Background: Smoking causes a global down-regulation in alveolar macrophage miRNA expression.
Results: Cigarette smoke exposure modifies the RNA endonuclease DICER, resulting in a microRNA processing defect.
Conclusion: Cigarette smoke alters alveolar macrophage microRNA expression, in part, by SUMOylation of DICER.
Significance: This is the first description of an environmental exposure causing changes in microRNA expression via post-translational modification of DICER.
Keywords: Chronic Obstructive Pulmonary Disease (COPD), Cigarette Smoke, DICER, Epigenetics, Macrophage, MicroRNA (miRNA), Small Ubiquitin-like Modifier (SUMO), SUMOylation
Abstract
Despite the fact that alveolar macrophages play an important role in smoking-related disease, little is known about what regulates their pathophysiologic phenotype. Evaluating smoker macrophages, we found significant down-regulation of multiple microRNAs (miRNAs). This work investigates the hypothesis that cigarette smoke alters mature miRNA expression in lung macrophages by inhibiting processing of primary miRNA transcripts. Studies on smoker alveolar macrophages showed a defect in miRNA maturation. Studies on the miRNA biogenesis machinery led us to focus on the cytosolic RNA endonuclease, DICER. DICER cleaves the stem-loop structure from pre-miRNAs, allowing them to dissociate into their mature 20–22-nucleotide single-stranded form. DICER activity assays confirmed impaired DICER activity following cigarette smoke exposure. Further protein studies demonstrated a decreased expression of the native 217-kDa form of DICER and an accumulation of high molecular weight forms with cigarette smoke exposure. This molecular mass shift was shown to contain SUMO moieties and could be blocked by silencing RNA directed at the primary SUMOylating ligase, Ubc9. In determining the cigarette smoke components responsible for changes in DICER, we found that N-acetylcysteine, an antioxidant and anti-aldehyde, protected DICER protein and activity from cigarette smoke extract. This massive down-regulation of miRNAs (driven in part by alterations in DICER) may be an important regulator of the disease-promoting macrophage phenotype found in the lungs of smokers.
Introduction
COPD2 is characterized by inflammation in both small airways and alveoli, eventually leading to irreversible airflow limitation and parenchymal emphysema. Lung pathology in patients with COPD has been largely attributed to neutrophil elastases and macrophage proteinases (1, 2). Although multiple lung cell types contribute to the pathogenesis of COPD, evidence strongly supports a key role for alveolar macrophages (3–5). Inhaled tobacco smoke triggers disease-relevant changes in alveolar macrophage gene expression (i.e. MMP12) (6, 7). Although the regulation of disease-relevant genes is complex and multifactorial, increasing experimental evidence suggests that macrophage gene expression is strongly influenced by miRNAs (8, 9). Our laboratory has demonstrated that cigarette smoking is associated with an impressive decrease in alveolar macrophage miRNA expression (10). Detailing the mechanism(s) behind this significant decrease in miRNA levels could shed light on the pathophysiology of smoking-related lung diseases and improve understanding of the adverse health effects of cigarettes in general.
The role of miRNA levels in regulating cellular genomic tone is an area of active investigation (11–13). Primary miRNAs are transcribed in the nucleus and processed into precursor miRNA (pre-miRNA) by a nuclear endoribonuclease, DROSHA, in complex with a double-stranded RNA-binding protein, Pasha (known as DGCR8 in vertebrates). The pre-miRNA is an ∼70-base pair double-stranded RNA stem-loop structure that contains the imbedded 22-nucleotide sequence complementary to the mRNA target of interest. Pre-miRNAs are exported from the nucleus, where the cytoplasmic endoribonuclease, DICER, excises the stem-loop, yielding a 20–25-base pair RNA duplex (14, 15). In the cytosol, DICER, in complex with other protein components (transactivating response RNA-binding protein (TRBP), protein kinase RNA activator (PACT), and Argonaut protein Ago2) binds the duplex, slices out the anti-complementary “passenger” RNA strand, and targets it for destruction. The mature miRNA and processing proteins form an RNA-induced silencing complex, which represses translation or enhances degradation of the targeted complementary mRNA. Thus, up- or down-regulation of an miRNA can result in specific regulation of gene expression. The miRNA biogenesis pathway has several potential points for regulatory control. In this paper, we present evidence that cigarette smoke exposure leads to a post-translational modification of DICER, resulting in decreased ribonuclease activity and contributing to a generalized decrease in miRNA expression in smokers' alveolar macrophages.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Antibodies used in this study were obtained from a variety of sources: anti-HA, Roche Applied Science, catalog no. 11867423001; anti-DICER, Abcam, catalog no. ab14601; anti-PACT, Abcam, catalog no. ab75749; anti-TRBP, Abcam, catalog no. ab42018; anti β-actin, Abcam, catalog no. ab8226; anti-UBC9, Abcam, catalog no. ab21193; anti-SUMO 2/3, Abcam, catalog no. ab3742; anti-ubiquitin, Cell Signaling, catalog no. 3936. Horseradish peroxidase-conjugated anti-rabbit (sc-2004), anti-mouse (sc-2005), and anti-goat (sc-2020) antibodies were all obtained from Santa Cruz Biotechnology, Inc. Culture media used in experiments included serum-free RPMI tissue culture medium with Glutamax (Invitrogen, catalog no. 61870-036) and DMEM (Invitrogen, catalog no. 11095) with 10% FCS (Hyclone Fetal Bovine Serum, SH30071).
Ethics Statement
The University of Iowa Institutional Review Board approved all procedures and protocols described in this work. Written informed consent was obtained, and all clinical investigation has been conducted according to the principles expressed in the Declaration of Helsinki.
Alveolar Macrophage Donors
Subjects were recruited from the community through the Iowa Institute for Clinical and Translational Science Clinical Core. Inclusion criteria for smoking subjects required at least a 10-pack year history of ongoing cigarette smoking, whereas the nonsmoker control subjects were self-reported never smokers. Subjects were excluded if they had any significant co-morbid conditions, such as pregnancy or other acute or chronic disease, such as preexisting asthma, interstitial lung disease, or cardiovascular disease. Subjects were also excluded if a baseline spirometry revealed that the forced expiratory volume in the first second was less than 60% of the predicted value based on the National Health and Nutrition Examination Survey III data set. None of the subjects were using inhaled corticosteroids.
Bronchoalveolar Lavage
After informed consent was obtained, subjects underwent standard flexible bronchoscopy (16). Local anesthesia with lidocaine instillation into the upper airway was followed by bronchoalveolar lavage, whereby 20 ml of normal saline was instilled into a tertiary bronchus up to five times in three different lung segments. The first collection out of five was discarded to avoid possible contamination with upper airway secretions or lidocaine. The remaining lavage was filtered through sterile gauze and centrifuged at 200 × g for 5 min to pellet cellular material. The resulting pellet was suspended in phosphate-buffered saline (PBS) and centrifuged at 200 × g for 5 min. A sample of the cells were labeled with Wright stain and microscopically examined to determine the proportion of the cells that were macrophages (17–20). Aliquots of 5 × 106 cells were stored at −80 °C until the RNA isolation procedure was performed. Cell yields from bronchoscopy of nonsmokers averaged 25 ± 3 × 106 cells and for smokers averaged 67 ± 4 × 106. In all three cohorts, the procedure generated a relatively pure population of alveolar macrophages with fewer than 5% neutrophils or lymphocytes in the bronchoalveolar lavage fluid.
CSE Production and Exposure
Cigarette smoke extract was prepared as described previously (21, 22). CSE solutions were prepared using a modification of the method of Blue and Janoff (23). 10 ml of sterile serum-free RPMI tissue culture media with Glutamax was drawn into a 50-ml plastic syringe. Then 40 ml of cigarette smoke was drawn into the syringe and mixed with the medium by vigorously shaking them together. The smoke was expelled, and the process was repeated until one cigarette was used up. The generated CSE solution was filtered (0.22 μm) to remove large particles (24, 25). The resulting solution was designated a 100% CSE solution and was used immediately after generation. For the CSE dialysis experiment, we dialyzed 5% CSE overnight using Thermo Scientific's 2000 and 10,000 molecular weight cut-off Slide-A-Lyzer Dialysis Cassette GP cassettes.
RNA Isolation
RNA was isolated from alveolar macrophages using the mirVana miRNA Isolation kit (Applied Biosystems (ABI)). The quantity and quality of the RNA samples was assessed using an Experion automated electrophoresis station (Bio-Rad). The RNA quality indicator was above 8 for all samples, where values of greater than 8 indicate primarily intact RNA on a scale of 1–10. After preparation, RNA samples were stored at −80 °C until use.
miRNA Expression Analysis
RNA from alveolar macrophages of nonsmokers and active smokers was reverse transcribed with MultiScribe reverse transcriptase (ABI) using Megaplex Primers version 2.0 (ABI). Changes in miRNA expression were then determined using human TaqMan Low Density Arrays version 2.0 (ABI). Ct values calculated using SDS version 2.4 (ABI) were exported to the Partek Genomics Suite to calculate smoker-to-nonsmoker expression ratios. Robust multiarray averaging (RMA)-normalized data were subjected to an analysis of variance model with linear contrasts to calculate p values. Individual miRNA-specific assays were done using ABI kits specific for the mature miRNA or for the primary transcript (before processing).
mRNA Expression Analysis
Genome-wide macrophage mRNA expression was obtained using the GeneChip Human Exon 1.0 ST Arrays (Affymetrix). Generation of labeled cDNA, hybridizations, and scanning of the microarray were performed under contract by the University of Iowa DNA facility. The resulting data were analyzed using the Partek Genomics Suite version 6.5 (Partek). The data were assessed for quality and subjected to RMA normalization. The normalized data were then analyzed using an analysis of variance model with linear contrasts to calculate p values and smoker/nonsmoker expression ratios. The false discovery rate step-up method (26) was applied to correct for multiple testing. The expression data have been deposited in the NCBI GEO repository (accession number GSE34517). To generate the data shown in Fig. 3E, we mined RNA array data performed on alveolar macrophages from 43 nonsmokers and 42 smokers. The log 2 values obtained by the initial Partek analysis for the gene DICER are shown in an individual value plot. Possible significance between the two groups was assessed by Student's t test.
