Abstract
Mechanosensitivity is an inherent property of virtually all cell types, allowing them to sense and respond to physical environmental stimuli. Stretch-activated ion channels represent a class of mechanosensitive proteins which allow cells to respond rapidly to changes in membrane tension; however their identity has remained elusive. The piezo genes have recently been identified as a family of stretch-activated mechanosensitive ion channels. We set out to determine the role of piezo1 during zebrafish development. Here we report that morpholino-mediated knockdown of piezo1 impairs erythrocyte survival without affecting hematopoiesis or differentiation. Our results demonstrate that piezo1 is involved in erythrocyte volume homeostasis, disruption of which results in swelling/lysis of red blood cells and consequent anemia.
Introduction
Mechanosensitivity confers a cell the ability to detect and, ultimately, to respond to a variety of physical stimuli. This attribute relies on the reaction of mechanosensory proteins to mechanical stimuli, such as the stretch-induced opening of an ion channel or force-induced protein conformational changes which disrupt protein-protein interactions.1 Although electrophysiological recordings of mechanically stimulated cells have indicated the existence of mechanosensitive ion channels, their identity has remained elusive.2 The Piezo family of proteins have recently been identified as bona fide mechanosensitive ion channels.3 These represent a family of mixed cationic non-selective ion channels gated by mechanical stimuli.3,4 Homologs of the piezo genes have been identified in a variety of species ranging from protozoa to vertebrates.3 In particular, mammals possess two piezo genes, piezo1 (fam38A) and piezo2 (fam38B). Both genes have been detected in a number of tissues in which mechanosensitivity plays a major role, such as kidney, lung and colon, although piezo1 shows a much higher expression in the skin while piezo2 expression is elevated in dorsal root ganglion cells.3
Mechanosensitive proteins have been linked to various pathophysiological situations such as hearing and deafness,5 cardiac physiological and hypertrophic responses,6 as well as kidney function or disease.1,7 Current research is now focusing on the functional role of the piezo genes in determining the biological processes in which they may be involved.8 A recent study in the fruit fly Drosophila melanogaster (which has one piezo homolog, Dmpiezo) has shown that piezo has a role in mechanical nociception,9 while in-depth genomic analysis has linked the human homolog of piezo1 to the blood disorder xerocytosis.10
Given its rapid external development, the zebrafish represents a powerful vertebrate model system for determining gene function during embryogenesis and early development. Using a morpholino-mediated knockdown approach we sought to elucidate the role of piezo1 during zebrafish development.
Methods
Zebrafish strains and husbandry
Zebrafish were maintained under standardized conditions and experiments were conducted in accordance with the European Community’s council directive of November 1986. Embryos were staged according to Kimmel et al.11 The transgenic lines used in this study were the CD41:GFP line, which has been described previously,12 the MPEG:mCherry line, which was generated in Lutfalla’s laboratory based on a previous report,13 and the Lmo2:dsRed line, which has been described previously.14
Morpholinos and injections
Morpholino oligonucleotides were obtained from Gene Tools (Philomath, OR, USA) and injected into one-cell stage embryos. Pz1 MO1 targets the start codon of piezo1, while Pz1 MO2 targets the splice site at the boundary between intron 1 and exon 2 of piezo1. The sequences and amounts of injected morpholino oligonucleotides were as follows:
Pz1 MO1: 5′-ATCCGCATACCACCTGAAGCTCCAT-3′; 8 ng
Pz1 MO2 : 5′-AATATGCAGGCTGTGGAAAACACAT -3′; 10 ng
Blood smears
Embryonic blood was obtained by cutting the tail of embryos 2 days post-fertilization (dpf) (10 wild-type embryos and 30 Pz1 morphant embryos). The blood was collected into a solution of 1x phosphate-buffered saline (PBS), 1% bovine serum albumin (BSA), and 10 U/mL heparin in a microtube and centrifuged for 3 min at 750 rpm. The pellet was resuspended in 5 mL of the same solution, deposited on a slide and air-dried overnight. The blood preparation was then stained with Wright-Giemsa solution following the manufacturer's instructions (Sigma). For immunostaining, blood smears were treated as described by Shafizadeh et al.15 Images were taken using a Zeiss AxioImager Z1.
