Scholar]
- 37.Yamada M, Ichikawa Y, Yamagishi S, Momiyama N, Ota M, Fujii S, et al. Amphiregulin is a promising prognostic marker for liver metastases of colorectal cancer. Clin Cancer Res. 2008;14:2351–6. doi: 10.1158/1078-0432.CCR-07-4499. [DOI] [PubMed] [Google Scholar]
- 38.Li XD, Miao SY, Wang GL, Yang L, Shu YQ, Yin YM. Amphiregulin and epiregulin expression in colorectal carcinoma and the correlation with clinicopathological characteristics. Onkologie. 2010;33:353–8. doi: 10.1159/000315380. [DOI] [PubMed] [Google Scholar]
- 39.Kuramochi H, Nakajima G, Kaneko Y, Nakamura A, Inoue Y, Yamamoto M, et al. Amphiregulin and Epiregulin mRNA expression in primary colorectal cancer and corresponding liver metastases. BMC Cancer. 2012;12:88. doi: 10.1186/1471-2407-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230–7. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 41.Dong A, Gupta A, Pai RK, Tun M, Lowe AW. The human adenocarcinoma-associated gene, AGR2, induces expression of amphiregulin through Hippo pathway co-activator YAP1 activation. J Biol Chem. 2011;286:18301–10. doi: 10.1074/jbc.M110.215707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yotsumoto F, Yagi H, Suzuki SO, Oki E, Tsujioka H, Hachisuga T, et al. Validation of HB-EGF and amphiregulin as targets for human cancer therapy. Biochem Biophys Res Commun. 2008;365:555–61. doi: 10.1016/j.bbrc.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 43.Park JK, Kim MA, Ryu JK, Yoon YB, Kim SW, Han HS, et al. Postoperative prognostic predictors of pancreatic ductal adenocarcinoma: clinical analysis and immunoprofile on tissue microarrays. Ann Surg Oncol. 2012;19:2664–72. doi: 10.1245/s10434-012-2277-7. [DOI] [PubMed] [Google Scholar]
- 44.Rego RL, Foster NR, Smyrk TC, Le M, O’Connell MJ, Sargent DJ, et al. Prognostic effect of activated EGFR expression in human colon carcinomas: comparison with EGFR status. Br J Cancer. 2010;102:165–72. doi: 10.1038/sj.bjc.6605473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galizia G, Lieto E, Ferraraccio F, De Vita F, Castellano P, Orditura M, et al. Prognostic significance of epidermal growth factor receptor expression in colon cancer patients undergoing curative surgery. Ann Surg Oncol. 2006;13:823–35. doi: 10.1245/ASO.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 46.Voss JS, Holtegaard LM, Kerr SE, Fritcher EG, Roberts LR, Gores GJ, et al. Molecular profiling of cholangiocarcinoma shows potential for targeted therapy treatment decisions. Hum Pathol. 2013;44:1216–22. doi: 10.1016/j.humpath.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Hezel AF, Deshpande V, Zhu AX. Genetics of biliary tract cancers and emerging targeted therapies. J Clin Oncol. 2010;28:3531–40. doi: 10.1200/JCO.2009.27.4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deshpande V, Nduaguba A, Zimmerman SM, Kehoe SM, Macconaill LE, Lauwers GY, et al. Mutational profiling reveals PIK3CA mutations in gallbladder carcinoma. BMC Cancer. 2011;11:60. doi: 10.1186/1471-2407-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levi S, Urbano-Ispizua A, Gill R, Thomas DM, Gilbertson J, Foster C, et al. Multiple K-ras codon 12 mutations in cholangiocarcinomas demonstrated with a sensitive polymerase chain reaction technique. Cancer Res. 1991;51:3497–502. [PubMed] [Google Scholar]
- 50.Wood LD, Hruban RH. Pathology and molecular genetics of pancreatic neoplasms. Cancer J. 2012;18:492–501. doi: 10.1097/PPO.0b013e31827459b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ottenhof NA, de Wilde RF, Maitra A, Hruban RH, Offerhaus GJ. Molecular characteristics of pancreatic ductal adenocarcinoma. Patholog Res Int. 2011;2011:620601. doi: 10.4061/2011/620601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–49. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 53.Schultz NA, Roslind A, Christensen IJ, Horn T, Hogdall E, Pedersen LN, et al. Frequencies and prognostic role of KRAS and BRAF mutations in patients with localized pancreatic and ampullary adenocarcinomas. Pancreas. 2012;41:759–66. doi: 10.1097/MPA.0b013e31823cd9df. [DOI] [PubMed] [Google Scholar]
- 54.Schonleben F, Qiu W, Allendorf JD, Chabot JA, Remotti HE, Su GH. Molecular analysis of PIK3CA, BRAF, and RAS oncogenes in periampullary and ampullary adenomas and carcinomas. J Gastrointest Surg. 2009;13:1510–16. doi: 10.1007/s11605-009-0917-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoang MP, Murakata LA, Katabi N, Henson DE, Albores-Saavedra J. Invasive papillary carcinomas of the extrahepatic bile ducts: a clinicopathologic and immunohistochemical study of 13 cases. Mod Pathol. 2002;15:1251–8. doi: 10.1097/01.MP.0000036450.61830.8E. [DOI] [PubMed] [Google Scholar]
- 56.Phipps AI, Buchanan DD, Makar KW, Burnett-Hartman AN, Coghill AE, Passarelli MN, et al. BRAF Mutation Status and Survival after Colorectal Cancer Diagnosis According to Patient and Tumor Characteristics. Cancer Epidemiol Biomarkers Prev. 2012;21:1792–8. doi: 10.1158/1055-9965.EPI-12-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dienstmann R, Serpico D, Rodon J, Saura C, Macarulla T, Elez E, et al. Molecular profiling of patients with colorectal cancer and matched targeted therapy in phase I clinical trials. Mol Cancer Ther. 2012;11:2062–71. doi: 10.1158/1535-7163.MCT-12-0290. [DOI] [PubMed] [Google Scholar]
- 58.Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–3. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]