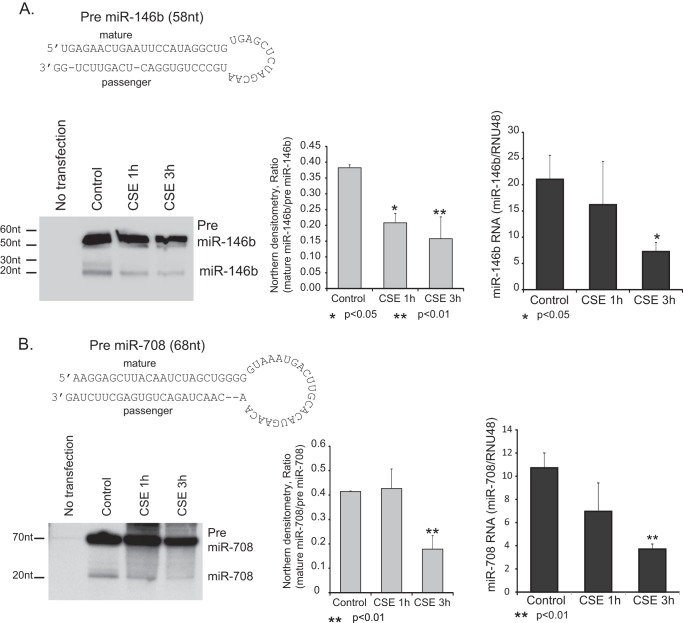
FIGURE 3.
Cigarette smoke exposure decreases pre-miRNA processing activity. Two highly CSE regulated miRNAs were chosen for an in vivo pre-miRNA processing assay. A and B, transfection with either pre-miR-146b (A) or pre-miR-708 (B) and processing of the respective miRNAs were evaluated by Northern blot and qRT-PCR. HeLa cells were transfected with a plasmid expressing pre-miRNAs under control of the constitutive H1 promoter. 24 h after transfection, cells were exposed to 1 or 3 h of CSE, and RNA was isolated. Northern analysis was performed to demonstrate the relative degree of pre-miRNA processing. qRT-PCR to compare pre-miRNA to mature miRNA levels was also performed. All assays were done in triplicate, and statistical analysis for both the densitometry data and qRT-PCR was performed (Student's t test). Error bars, S.E.
Whole Cell Protein Isolation
Whole cell protein was obtained by lysing the cells on ice for 20 min in 200 μl of lysis buffer (0.05 m Tris, pH 7.4, 0.15 m NaCl, 1% Nonidet P-40 with added protease and phosphatase inhibitors: 1 protease Minitab (Roche Applied Science)/10 ml and 100 μl of 100× phosphatase inhibitor mixture (Calbiochem)/10 ml. The lysates were sonicated for 20 s, kept at 4 ºC for 30 min, and spun at 15,000 × g for 10 min, and the supernatant was saved. Protein determinations were made using the Bradford protein assay (Bio-Rad). Cell lysates were stored at −70° until use.
Western Analysis
Whole cell protein was obtained as described previously (27). Following protein purification, Western analysis was performed following protocols as described by Reisetter et al. (16). The following antibodies were used: anti-HA, Roche Applied Science, catalog no. 11867423001; anti-DICER, Abcam, catalog no. ab14601; anti-PACT, Abcam, catalog no. ab75749; anti-TRBP, Abcam, catalog no. ab42018; anti-β-actin, Abcam, catalog no. ab8226; anti-UBC9, Abcam, catalog no. ab21193; anti-SUMO 2/3, Abcam, catalog no. ab3742; anti-ubiquitin, Cell Signaling, catalog no. 3936.
Immunoprecipitation
Immunoprecipitation of DICER was performed on alveolar macrophages or THP-1 cells. Immunoprecipitation of cellular protein was performed using the protein G Dynabead immunoprecipitation kit (Invitrogen) as per the manufacturer's instructions. Immunoprecipitation was used in the protein modification assays and in the DICER activity assay. DICER was pulled down using an antibody to DICER (Abcam, catalog no. ab14601).
In Vitro DICER Activity Assay
An in vitro DICER activity assay was used to test the effect of smoking on DICER activity (28). Briefly, whole cell protein extracts were adjusted to 1 mg/ml and 25 μl of the lysate incubated with 125 pmol of a 27-mer RNA duplex DICER substrate (DsiRNAs, IDT) and incubated at 37 °C for 8 h. To evaluate the conversion of 27-mer dsRNA substrate to 21-mer dsRNA product, 2.5 μl of extract postincubation was resolved on a 3.5–20% non-denaturing polyacrylamide gel and visualized with SYBR Green fluorescent dye. DICER activity was calculated based on the percentage of 27-mer converted to a 21-mer RNA strand. The fluorescent image from a Bio-Rad VersaDoc imaging system was converted to a tiff file and analyzed for pixels per band using ImageJ, a public domain Java image-processing program inspired by NIH Image (National Institutes of Health).
In Vivo DICER Activity Assay
To measure DICER activity within cells, vectors were made expressing pre-miRNAs for two of the highly smoking-altered miRNAs (miR-146b and miR-708). HeLa cells were transfected with the pre-miRNA constructs, and expression was allowed to proceed overnight. Cells were then exposed to CSE for a short time (1 and 3 h, chosen to show DICER altered but not depleted), and RNA was harvested. Generation of mature miRs was determined by both qRT-PCR and Northern analysis.
Pre-miRNA Cloning
Primers for the H1 promoter that included sequence for the pre-miR of interest were obtained from Integrated DNA Technologies (Coralville, IA). These included H1 promoter forward primer (5′-ccatggaattcgaacgctgacg-3′) and reverse primers for either miR-146b (5′-aaaactcgagaaaaaacagaactgagtccacagggcattgctagagctcacagcctatggaattcagttctcagggaaagagtggtctcagacagaac-3′) or miR-708 (3′-aaaactcgagaaaaaactagaagctcacagtctagttgtgttcatgtgcaagtcatttacccccagctagattgtaagctccttgggaaagagtggtctcagacagaac-3′). PCR was performed using the H1 plasmid as a substrate (as described previously (29). The resulting product (H1 promoter followed by the specific pre-miR) was cloned into a TOPO cloning vector (pCR 4-TOPO TA cloning vector) using the manufacturer's protocol. Kanamycin/ampicillin-resistant clones were selected and amplified, and DNA was isolated. Selected clones were grown and sequenced to verify the insert.
Northern Analysis
Isolated RNA was run on a 12% polyacrylamide gel (19:1 bis/7 m urea) in 0.5× TBE. The gel was blotted onto Hybond N+ membrane and probed with a 32P end-labeled oligonucleotide designed to bind both the mature miR and pre-miRNA sequence (miR-146b, 5′-GAGCCTATGGAATTCAGTTCTCA and 3′GC AGAACTGAGTCCACTGGGCA; miR-708, 5′-GAGCCTATGGAATTCAGTTCTCA and 3′-GCCAGAACTGAGTCCACAGGGCA). For both miR146b and miR-708, the 5′ arm of the duplex produces the major mature product. The 3′ probes were used as a control. Blots were hybridized overnight and washed, and images were obtained with x-ray film.
Ubc9 Depletion with siRNA
Control siRNA (Santa Cruz Biotechnology, catalog no. 44230) or target-specific 19–25-nucleotide siRNA designed to knock down Ubc9 was transfected into HeLa cells at a final concentration of 50 nm using Lipofectamine 2000 (Invitrogen). Transfection mixtures were assembled as described previously (30). Cells were incubated with 200 μl of transfection mixture and 2 ml of antibiotic-free medium for 16–18 h. Transfection efficiency was assessed by using a fluorescent control siRNA (Santa Cruz Biotechnology, catalog no. 36869). Knockdown of Ubc9 was confirmed with Western blotting.
Glutathione Assay
A glutathione assay was performed on whole cell lysates using the GSH-Glo glutathione Assay from Promega (catalog no. V6911) according to the manufacturer's instructions.
Statistical Analysis
Unpaired Student's t test and one-way analysis of variance were used to determine significance between experimental groups. Data are presented as means ± S.E. The program used for data analysis was GraphPad Prism version 5.00 (San Diego, CA).
RESULTS
Alveolar Macrophages from Chronic Active Cigarette Smokers Demonstrate Decreased Mature miRNA Transcript Expression When Compared with Cells from Nonsmokers
Our laboratory has previously shown that smokers' alveolar macrophages are characterized by a decrease in expression of a wide variety of miRNAs (10). In Fig. 1A, we show an analysis of the changes in miRNA that are linked to mRNA changes between nonsmoker and smoker alveolar macrophages. These values were extracted from the larger data set reported previously (10). The data for this figure were generated from lists of altered miRNAs (>2-fold change) and their potential targets (determined by Target Scan and TarBase) and significantly altered mRNAs (q ≤ 0.10) (Gene Chip Human Exon Array 1.0 ST arrays). The q value (false discovery rate) for the mRNAs was calculated using the Partek GS implementation of the step-up method. Gene IDs common to both lists (miRNA targets and mRNAs) were plotted. Each dot represents a gene/miRNA pair as predicted by Target Scan and/or TarBase.