Erythrocyte counting
To count the cells, ten movies of wild-type and ten movies of piezo1 morphants were taken using a Zeiss stereomicroscope and a 20X M PLAN APO 0.42 objective. For each movie, five time-frames were picked randomly and cells were counted using the Cell-count ImageJ plug-in.
Erythrocyte volume calculation
Wild-type erythrocytes were assumed to be ellipsoid with the diameters of the major and minor axes being equal. The following formula was used to calculate the volume of wild-type erythrocytes.
V=π/6(abc), a=major axis, b=minor axis and c=vertical axis
Piezo1 morphant erythrocytes were assumed to be spherical with equal radii. The following formula was used to calculate the volume of piezo1 morphant erythrocytes.
V = (4/3) × π × r3
In situ hybridisation
In situ hybridization was performed as described previously.16
Immunohistochemistry
Embryos were fixed with 4% paraformaldehyde (Sigma) in PBS for 2 h at room temperature, rinsed in PBS-Tween 0.1% and permeabilized by PBS-Tween 0.1%-Triton 1%. Embryos were then blocked for 2 h in 10% sheep serum/PBSDT (PBS, 1% DMSO, 0.1% Triton) at room temperature, incubated overnight with anti-α-tubulin (Sigma, mouse, 1:1000) at 4°C, and washed with PBSDT for 6 h at room temperature. Secondary antibody (Invitrogen, anti-mouse, 1:500) was applied overnight then rinsed in PBS-Tween 0.1% for 4 h and observed by confocal fluorescence microscopy.
Results and Discussion
The zebrafish homologs piezo1 and piezo2a have been previously identified (accession numbers- XP_696355.4 and XP_002666625, respectively3). However, in silico analysis using the Ensembl database indicates that a third homolog, piezo2b (accession number-XP_003198010), is also present in zebrafish. Gene duplication is not uncommon in zebrafish and is most likely caused by a whole genome duplication event during the animal’s evolution.17 Protein sequence analysis indicates that, overall, zebrafish PIEZO1 shares 40.63% homology with zebrafish PIEZO2A and 46.62% homology with PIEZO2B (Online Supplementary Figure S1). Comparison with its mammalian counterpart indicates that zebrafish PIEZO1 shares 56% homology with human PIEZO1 (Online Supplementary Figure S2). In order to determine the expression of piezo1 during early development we designed an antisense RNA probe covering the initial 500 bp of this gene. In situ hybridization analysis revealed that at 30 h post-fertilization (hpf), piezo1 is expressed in areas containing primitive erythrocytes, similar to previously reported primitive erythrocyte-specific genes such as hbbe118 (Figure 1A–E).
Figure 1.
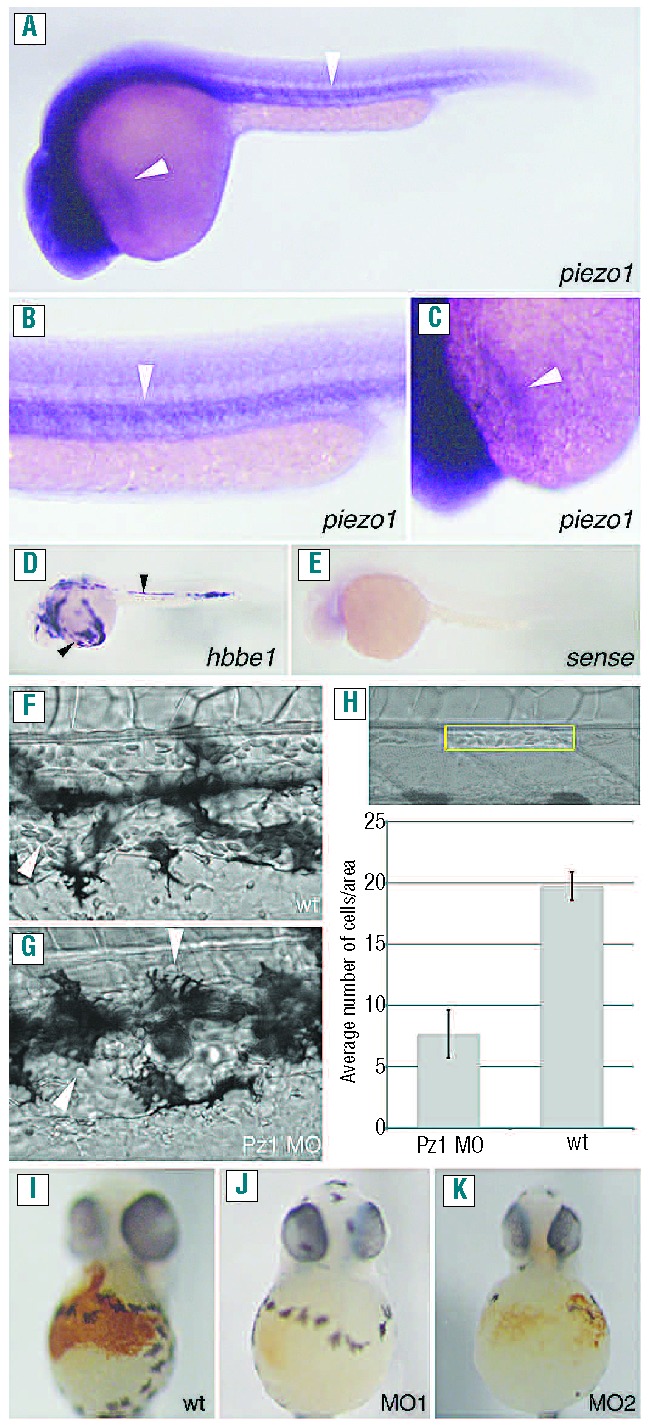
Piezo1 regulates erythrocyte development/homeostasis. (A–C) In situ hybridization analysis using an anti-sense piezo1 probe. Piezo1 is expressed in areas containing primitive erythrocytes (indicated by arrows) at 30 hpf. (B, C) Higher magnification of the areas indicated in (A). (D) In situ hybridization analysis using an anti-sense hbbe1 probe labeling differentiated primitive erythrocytes. Hbbe1 is also expressed in areas containing primitive erythrocytes (indicated by arrows) at 30 hpf. (E) In situ hybridization analysis using a sense piezo1 probe. (F, G) Brightfield images of the dorsal aorta and cardinal vein of 3 dpf wild-type (F) or piezo1 morphant embryos (G), the arrow heads indicate individual circulating cells. (H) Brightfield image of the area used to count the number of erythrocytes (yellow rectangle). The graph indicates the average number of erythrocytes counted in this region in either wild-type (wt) or piezo1 morphants (Pz1 MO). (I-K) Two dpf wildtype and piezo1 morphant embryos treated with the erythrocyte marker o-dianisidine. Wild-type (n=47) (I), piezo1 MO1 (n=52) (J) and piezo1 MO2 (n=27) (K). Piezo1 morphant embryos show a markedly reduced amount of o-dianisidine staining, indicating that erythrocyte development/homeostasis has been disrupted.
To assess the role of piezo1 during zebrafish development we used antisense morpholino oligonucleotides targeting either the start codon (piezo1 MO1) or the intron1/exon2 splice site (piezo1 MO2) of piezo1; due to the differences in homology neither of these two morpholinos is predicted to target either piezo2a or piezo2b (Online Supplementary Figure S3). We titrated morpholinos to concentrations which did not cause any gross developmental defects/necrosis. Subsequently, we found that piezo1 morphants displayed a severe reduction in the number of circulating erythrocytes compared to the number of erythrocytes in wild-type controls (Figure 1F–H and Online Supplementary Movies S1 and S2). We confirmed this phenotype using o-dianisidine, which stains hemoglobin (Figure 1I–K).