FIGURE 1.
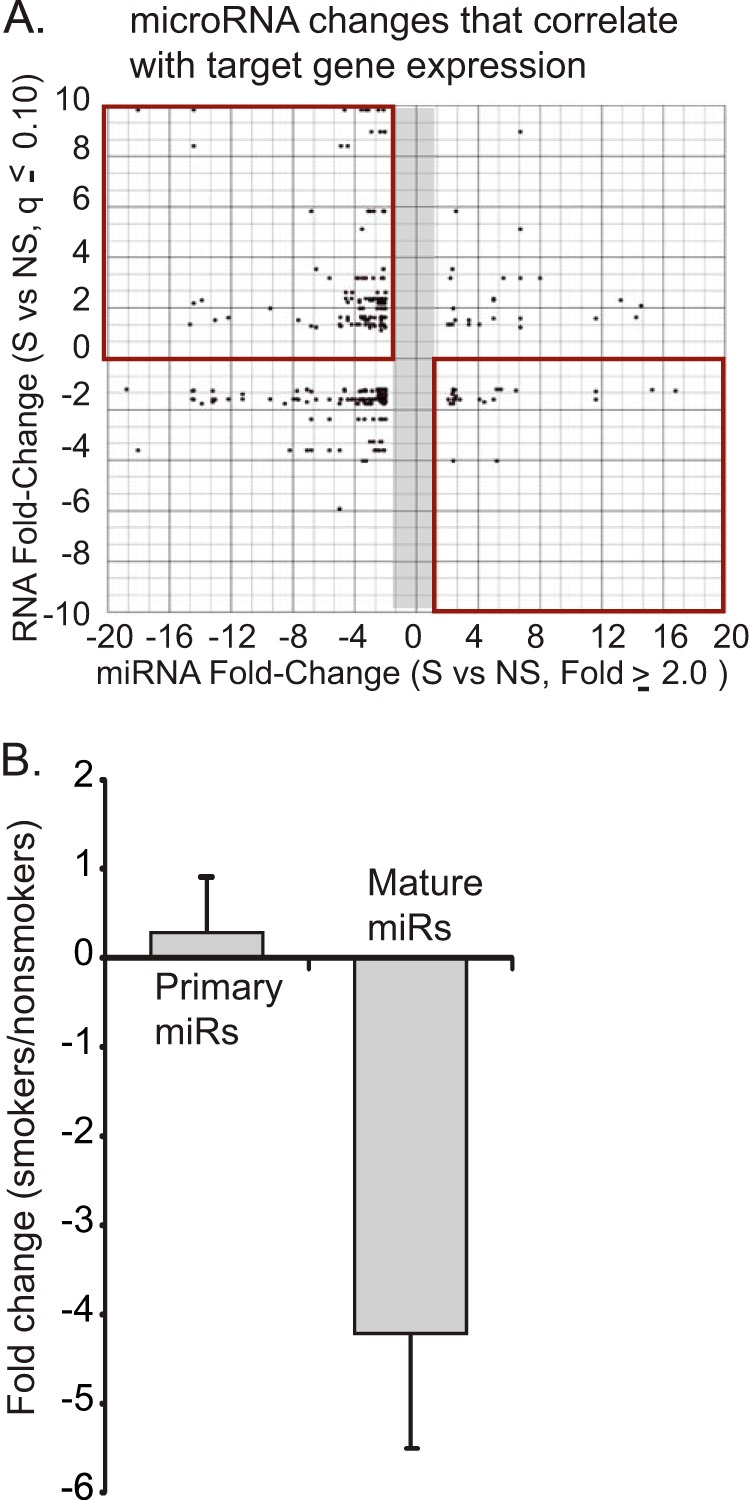
In vivo cigarette smoke exposure alters miRNA profiles in alveolar macrophages. A, differentially expressed miRNAs in smokers' alveolar macrophages are down-regulated and link to up-regulated mRNAs. Alveolar macrophages were isolated from four active smokers (>10 pack years) and four never smokers. An analysis of mRNA expression was performed using Human Exon Array 1.0 ST arrays from Affymetrix. miRNA expression was analyzed using TaqMan Low Density Arrays version 2.0 (ABI). Altered miRNAs (>2-fold change) were analyzed for potential mRNA targets using TargetScan and TarBase. The miRNA target information was compared with significantly altered mRNAs (q ≤ 0.10). The q value (false discovery rate) for the mRNAs was calculated using the Partek GS implementation of the step-up method. Gene IDs common to both lists (miRNA targets and mRNAs) were plotted. Each dot represents a gene/miRNA pair as predicted by Target Scan and/or TarBase. The top right quadrant is of particular interest because it identifies down-regulated miRNAs that link to up-regulated mRNAs. B, miRNAs that are down-regulated in smokers' alveolar macrophages have intact transcriptional profiles. A panel of down-regulated miRNAs (miR-708, miR-200a, miR-210, miR-187, miR-149, miR-429, miR-146b-3p, and miR-200c) was tested in RNA isolated from smoker and never smoker alveolar macrophages. Each miRNA was tested using primers specific for the mature miRNA and primers specific for the primary miRNA transcript (the stem loop structure with accompanying 5′- and 3′-ends). The data are a composite of results from four smokers and four never smokers with significance determined by Student's t test. Error bars, S.E.
Of particular note are the gene/miRNA pairs identified in the top left and bottom right quadrants (Fig. 1A). The top left (more mRNA and less linked miRNA) and bottom right (less mRNA and more linked miRNA) quadrants demonstrate multiple reciprocal relationships between miRNA changes and mRNA changes with smoking. Some of the genes included in the top left quadrant of this figure include MMP12, a matrix metalloproteinase linked to emphysema; SPRY2, a possible regulator of the DICER complex; SPARC, a regulator of extracellular matrix; and γ-glutamylcysteine synthetase, the first rate-limiting enzyme in glutathione synthesis. The data were significant both in the consistency of the changes caused by smoking and by the presence of smoking-linked changes in genes involved in disease (i.e. MMP12, other matrix-related genes, inflammatory genes, and angiogenesis factors) (4, 5, 31–35). Thus, the decrease in miRNA expression appears to have consequences for mRNA levels and, probably, protein expression.
In order to dissect the mechanism underlying this significant decrease in expression of miRNAs, we selected eight miRNAs that were significantly down-regulated in our published data set (miR-708, miR-200a, miR-210, miR-187, miR-149, miR-429, miR-146b-3p, and miR-200c) (10). Using alveolar macrophages from four nonsmoking control subjects and four active smokers, we performed qRT-PCR for both mature miRNA levels and primary transcripts. Primary miRNA transcripts are transcribed from the genome and include flanking regions and one or more stem loop structures; a difference in primary miRNA levels would imply a change in nuclear miRNA transcription rate or alteration in primary miRNA stability. Our assay for the mature transcript measured only the 21/22-nucleotide miRNA; a change in levels of only the mature miRNAs would imply alterations in processing or stability. Fig. 1B demonstrates no decrease in the amount of primary miRNA transcripts in smoker alveolar macrophages. If anything, there may have been a subtle accumulation, as has been reported previously with inhibition of DICER (36). In contrast, consistent with our prior observations, there was a significant comparative decrease in the level of the mature miRNA transcripts.
Cigarette Smoke Exposure Reduces DICER Complex Activity
Because cigarette smoke-induced alterations in miRNA levels occurred somewhere between nuclear transcription and loading onto the RNA-induced silencing complex, we initiated studies to explore miRNA processing activity. Because these studies required quantities of cellular protein difficult to consistently isolate from the number of macrophages retrieved from nonsmokers, we used the human monocytic leukemia cell line, THP-1, as an in vitro model. In this bioactivity assay, FITC-labeled RNA oligomers (27-mer) are incubated with aliquots of whole cell lysates. DICER-like activity is detected by demonstrating cleavage of the oligomers to 21-mer strands with a band shift on gel electrophoresis. Fig. 2A shows a method development assay, showing a dose response in the ability of whole cell lysates to cleave the 27-mer to a 21-mer. There is clear cleavage of the 27-mer that varies in proportion with the total cell protein. In Fig. 2B, THP-1 cells were cultured for 6 h with or without 5% CSE. Whole cell lysates were collected, and an activity assay was run using 10 μg of protein. Shown in Fig. 2B is a representative gel and quantification from three identical experiments. To more directly attribute the measured endonuclease activity to DICER, lysates from untreated and CSE-exposed cells were subject to immunoprecipitation using a DICER-specific antibody. As a control, parallel samples went through the immunoprecipitation protocol with a nonspecific IgG. 10% of the recovered sample was incubated with a set amount of the FITC-labeled 27-mer. As shown in Fig. 2C, we observed a significant decrease in DICER activity in the immunoprecipitated sample recovered from smoke-exposed cells (shown on the right is densitometry from three separate experiments). Cigarette smoke exposure reduces DICER-like RNA endonuclease activity.
FIGURE 2.