To ensure these observations were due to knockdown of piezo1, we isolated RNA from piezo1 MO2 morphants and performed reverse transcriptase polymerase chain reaction analysis. Subsequently we found that the splicing of piezo1 mRNA transcripts had been disrupted in these embryos, resulting in the observed phenotype (Figure 2A, B). To further verify that the observed phenotype was specific to knockdown of piezo1 we co-injected suboptimal concentrations of both piezo1 morpholinos (Online Supplementary Figure S4). O-dianisidine staining revealed that at suboptimal concentrations the individual morpholinos had little effect; however, co-injection of both morpholinos at these concentrations produced a marked loss of o-dianisdine staining (Online Supplementary Figure S4). To understand how knockdown of piezo1 leads to such a dramatic reduction in the number of circulating erythrocytes, we first investigated whether increased apoptosis could explain this phenotype. However, utilizing the vital dye acridine orange, which stains apoptotic cells,19 we were unable to detect any discernible differences between wild-type and piezo1 morphants, either prior to the onset of circulation (24 hpf) or at later stages (2 dpf) (Figure 2G–N). Another possible explanation for the reduced number of erythrocytes observed in piezo1 morphants is that primitive hematopoiesis was in some way disrupted. For example, knockdown of the hematopoietic gene tal1/scl results in a similar reduction of erythrocytes.20 To investigate this possibility we performed in situ hybridization analysis on piezo1 morphants using a battery of hematopoietic markers, namely: tal1/scl (hematopoietic stem cells), gata1 (early erythrocytes), and hbbe1 and band3 (terminally differentiated erythrocytes).15 We were unable to detect any discernible differences in the expression patterns of these genes when comparing piezo1 morphants with wild-type embryos (Figure 2C–F). Consequently, it appears that hematopoiesis is unperturbed following piezo1 knockdown and subsequent erythrocyte differentiation proceeds normally. Taken together these results indicate that the loss of erythrocytes observed in piezo1 morphants is not caused by either an increase in erythrocyte apoptosis or a perturbation of primitive hematopoiesis, but is most likely due to hemolysis of mature erythrocytes.
Figure 2.
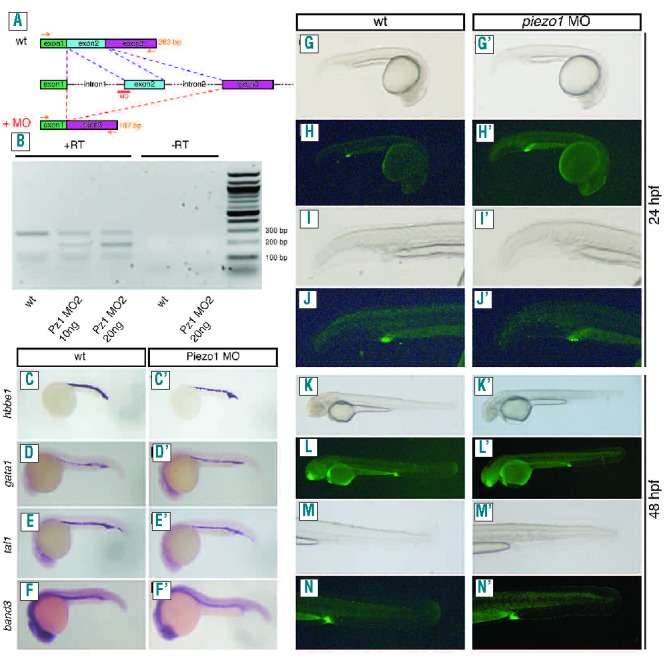
Piezo1 knockdown does not affect primitive hematopoiesis, erythrocyte differentiation or induce apoptosis. (A, B) RT-PCR analysis of piezo1 MO2 morphant embryos. The piezo1 MO2 targets the intron1/exon2 splice site resulting in skipping of this exon (A). Primers designed to the start of exon 1 and the end of exon 3 are able to amplify the correct 283 bp fragment from wild-type embryo cDNA (lane 1). With morphant embryo cDNA the intensity of this 283 bp band is reduced and is accompanied by the appearance of a 187 bp fragment (lane 2), indicating that exon 2 has been excluded. This observation is also dependent on the concentration of morpholino injected (lane 3). Lanes 4 and 5 are negative controls in which no reverse transcriptase was used in the initial reaction indicating that these results are specific to the RNA that has been isolated (B). (CF′) In situ hybridization analysis on either wild-type (C–F) or piezo1 morphant embryos (C′–F′). No differences were observed between wild-type and piezo1 morphants using probes detecting hbbe1 (C, C′), gata1 (D, D′), tal1/scl(E, E′) and band3 (F, F′). (G–N) Acridine orange staining of wild-type 24 hpf (G–J) and 48 hpf embryos (K–N). No differences were observed in piezo1 morphant embryos analyzed at similar time points, 24 hpf (G′–J′) and 48 hpf (K′–N′). Images were obtained using an Olympus SZX16 fluorescent microscope.
We next sought to determine whether piezo1 is involved in the development/maintenance of other types of blood cells. To do this we injected piezo1 morpholinos into a number of different transgenic reporter lines (Figure 3A–H). The CD41:GFP transgenic line expresses green fluorescent protein in both thrombocytes (high expression) and hematopoietic stem cells (low expression).21 We could not detect any discernible differences in the quantity of either thrombocytes or hematopoietic stem cells in CD41:GFP piezo1 morphants compared to control embryos (Figure 3C, D), although we did observe a reduction in circulating erythrocytes (Online Supplementary Figure S5). The MPEG:mCherry transgenic line specifically labels the macrophage population in developing zebrafish embryos.13 We found that although the concentration of erythrocytes was reduced (Online Supplementary Figure S5), macrophages were still present in MPEG:mCherry piezo1 morphants, indicating that knockdown of this gene does not affect macrophage homeostasis (Figure 3E, F). To assess whether neutrophils had been affected in piezo1 morphants we used a previously described Sudan Black stain.22 We did not detect any differences in the neutrophil population between wild-type and piezo1 morphant embryos (Figure 3G, H). Taken together these data suggest that loss of piezo1 does not have any significant effect on the development/homeostasis of other blood cell lineages. In order to ascertain what was causing the loss of erythrocytes observed in piezo1 morphants, we injected piezo1 morpholinos into a Lmo2:dsRed reporter line which specifically labels erythrocytes and blood vessels.14 In control embryos we detected circulating erythrocytes with a typical flattened elliptical morphology23 (Figure 3I, J). Conversely, the few remaining erythrocytes we were able to detect in Lmo2:dsRed piezo1 morphants had a spherical morphology with obvious signs of membrane deformation (Figure 3K, L). Further analysis showed that the dimensions of piezo1 morphant erythrocytes had shifted from an elliptical 3:1 ratio (average diameter=9.29 μm ±1.16 SD, average height=2.89 μm ±0.62 SD, n=50) to a spherical 1:1 ratio (average diameter=7.48 μm ±0.5 SD, average height=7.45 μm ±0.58SD, n=50) indicating that knockdown of piezo1 results in an increase in erythrocyte volume (average wild-type erythrocyte volume=130 μm3, average piezo1 morphant erythrocyte volume=219 μm3) (Figure 3I, K). To investigate these observations further, we analyzed blood smears prepared from wild-type or piezo1 morphant embryos. Wright and Giesma stained blood from piezo1 morphant erythrocytes appeared markedly different from that of their wild-type counterparts, having deformed/ruptured plasma membranes and swollen nuclei (Figure 4A–D.). Furthermore, immunohistochemical analysis using an α-tubulin antibody to label the marginal band of microtubules, phalloidin (actin cytoskeleton) and Hoechst (nucleus) confirmed these observations (Figure 4E–L). The cellular morphology observed in these piezo1 morphant erythrocyte preparations differed somewhat from the spherical/deformed shape we had observed in vivo. We surmised that this may be due to an increase in fragility of these cells, such that the preparation of the blood smear may have induced the observed rupture/lysis. To circumvent this possibility we performed similar immunohistochemistry analyses on whole mount piezo1 morphant embryos obtained from the Lmo2:dsRed transgenic line. This indicated that although piezo1 morphant erythrocytes appear spherical with signs of membrane deformation, they do not have such a dramatic ruptured phenotype as that observed in the blood smear preparation (Figure 4M–R). The marginal band of microtubules appears to be disrupted when compared to that of the control erythrocytes. Rather than encircling the erythrocytes in a single plane it appears more diffuse (Figure 4M, P). Taken together these results indicate that knockdown of piezo1 leads to erythrocyte swelling. These cells also adopt a spherical shape and exhibit signs of membrane deformation, along with disruption of the marginal band of microtubules.
Figure 3.