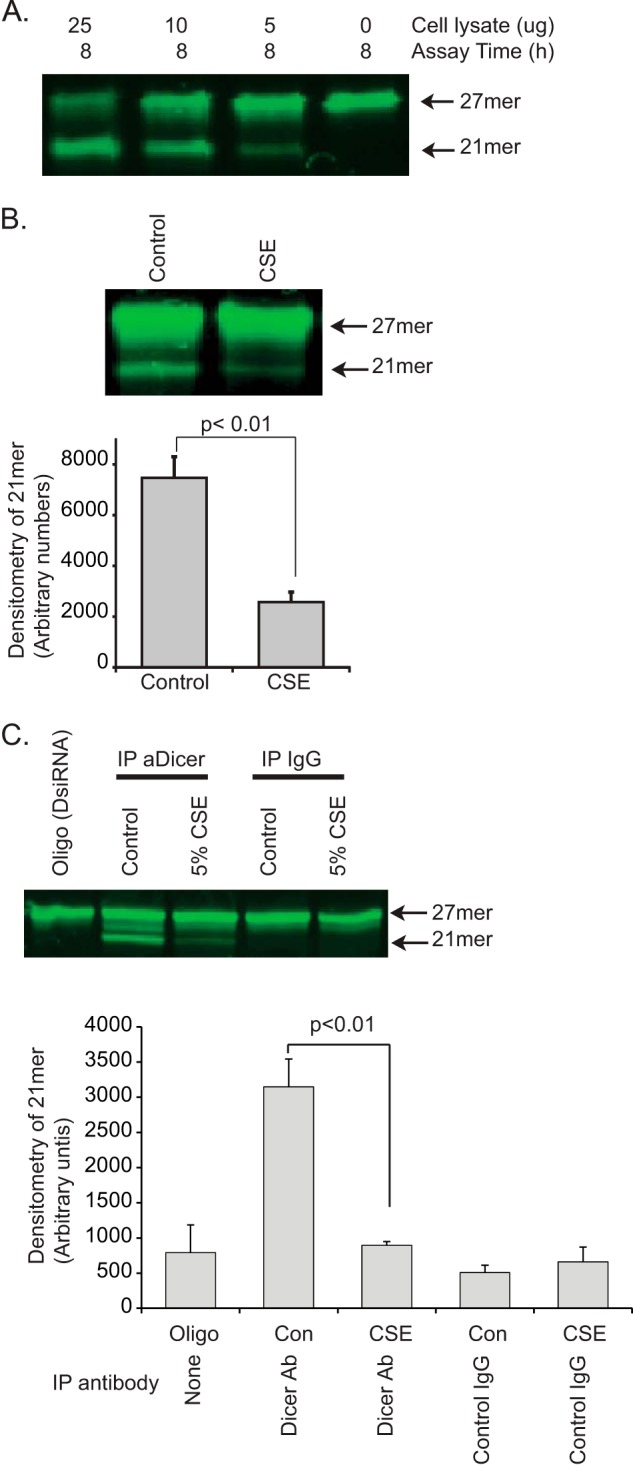
Cigarette smoke exposure decreases dsRNA endonuclease activity. To test the effect of cigarette smoke on DICER activity, we used an in vitro assay of endonuclease cleavage of a fluorescent 27-mer target. A, to validate the assay, varying amounts of whole cell lysate protein (THP-1 cells) were incubated with the FITC-27-mer. Shown is a gel demonstrating a dose-dependent cleavage of the 27-mer target dsRNA. B, whole cell lysates from control THP-1 cells and CSE-exposed THP-1 cells (5% CSE for 6 h) were incubated with the FITC-27-mer. Shown is a fluorescent image of the DNA gel and densitometry from three separate experiments. Significance was determined using Student's t test. Error bars, S.E. C, DICER was immunoprecipitated (IP) from 500 μg of THP-1 protein (control and CSE-exposed, 5% for 6 h). 20% of the resulting protein-coated beads (pulled down with an anti-DICER antibody or a nonspecific IgG) were mixed with the FITC-27-mer to assay for DICER-specific ribonuclease activity. Shown is the fluorescent image of a DNA gel showing both the original 27-mer and the cleaved 21-mer product. Densitometry is from three separate experiments.
To confirm the effect of cigarette smoke exposure on miRNA processing in an in vivo model, HeLa cells were transfected with a construct expressing either pre-miR-146b or pre-miR-708. 24 h after transfection, cells were exposed to CSE for varying times. RNA was isolated, and pre-miRNA processing was assessed by both Northern analysis and qRT-PCR. Fig. 3 demonstrates that CSE exposure leads to a decrease in processing of pre-miRNAs into their respective mature transcripts (Fig. 3, A (miR-146b) and B (miR-708)). Experiments using the 3′ Northern probes showed extremely low levels of mature miRNAs, consistent with what is known about the respective miRNAs (data not shown). Both the Northern blot and qRT-PCR demonstrated decreased processing of the pre-miRNAs after CSE exposure. This in vivo assay of pre-miRNA processing demonstrates that cigarette smoke exposure reduces stem-loop cleavage activity and specific mature miRNA levels. Although we cannot exclude a concurrent effect at the initial primary miRNA processing step in the nucleus (e.g. DROSHA), it seems clear that smoke exposure disrupts extranuclear, terminal miRNA processing.
Cigarette Smoke Decreases DICER Protein Levels in Exposed Cells
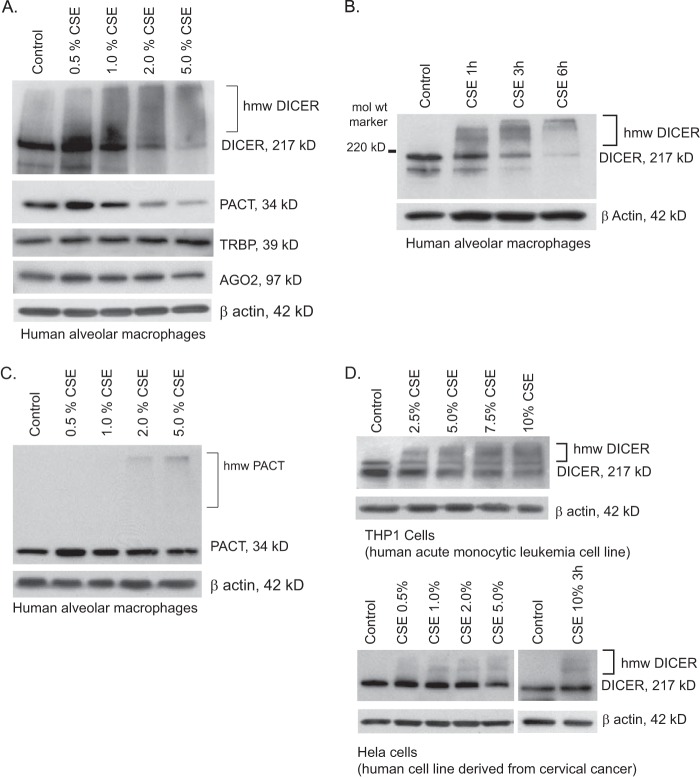
Given the dramatic decreases in both the levels of mature miRNA and DICER activity after smoke exposure, we further investigated the proteins involved in the terminal steps of miRNA processing. Newly isolated human alveolar macrophages retrieved from nonsmokers were incubated for 6 h in cell culture medium supplemented with freshly prepared CSE at final concentrations ranging from 0 to 5%. Whole cell lysates were subjected to standard Western analysis using antibodies to human DICER, PACT, TRBP, and Ago2, with constitutively expressed β-actin serving as a control for equal protein loading. CSE-exposed alveolar macrophages from nonsmokers demonstrated a dose-dependent change in the molecular mass of DICER-immunoreactive protein even at very low concentrations of CSE exposure (Fig. 4A). Fig. 4B demonstrates that as early as 1 h after in vitro exposure to 2% CSE, there was a progressive loss of the 217 kDa band and appearance of multiple higher molecular weight bands. For other proteins involved in the cytosolic processing of pre-miRNAs, we found no change in the levels of TRBP, Ago2, or β-actin, demonstrating that the DICER changes were not a nonspecific cytotoxic effect from the smoke extract. PACT was the only other complex protein to show a change with CSE exposure. PACT also demonstrated a relative decrease at the higher concentrations of CSE, although this was less pronounced than the effect on DICER (Fig. 4C). Interestingly, PACT also manifests a shift to a higher molecular mass form after smoke exposure similar to that seen in DICER (Fig. 4C). Because the effect of CSE on DICER was more pronounced that the changes in PACT, subsequent experiments focused on DICER. Along with the effect of CSE on DICER protein in alveolar macrophages, similar decreases in native sized DICER (271 kDa) and the appearance of higher molecular weight immunoreactive DICER forms were also found in cell lysates from THP-1 and HeLa cells (Fig. 4D). Because the Western blots in all of Fig. 4 were run under standard reducing conditions, the shift to a higher molecular mass DICER was not due to protein-protein aggregation but more likely a smoke exposure-induced post-translational modification of the DICER protein.
FIGURE 4.
DICER protein is modified by cigarette smoke exposure. A, human alveolar macrophages freshly isolated from nonsmokers were exposed to varying concentrations of CSE for 6 h (0.5–5.0%). Whole cell lysates were obtained, and Western analysis was performed for DICER, PACT, TRBP, and AGO2. Equal loading of the gel was determined by staining for β-actin. B, normal human alveolar macrophages were exposed to CSE (2%) for varying times (1–6 h). Whole cell lysates were obtained, and Western analysis performed for DICER. C, normal human alveolar macrophages were exposed to CSE at varying concentrations, and whole cell lysates were run on an SDS-7.5% polyacrylamide gel to optimize transfer of high molecular weight (hmw) proteins. PACT protein levels were determined by Western analysis. D, THP-1 cells were exposed to varying concentrations of CSE for 6 h; HeLa cells were exposed to CSE for 3 and 6 h. Whole cell lysates were obtained, and Western analysis was performed for DICER.