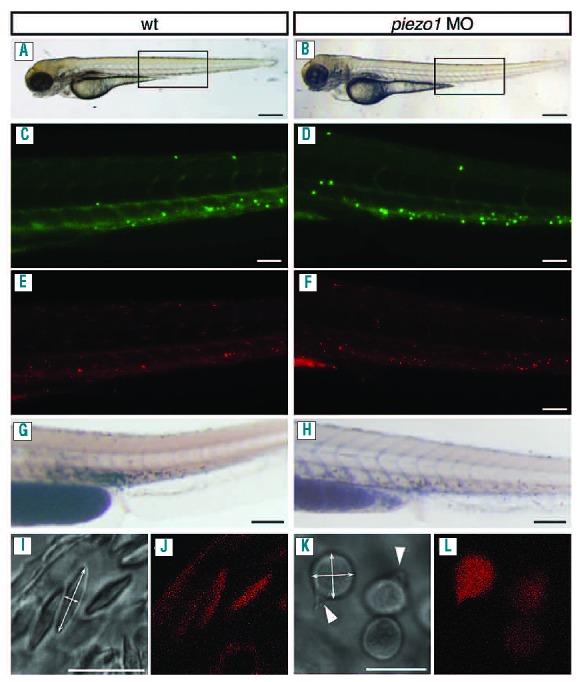
Piezo1 knockdown perturbs erythrocyte volume homeostasis without affecting other blood cell lineages. (A, B) Brightfield images of either wild-type or piezo1 morphants, the black box indicates the areas depicted in (C–H). CD41:GFP expression in either wild-type (n=20) (C) or piezo1 morphants (n=32) (D). Thrombocytes have high GFP expression and hematopoietic stem cells have low GFP expression. MPEG:mCherry expression in either wild-type (n=20) (E) or piezo1 morphants (n=32) (F). Macrophages are labeled with mCherry. Sudan Black staining of either wild-type (n=15) (G) or piezo1 morphants (n=17) (H). Sudan Black labels the neutrophil population. Brightfield and fluorescent images of erythrocytes in either wild-type Lmo:dsRed (I, J) or piezo1 morphant Lmo:dsRed embryos (K, L). Double-headed arrows in (I, K) indicate how erythrocyte dimensions were calculated: the arrow heads in (K) indicate membrane deformation. Scale bars: AB 150 μm, C–H 35 μm and I–L 10 μm.
Figure 4.
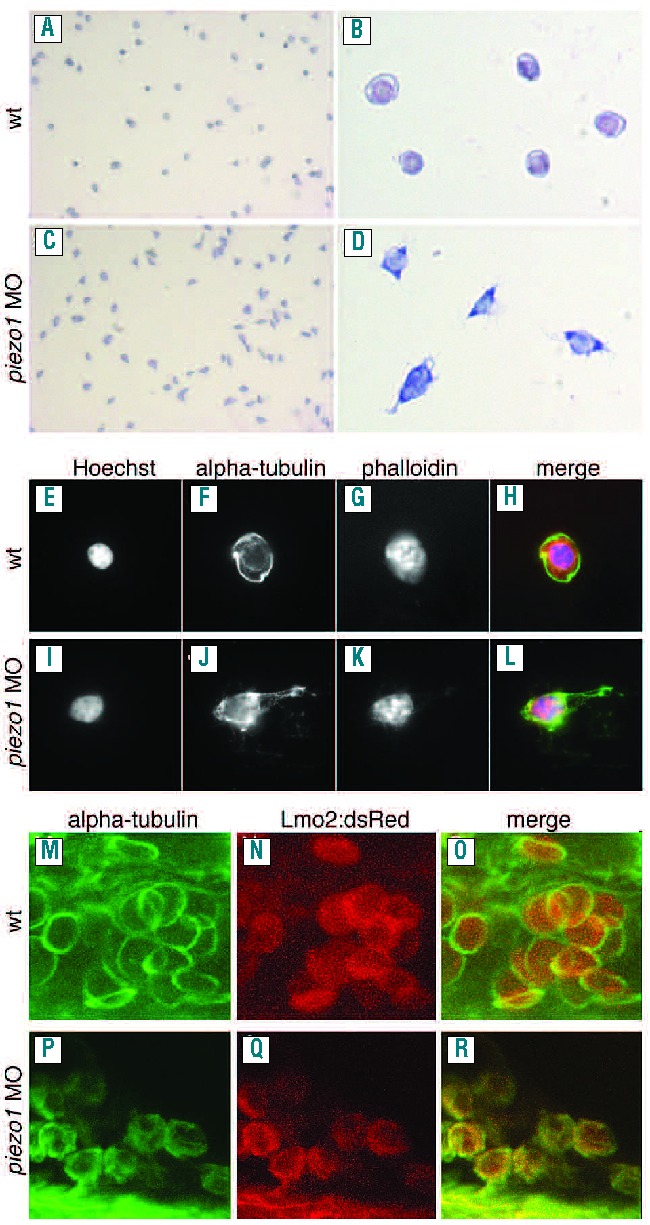
Piezo1 knockdown results in deformed erythrocytes and disruption of the marginal band of microtubules. (A–D) Wright-Giesma stained blood smears prepared from wild-type (A, B) and piezo1 morphant embryos (C, D). Piezo1 morphant erythrocytes appear deformed with signs of membrane rupture and swollen nuclei (D). Immunohistochemistry analysis of either wild-type (E-H) or piezo1 morphant embryos (I-L), utilizing Hoechst to stain the nuclei (E, I), an anti-α−tubulin to label the marginal band of microtubules (F, J) and phalloidin to label the cytoskeleton (G, K) and merged images (H, L). Piezo1 morphant erythrocytes have swollen deformed nuclei (I) and a deformed/ruptured membrane (J). In vivo immunohistochemistry analysis of either wild-type (M–O) or piezo1 morphant embryos (P–R) obtained Lmo:dsRed transgenic zebrafish. The marginal band is labeled with anti α−tubulin (M, P) and erythrocytes express dsRed (N, Q). Piezo1 morphant erythrocytes appear spherical and have a disrupted/diffuse marginal band of microtubules (P, R) when compared to control erythrocytes in which the marginal band encircles the erythrocytes in a single plane (M, O).