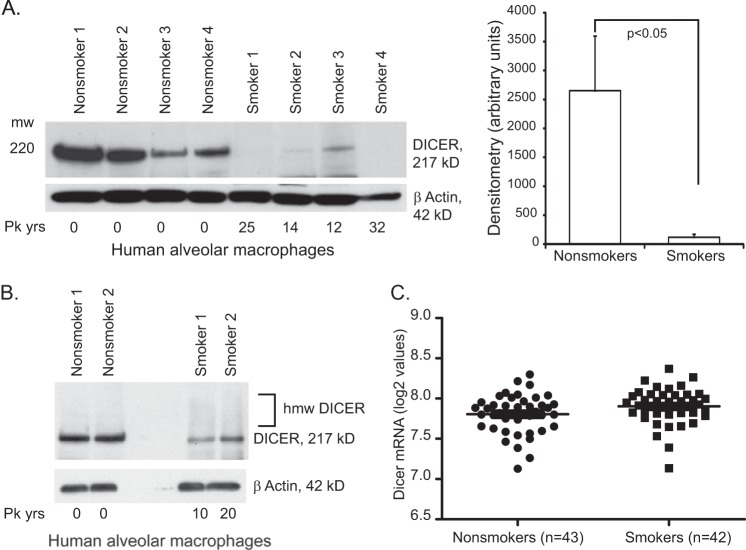
To assess the effect of chronic in vivo cigarette smoke exposure on DICER protein, whole cell lysates of alveolar macrophages were obtained from active smokers and nonsmokers and analyzed for DICER expression. Western blot analysis shown in Figs. 5, A and B, confirms that freshly isolated alveolar macrophages from active smokers contain significantly less DICER protein than nonsmokers' cells. The higher molecular weight DICER bands were also found in cells from chronic smokers although at seemingly lower levels than after in vitro exposure (Fig. 3 versus Fig. 4B). Expression array analysis of freshly isolated alveolar macrophages showed no difference in DICER mRNA levels between smokers and nonsmokers, confirming that smoke-induced reductions in DICER gene transcription were not a major contributor to our observations (Fig. 5C). Thus, cigarette smoke exposure results in a broad decrease in mature miRNA transcripts. This is associated with a post-translational modification resulting in functional, quantitative, and qualitative changes in the DICER protein.
FIGURE 5.
Smoking alters alveolar macrophage DICER protein and not DICER mRNA. A, human alveolar macrophages were obtained from active smokers and never smokers. Whole cell lysates were obtained immediately (baseline sample), and Western analysis was performed for DICER. Shown on the right is densitometry. Significance was determined using Student's t test. B, human alveolar macrophages were obtained from active smokers and never smokers. Fresh whole cell lysates were immediately analyzed by Western blotting and probed for DICER. The gel was optimized to maximize visualization of the high molecular weight bands (5% gel with a long transfer). C, total RNA was obtained from freshly isolated human alveolar macrophages (43 nonsmoker subjects and 42 smoker subjects). The mRNA levels for DICER were analyzed using an Affymetrix array (Gene Chip Human Exon Array 1.0 ST arrays). Shown are the log 2 values for DICER, as determined by Partek analysis of the raw data. There is no difference in DICER mRNA levels between smokers and nonsmokers. Error bars, S.E.
Cigarette Smoke Exposure Induces Post-translational SUMOylation of DICER
The dramatic change in apparent molecular mass on reducing gel analysis suggested a post-translational modification. Previously work from our laboratory identified a block in the autophagy pathway of smoke-exposed alveolar macrophages with accumulation of high molecular mass protein complexes targeted for degradation. These complexes contained both ubiquitin and SUMO 2/3 polymers. This directed us to explore whether this high molecular mass DICER also contained ubiquitin or SUMO 2/3 adducts.
Freshly isolated alveolar macrophages were held in culture with or without media supplemented with CSE. Equal amounts of protein from whole cell lysates were subjected to immunoprecipitation with anti-DICER antibody or a control IgG. The proteins retrieved were separated using reducing gel electrophoresis and Western blots probed with anti-DICER, anti-SUMO, or anti-ubiquitin antibodies. As seen in Fig. 6A, CSE resulted in a notable reduction in immunoprecipitable DICER with appearance of faint higher molecular mass bands; no similar proteins were retrieved using a nonspecific control antibody. When anti-DICER immunoprecipitates were probed with antibody recognizing SUMO 2/3, there was considerable SUMO immunoreactivity clearly detected within the high molecular mass bands (Fig. 6A). Interestingly, although there was a reduction in total DICER protein after CSE exposure, the SUMO 2/3 antibody suggested that much of this native DICER (217 kDa) was also SUMOylated. Antibody recognizing ubiquitin moieties also detected immunoreactivity in the high molecular mass DICER forms, albeit at comparatively lower levels than seen with anti-SUMO 2/3.
FIGURE 6.
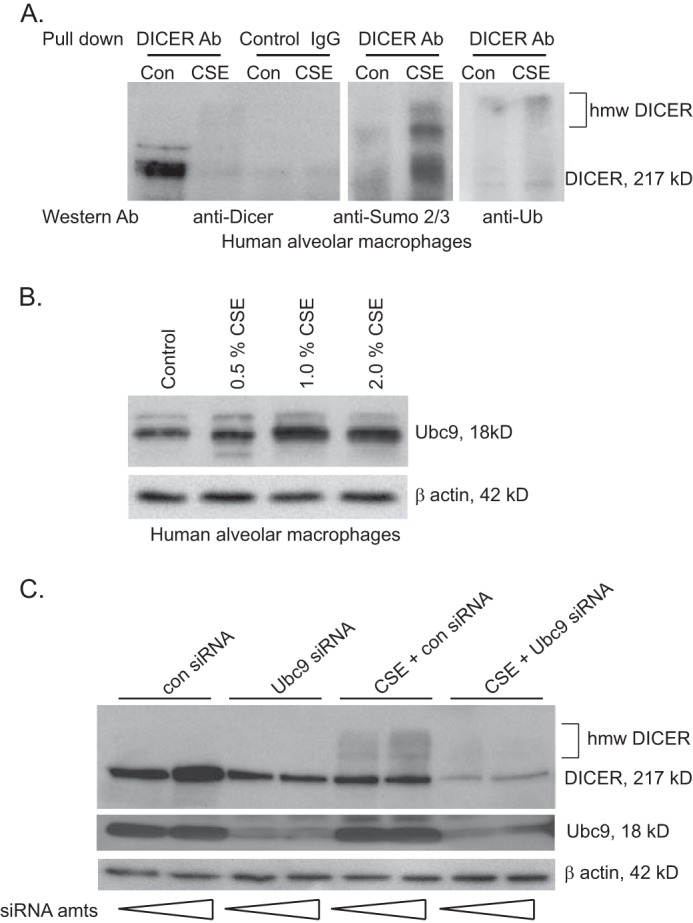
Cigarette smoke exposure increases conjugation of SUMO chains to the protein DICER. A, alveolar macrophages from nonsmokers were exposed to 2% CSE in vitro for 6 h. Whole cell lysates were isolated, and 500 μg of protein was used for immunoprecipitation using an anti-DICER antibody. The resulting samples were divided into thirds, and Western analysis was performed for DICER, SUMO 2/3, and ubiquitin. As a control, 500 μg of whole cell lysate protein was also subjected to immunoprecipitation with a nonspecific IgG. B, human alveolar macrophages were exposed to varying concentrations of CSE. Western analysis was performed for Ubc9. C, HeLa cells were incubated with Ubc9-specific siRNA or a control siRNA. The cells were then exposed to 5% CSE for 6 h, and whole cell protein was obtained. Western analysis was performed for DICER, Ubc9 (verifying knockdown), and β-actin.
DICER contains several candidate lysine SUMOylation sites (see supplemental Table 1). We next asked if blocking a key enzyme in the SUMOylation cascade would alter the appearance of high molecular weight DICER forms after cigarette smoke exposure. The SUMO-conjugating (E2) enzyme Ubc9 catalyzes the formation of isopeptide bonds between the C terminus of SUMO and the amino group of lysine in the target protein. HeLa cells were treated with varying concentration of silencing RNA (siRNA) specific to Ubc9 or a control siRNA and then exposed to CSE. Whole cell lysates were then extracted and analyzed by Western blotting. As demonstrated in Fig. 6C, the siRNA specific for Ubc9 reduced levels of immunoreactive Ubc9 in control and CSE-exposed cells. CSE exposure reduced levels of immunoreactive DICER and again resulted in the appearance of higher molecular mass forms in the cells not exposed to smoke. Interestingly, although the Ubc9 siRNA attenuated the CSE-induced change in molecular mass of DICER, it did not appear to block the reduction in immunoreactive native DICER (217 kDa). The reason for this unexpected result is part of ongoing studies in the laboratory. We also found that smoke exposure resulted in minor increases in Ubc9 mass detected by Western blotting (Fig. 6B) that might account for some SUMOylation change. This finding certainly does not exclude a concurrent smoke-induced alteration in Ubc9 activity and/or effects on function of SUMOlase enzymes. As a composite, these data show that cigarette smoke exposure results in post-translation modification of DICER protein that is due, at least in part, to changes in SUMOylation.
Cigarette Smoke Extract-induced DICER Modification Is Blocked by NAC and Reproduced by Acrolein
Cigarette smoke contains hundreds of known toxic chemicals that exist in both vapor and particulate phases. Because our cigarette smoke extract was generated using an aqueous medium, we knew the target compound was water-soluble. Fig. 7A demonstrates that overnight incubation does not impact the ability of CSE to induce a shift to higher molecular mass forms of DICER in THP-1 cells. The DICER-modifying activity is lost when CSE is dialyzed overnight using membranes with a molecular mass cut-off of >2000 kDa. Thus, the active ingredient(s) in CSE are low molecular weight, water-soluble, and stable over many h.
FIGURE 7.
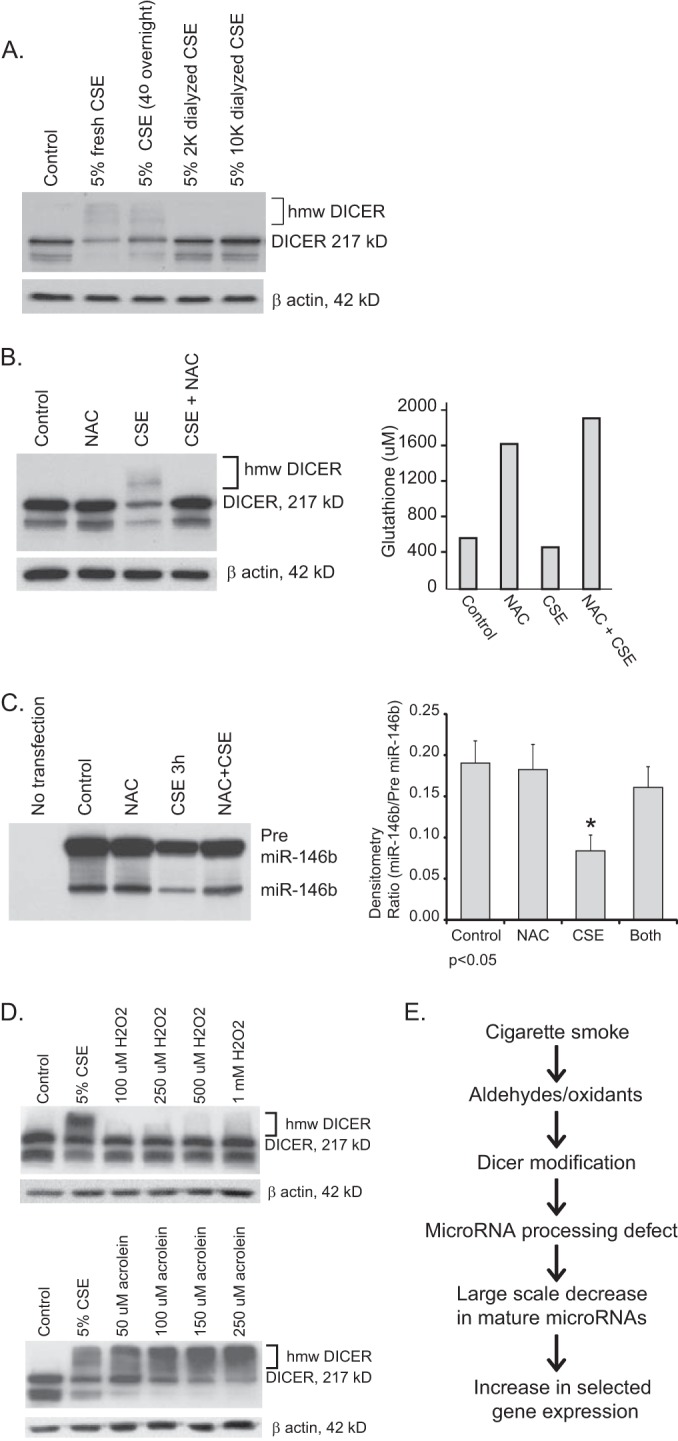
DICER modification by cigarette smoke is inhibited by N-acetylcysteine. A, to explore the chemical properties of the DICER-active component of cigarette smoke, CSE was dialyzed overnight in tubing with cut-offs of 2 or 10 kDa. THP-1 cells were then exposed to freshly prepared CSE, CSE that sat in the refrigerator overnight (control for dialysis), or the dialyzed CSE for 6 h. Western analysis for DICER was performed on whole cell lysates. B, THP-1 cells were exposed to CSE alone (2%) or CSE in the presence of NAC (2 mm) for 6 h. Duplicate assays were set up. One set of cells was utilized for glutathione assays, and the other set of cells were lysed, and whole cell protein was obtained for Western analysis for DICER. C, HeLa cells were transfected with a plasmid that expresses pre-miRNA-146b under control of the constitutive H1 promoter. 24 h following transfection, cells were exposed to CSE (10%) with and without added NAC (2 mm) for 3 h. RNA was isolated, and Northern analysis for miRNA-146b was performed. Shown is a representative blot and densitometry from three separate experiments. Error bars, S.E. D, THP-1 cells were exposed to varying concentrations of H2O2 or acrolein for 6 h. Whole cell lysates were obtained, and Western analysis for DICER was performed. E, this diagram represents an overview of the conclusions supported by the study data.
Because many of the toxic compounds generated by the combustion of tobacco are potent oxidants, we examined whether DICER could be protected from CSE effects using the potent cellular antioxidant, NAC. Cells were incubated with or without 2% CSE as per prior experiments, and the media were concurrently supplemented with NAC at a final concentration of 2 mm for 6 h. Whole cell lysates were subjected to Western blotting and probed with anti-DICER antibody. As seen in Fig. 7B, despite the brief incubation period, NAC supplementation increased intracellular glutathione levels in both control and CSE-exposed cells. Importantly, NAC treatment prevented the CSE-mediated decrease in DICER protein levels and blocked the shift to higher molecular mass forms.
We next tested whether the DICER protein protection seen with NAC led to increased DICER activity. Using the in vivo activity assay shown in Fig. 3, we transfected a pre-miRNA-146b-expressing vector into HeLa cells. Cells were exposed to CSE with and without NAC (added to the cell cultures 15 min before the CSE). Fig. 7C shows that NAC reverses the loss of pre-miRNA-146b cleavage seen with CSE (shown is a representative Northern blot and densitometry from three separate experiments).
Because increasing cellular glutathione levels lead to protection from many forms of oxidant stress, we tested two classic candidate molecular species commonly associated with cigarette smoke, hydrogen peroxide (H2O2) and acrolein (a pure aldehyde compound). As shown in Fig. 7D, supplementing the media of THP-1 cells with concentrations of H2O2 approaching the limits of cellular viability did not reproduce the effect of CSE on immunoreactive DICER levels. In contrast, acrolein at concentrations within the range seen in cigarette smoke vapor and in the epithelial lining fluid of active smokers (37–39) faithfully reproduced both the decrease in DICER protein levels and the appearance of higher molecular mass forms seen after CSE exposure.
DISCUSSION
The major conclusions of the current study are that cigarette smoke exposure alters miRNA expression in human alveolar macrophages via disruption of the complex maturation process that occurs between miRNA transcription and generation of the mature transcript. In addition, we demonstrate that the RNA endonuclease DICER, an essential component of the miRNA biogenesis pathway, is altered by cigarette smoke exposure. These conclusions are based on the following experimental evidence. We show that a group of miRNAs with significantly decreased expression in smokers' cells is not decreased at the transcriptional level. This suggests a defect in the processing of the miRNA transcript. We demonstrate that the lysates of smoke-exposed cells contain less dsRNA endonuclease activity with both an in vitro and in vivo assay. Transfecting in expression plasmids for pre-miRNAs, we demonstrate that smoke exposure decreases the conversion of overexpressed pre-miR146b and pre-miR708 to mature miRNA.
In smokers' alveolar macrophages, we found alterations in DICER, the RNA endonuclease responsible for the final processing of the miRNA stem-loop structure. Exposure to cigarette smoke led to decreased activity and amounts of the endonuclease DICER. We demonstrated that cigarette smoke-exposed cells expressed an immunoreactive DICER of increased molecular mass (in reducing conditions). This change in mass appears to be linked to the addition of SUMO chains to DICER. Finally, we demonstrated that the smoking-induced modifications to intracellular DICER were blocked by incubation with N-acetylcysteine, were reproduced by exposure to acrolein, and were less evident with exposure to H2O2; these data robustly support a role for aldehydes in cigarette smoke-induced DICER protein modification and consequent decrease in miRNA expression. Collectively, this evidence strongly supports the concept that alveolar macrophage phenotype in smokers' lungs is determined, in part, by gene expression changes driven by a DICER-linked processing defect in the miRNA system (Fig. 7E).
There is increasing evidence that cigarette smoking impacts pulmonary pathophysiology through alterations in miRNA expression in the lung. We have shown previously that miRNAs are decreased in alveolar macrophages from smokers (10). In that paper, we found that smoking down-regulated one-third of the tested miRNAs. In addition, we found a number of correlations between down-regulated miRNAs and up-regulated mRNAs, including the emphysema-relevant gene MMP12. Other studies have supported our discovery of reduced miRNAs in smoker lung cells. Schembri et al. (40) demonstrated differences in miRNA expression in epithelial cells isolated from the airways of smokers as compared with cells from never smokers. Similarly, the induced sputum from active smokers contained a pattern of miRNA different from that in the sputum from nonsmokers (41). In both of these studies, a general decrease in miRNA expression was noted in the smoking subjects. No clear differences could be identified when smokers with and without clinical COPD were compared, although both groups differed from nonsmokers (41). Our findings in the alveolar macrophages retrieved from active smokers show that miRNA down-regulation in response to cigarette smoke is not restricted to the airway epithelium. This is in line with the findings of whole lung miRNA profiles from smoke-exposed rats (42). Furthermore, our findings that both THP-1 and HeLa cells manifest similar DICER modifications offer the possibility that cigarette smoke-induced defects in miRNA processing may be a more universal cellular response.
These changes in miRNA levels are probably not simply epiphenomena because all four laboratories have found reciprocal changes in mRNA coding genes relevant to smoking-induced lung disease, including inflammatory cytokine receptors, nuclear transcription factors, and metalloproteinases. In this study, we extend our initial work to demonstrate that the decrease in miRNAs targeting some of these genes of interest is not due to a change in miRNA transcription or intranuclear stability because there was little difference in the levels of primary miRNA transcripts between smokers and nonsmokers despite a dramatic decrease in mature miRNA strands. Thus, the smoking-induced decrease in miRNA levels results from changes in miRNA maturation.
The importance of miRNAs in modulating health and disease is well accepted. However, how miRNA levels are modulated remains incompletely defined. The biogenesis of miRNAs is complex but involves a process leading from nuclear transcription through a final cytosolic maturational processing. A key step in this maturation is the cleavage of the stem loop structure by the cytoplasmic RNA endonuclease, DICER. Our demonstration that DICER activity is reduced in smoke-exposed cells led us to focus on this enzyme. The regulation of DICER appears to be complex with evidence for autophagy-mediated protein degradation as well as post-transcriptional repression of DICER mRNA by the miRNA let-7a (43). Our laboratory has previously established that cigarette smoke induces an autophagic block in alveolar macrophages, and we found no change in DICER mRNA in smoke-exposed cells (9, 20).
This led us to postulate that cigarette smoke must induce some novel change in DICER protein itself. Immunoblotting of cell lysates demonstrated the appearance of DICER species in Western gels (in reducing conditions) of higher molecular mass, suggesting a post-translational protein modification. This was followed at later times by a decrease in total DICER mass. By overexpressing pre-miRNAs, we were able to show a defect in DICER/complex activity and impaired maturation at time points when higher molecular weight DICER species were apparent without notable change in total DICER mass.
Further analysis of DICER protein provided evidence for low level ubiquitination that might lead to decreased activity or proteasome targeting of DICER. However, a more significant finding was an increase in SUMO 2/3 staining of immunoprecipitated DICER (217 kDa). The high molecular weight species generated by cigarette smoke exposure were blocked with inhibition of the dedicated SUMO ligase, Ubc9. SUMO protein adducts are seen in response to a variety of cellular stresses, including oxidant injury, heat shock, and DNA strand damage (44–46). DICER has 10 lysine moieties that fulfill described SUMO consensus sequences (ΨKX(D/E), where Ψ is a hydrophobic residue) (47, 48) (supplemental Table 1). Although Ubc9 may modify many proteins as part of their respective regulatory processes, this represents the first description of a change in DICER function due to post-translational SUMOylation. Further studies are needed to dissect whether this may be a potential target for manipulation of miRNA expression.
Cigarette smoke is a complex potpourri of gas phase chemicals and particulate biotoxins. These include potent oxidants and reactive aldehydes (49, 50). We found that smoke-induced quantitative and qualitative changes in cellular DICER were abrogated by the concurrent administration of the potent antioxidant and aldehyde-neutralizing agent, NAC. In addition, the smoke effects on DICER were faithfully reproduced with acrolein exposure but not hydrogen peroxide. Thus, acrolein delivered via cigarette smoke inhalation might reduce cellular DICER activity, leading to alterations in miRNA levels and, ultimately, gene expression. Such a model has been described in cultured endothelial cells where acrolein exposure resulted in decreases in miRNA and increased expression of their respective mRNA targets (39). Because acrolein is a by-product of the incomplete combustion of many natural and human-made materials, aldehyde-induced changes in miRNA expression due to alterations in DICER activity may prove to be a universal mechanism of inhalational pollutant toxicity (37).
The accumulation of alveolar macrophages in the distal air space is a defining characteristic of several smoking-associated lung diseases. The alveolar macrophage is poised as an ideal cell to reflect the toxic effects of inhaled smoke and situated to respond in ways that may initiate and/or perpetuate disease. Cigarette smoke has been shown to have profound effects on gene expression profiles of many cells, including alveolar macrophages (5, 51–53). The mechanisms behind this response are complex and probably due to both genetic and epigenetic responses. This study describes a novel impact of cigarette smoke exposure on the miRNA-processing pathway in alveolar macrophages. Smoking-induced alterations in miRNA maturation could significantly regulate changes in the expression of disease-relevant genes and represent a new target for potential therapeutic intervention.
Supplementary Material
Acknowledgments
We thank Jiselle Vertinez and Richard Starr for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 HL079901, RO1 HL096625, and R21 HL109589 (to M. M.). This research was also supported in part by the NIEHS, National Institutes of Health, through the University of Iowa Environmental Health Sciences Research Center, NIEHS/National Institutes of Health Grant P30 ES005605, and NCRR/National Institutes of Health Grant UL1RR024979.

This article contains supplemental Table 1.
- COPD
- chronic obstructive pulmonary disease
- CSE
- cigarette smoke extract
- SUMO
- small ubiquitin-like modifier
- NAC
- N-acetyl cysteine
- miRNA and miR
- microRNA
- TRBP
- transactivating response RNA-binding protein
- PACT
- protein kinase RNA activator
- RMA
- robust multiarray averaging
- qRT-PCR
- quantitative RT-PCR.
REFERENCES
- 1. Martin T. R., Raghu G., Maunder R. J., Springmeyer S. C. (1985) The effects of chronic bronchitis and chronic air-flow obstruction on lung cell populations recovered by bronchoalveolar lavage. Am. Rev. Respir. Dis. 132, 254–260 [DOI] [PubMed] [Google Scholar]
- 2. Janoff A., Scherer J. (1968) Mediators of inflammation in leukocyte lysosomes. IX. Elastinolytic activity in granules of human polymorphonuclear leukocytes. J. Exp. Med. 128, 1137–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shapiro S. D., Ingenito E. P. (2005) The pathogenesis of chronic obstructive pulmonary disease: advances in the past 100 years. Am. J. Respir. Cell Mol. Biol. 32, 367–372 [DOI] [PubMed] [Google Scholar]
- 4. Tetley T. D. (2002) Macrophages and the pathogenesis of COPD. Chest 121, 156S–159S [DOI] [PubMed] [Google Scholar]
- 5. Shaykhiev R., Krause A., Salit J., Strulovici-Barel Y., Harvey B. G., O'Connor T. P., Crystal R. G. (2009) Smoking-dependent reprogramming of alveolar macrophage polarization: implication for pathogenesis of chronic obstructive pulmonary disease. J. Immunol. 183, 2867–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hautamaki R. D., Kobayashi D. K., Senior R. M., Shapiro S. D. (1997) Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 277, 2002–2004 [DOI] [PubMed] [Google Scholar]
- 7. Shapiro S. D. (1999) The macrophage in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 160, S29–S32 [DOI] [PubMed] [Google Scholar]
- 8. Chaudhuri A. A., So A. Y., Sinha N., Gibson W. S., Taganov K. D., O'Connell R. M., Baltimore D. (2011) MiRNA-125b potentiates macrophage activation. J. Immunol. 187, 5062–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graff J. W., Dickson A. M., Clay G., McCaffrey A. P., Wilson M. E. (2012) Identifying functional miRNAs in macrophages with polarized phenotypes. J. Biol. Chem. 287, 21816–21825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Graff J. W., Powers L. S., Dickson A. M., Kim J., Reisetter A. C., Hassan I. H., Kremens K., Gross T. J., Wilson M. E., Monick M. M. (2012) Cigarette smoking decreases global miRNA expression in human alveolar macrophages. PLoS One 7, e44066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baek D., Villén J., Shin C., Camargo F. D., Gygi S. P., Bartel D. P. (2008) The impact of miRNAs on protein output. Nature 455, 64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farh K. K., Grimson A., Jan C., Lewis B. P., Johnston W. K., Lim L. P., Burge C. B., Bartel D. P. (2005) The widespread impact of mammalian MiRNAs on mRNA repression and evolution. Science 310, 1817–1821 [DOI] [PubMed] [Google Scholar]
- 13. Selbach M., Schwanhäusser B., Thierfelder N., Fang Z., Khanin R., Rajewsky N. (2008) Widespread changes in protein synthesis induced by miRNAs. Nature 455, 58–63 [DOI] [PubMed] [Google Scholar]
- 14. Macrae I. J., Zhou K., Li F., Repic A., Brooks A. N., Cande W. Z., Adams P. D., Doudna J. A. (2006) Structural basis for double-stranded RNA processing by Dicer. Science 311, 195–198 [DOI] [PubMed] [Google Scholar]
- 15. Park J. E., Heo I., Tian Y., Simanshu D. K., Chang H., Jee D., Patel D. J., Kim V. N. (2011) Dicer recognizes the 5′ end of RNA for efficient and accurate processing. Nature 475, 201–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reisetter A. C., Stebounova L. V., Baltrusaitis J., Powers L., Gupta A., Grassian V. H., Monick M. M. (2011) Induction of inflammasome-dependent pyroptosis by carbon black nanoparticles. J. Biol. Chem. 286, 21844–21852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Monick M. M., Carter A. B., Gudmundsson G., Geist L. J., Hunninghake G. W. (1998) Changes in PKC isoforms in human alveolar macrophages compared with blood monocytes. Am. J. Physiol. 275, L389–L397 [DOI] [PubMed] [Google Scholar]
- 18. Monick M. M., Powers L. S., Barrett C. W., Hinde S., Ashare A., Groskreutz D. J., Nyunoya T., Coleman M., Spitz D. R., Hunninghake G. W. (2008) Constitutive ERK MAPK activity regulates macrophage ATP production and mitochondrial integrity. J. Immunol. 180, 7485–7496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Monick M. M., Powers L. S., Gross T. J., Flaherty D. M., Barrett C. W., Hunninghake G. W. (2006) Active ERK contributes to protein translation by preventing JNK-dependent inhibition of protein phosphatase 1. J. Immunol. 177, 1636–1645 [DOI] [PubMed] [Google Scholar]
- 20. Monick M. M., Powers L. S., Walters K., Lovan N., Zhang M., Gerke A., Hansdottir S., Hunninghake G. W. (2010) Identification of an autophagy defect in smokers' alveolar macrophages. J. Immunol. 185, 5425–5435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nyunoya T., Monick M. M., Klingelhutz A., Yarovinsky T. O., Cagley J. R., Hunninghake G. W. (2006) Cigarette smoke induces cellular senescence. Am. J. Respir. Cell Mol. Biol. 35, 681–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nyunoya T., Monick M. M., Klingelhutz A. L., Glaser H., Cagley J. R., Brown C. O., Matsumoto E., Aykin-Burns N., Spitz D. R., Oshima J., Hunninghake G. W. (2009) Cigarette smoke induces cellular senescence via Werner's syndrome protein down-regulation. Am. J. Respir. Crit. Care Med. 179, 279–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blue M. L., Janoff A. (1978) Possible mechanisms of emphysema in cigarette smokers. Release of elastase from human polymorphonuclear leukocytes by cigarette smoke condensate in vitro. Am. Rev. Respir. Dis. 117, 317–325 [DOI] [PubMed] [Google Scholar]
- 24. Panayiotidis M. I., Stabler S. P., Allen R. H., Ahmad A., White C. W. (2004) Cigarette smoke extract increases S-adenosylmethionine and cystathionine in human lung epithelial-like (A549) cells. Chem. Biol. Interact. 147, 87–97 [DOI] [PubMed] [Google Scholar]
- 25. Carnevali S., Petruzzelli S., Longoni B., Vanacore R., Barale R., Cipollini M., Scatena F., Paggiaro P., Celi A., Giuntini C. (2003) Cigarette smoke extract induces oxidative stress and apoptosis in human lung fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 284, L955–L963 [DOI] [PubMed] [Google Scholar]
- 26. Benjamini Y., Krieger A., Yekutieli D. (2006) Adaptive linear step-up procedures that control the false discovery rate. Biometrika 93, 491–507 [Google Scholar]
- 27. Philibert R. A., Sears R. A., Powers L. S., Nash E., Bair T., Gerke A. K., Hassan I., Thomas C. P., Gross T. J., Monick M. M. (2012) Coordinated DNA methylation and gene expression changes in smoker alveolar macrophages: specific effects on VEGF receptor 1 expression. J. Leukoc. Biol. 92, 621–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim D. H., Behlke M. A., Rose S. D., Chang M. S., Choi S., Rossi J. J. (2005) Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nat. Biotechnol. 23, 222–226 [DOI] [PubMed] [Google Scholar]
- 29. Boudreau R. L., Martins I., Davidson B. L. (2009) Artificial miRNAs as siRNA shuttles: improved safety as compared to shRNAs in vitro and in vivo. Mol. Ther. 17, 169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manzel L. J., Chin C. L., Behlke M. A., Look D. C. (2009) Regulation of bacteria-induced intercellular adhesion molecule-1 by CCAAT/enhancer binding proteins. Am. J. Respir. Cell Mol. Biol. 40, 200–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Houghton A. M., Quintero P. A., Perkins D. L., Kobayashi D. K., Kelley D. G., Marconcini L. A., Mecham R. P., Senior R. M., Shapiro S. D. (2006) Elastin fragments drive disease progression in a murine model of emphysema. J. Clin. Invest. 116, 753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Belaaouaj A., Shipley J. M., Kobayashi D. K., Zimonjic D. B., Popescu N., Silverman G. A., Shapiro S. D. (1995) Human macrophage metalloelastase. Genomic organization, chromosomal location, gene linkage, and tissue-specific expression. J. Biol. Chem. 270, 14568–14575 [DOI] [PubMed] [Google Scholar]
- 33. Robbins C. S., Bauer C. M., Vujicic N., Gaschler G. J., Lichty B. D., Brown E. G., Stämpfli M. R. (2006) Cigarette smoke impacts immune inflammatory responses to influenza in mice. Am. J. Respir. Crit. Care Med. 174, 1342–1351 [DOI] [PubMed] [Google Scholar]
- 34. Birrell M. A., Wong S., Catley M. C., Belvisi M. G. (2008) Impact of tobacco-smoke on key signaling pathways in the innate immune response in lung macrophages. J. Cell. Physiol. 214, 27–37 [DOI] [PubMed] [Google Scholar]
- 35. Yang S. R., Chida A. S., Bauter M. R., Shafiq N., Seweryniak K., Maggirwar S. B., Kilty I., Rahman I. (2006) Cigarette smoke induces proinflammatory cytokine release by activation of NF-κB and posttranslational modifications of histone deacetylase in macrophages. Am. J. Physiol. Lung Cell Mol. Physiol. 291, L46–L57 [DOI] [PubMed] [Google Scholar]
- 36. Koscianska E., Starega-Roslan J., Krzyzosiak W. J. (2011) The role of Dicer protein partners in the processing of miRNA precursors. PLoS One 6, e28548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stevens J. F., Maier C. S. (2008) Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res. 52, 7–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eiserich J. P., van der Vliet A., Handelman G. J., Halliwell B., Cross C. E. (1995) Dietary antioxidants and cigarette smoke-induced biomolecular damage: a complex interaction. Am. J. Clin. Nutr. 62, 1490S–1500S [DOI] [PubMed] [Google Scholar]
- 39. Talbot P., DiCarlantonio G., Knoll M., Gomez C. (1998) Identification of cigarette smoke components that alter functioning of hamster (Mesocricetus auratus) oviducts in vitro. Biol. Reprod. 58, 1047–1053 [DOI] [PubMed] [Google Scholar]
- 40. Schembri F., Sridhar S., Perdomo C., Gustafson A. M., Zhang X., Ergun A., Lu J., Liu G., Zhang X., Bowers J., Vaziri C., Ott K., Sensinger K., Collins J. J., Brody J. S., Getts R., Lenburg M. E., Spira A. (2009) miRNAs as modulators of smoking-induced gene expression changes in human airway epithelium. Proc. Natl. Acad. Sci. U.S.A. 106, 2319–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Van Pottelberge G. R., Mestdagh P., Bracke K. R., Thas O., van Durme Y. M., Joos G. F., Vandesompele J., Brusselle G. G. (2011) MiRNA expression in induced sputum of smokers and patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care. Med. 183, 898–906 [DOI] [PubMed] [Google Scholar]
- 42. Izzotti A., Calin G. A., Arrigo P., Steele V. E., Croce C. M., De Flora S. (2009) Downregulation of miRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J. 23, 806–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gibbings D., Mostowy S., Jay F., Schwab Y., Cossart P., Voinnet O. (2012) Selective autophagy degrades DICER and AGO2 and regulates miRNA activity. Nat. Cell Biol. 14, 1314–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bossis G., Melchior F. (2006) SUMO: regulating the regulator. Cell Div. 1, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bossis G., Melchior F. (2006) Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol. Cell 21, 349–357 [DOI] [PubMed] [Google Scholar]
- 46. Tempé D., Piechaczyk M., Bossis G. (2008) SUMO under stress. Biochem. Soc. Trans. 36, 874–878 [DOI] [PubMed] [Google Scholar]
- 47. Teng S., Luo H., Wang L. (2012) Predicting protein sumoylation sites from sequence features. Amino Acids 43, 447–455 [DOI] [PubMed] [Google Scholar]
- 48. Sampson D. A., Wang M., Matunis M. J. (2001) The small ubiquitin-like modifier-1 (SUMO-1) consensus sequence mediates Ubc9 binding and is essential for SUMO-1 modification. J. Biol. Chem. 276, 21664–21669 [DOI] [PubMed] [Google Scholar]
- 49. Pryor W. A., Stone K. (1993) Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann. N.Y. Acad. Sci. 686, 12–27; discussion 27–18 [DOI] [PubMed] [Google Scholar]
- 50. Facchinetti F., Amadei F., Geppetti P., Tarantini F., Di Serio C., Dragotto A., Gigli P. M., Catinella S., Civelli M., Patacchini R. (2007) α,β-unsaturated aldehydes in cigarette smoke release inflammatory mediators from human macrophages. Am. J. Respir. Cell Mol. Biol. 37, 617–623 [DOI] [PubMed] [Google Scholar]
- 51. Spira A., Beane J., Shah V., Liu G., Schembri F., Yang X., Palma J., Brody J. S. (2004) Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc. Natl. Acad. Sci. U.S.A. 101, 10143–10148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Spira A., Schembri F., Beane J., Shah V., Liu G., Brody J. S. (2004) Impact of cigarette smoke on the normal airway transcriptome. Chest 125, 115S. [DOI] [PubMed] [Google Scholar]
- 53. Zeskind J. E., Lenburg M. E., Spira A. (2008) Translating the COPD transcriptome: insights into pathogenesis and tools for clinical management. Proc. Am. Thorac. Soc. 5, 834–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.