Here we have shown that knockdown of piezo1 leads to a drastic reduction in the number of circulating erythrocytes, which is not due to perturbed primitive hematopoiesis or erythrocyte differentiation. Our observations indicate that piezo1 morphant erythrocytes become spherical and display a disrupted marginal band of microtubules, similar to observations made in zebrafish models of spherocytosis.15,24 When components of the erythrocyte cell membrane, such as protein 4.1 or β spectrin, are disrupted, this leads to a reduction in the cell surface (due to membrane blebbing) and failure to maintain an elliptical shape. Consequently, this results in the contraction of erythrocytes to the spherical form observed in spherocytosis.25 However, although piezo1 morphant erythrocytes adopt a spherical shape they appear to be swollen, increasing their overall volume in the process. Furthermore, we did not observe the increased apoptosis associated with spherocytosis, which indicates that the phenotype observed in piezo1 morphants is more akin to conditions such as hydrocytosis.24,26 Osmotic induced swelling of erythrocytes leads to changes in cell membrane tension.27 This will induce the stretch-activated channel piezo1 to open and ultimately counteract this increase in water influx to avoid erythrocyte lysis. If, however, piezo1 is missing, the erythrocyte will continue to swell and ultimately lyse. Interestingly, we observed that the marginal band of microtubules was disrupted in piezo1 morphants. Although we cannot rule out that this is just a consequence of swelling-induced lysis, it may indicate that piezo1 also has a structural role, similar to the anion exchanger band3. Indeed, observations similar to those in piezo1 morphants have been made in frog red blood cells. Frog erythrocytes can be induced to swell using specific toxins that induce water influx. However, these cells still retain an elliptical form. Subsequent disruption of the cytoskeleton using cytochalasin D leads to the development of a swollen spherical shape prior to lysis.28 Thus the defects associated with knockdown of piezo1 may also be due, in part, to disruption of the cytoskeleton.
Recent reports have linked piezo1 to the hereditary blood disorder xerocytosis in humans, and established that piezo1 is present in the membranes of erythrocytes.10,29 Xerocytosis is an autosomal dominant hemolytic anemia characterized by a defect in the cell membrane leading to potassium efflux and resulting in rigidity, hemolysis and reduced erythrocyte survival.30 However, our data suggests that loss of piezo1 results in erythrocyte swelling and lysis which is more consistent with conditions such as hydrocytosis rather than the dehydration observed in xerocytosis.31,32 Interestingly, it has recently been established that the mutations in piezo1 associated with xerocytosis lead to delayed channel inactivation. This means the channel will stay open for a longer time, resulting in dehydrated erythrocytes rather than the swollen cells we observed when piezo1 is knocked down.33,34
Undoubtedly, the severe phenotype we observe in piezo1 morphants would be lethal in mammals and it is unlikely that complete disruption of this gene could be tolerated. However, mutations may exist which could reduce the functionality of the channel, resulting in the swelling of erythrocytes associated with conditions such as hydrocytosis. Furthermore, if piezo1 also plays a structural role, there may also be mutations that affect this function while still retaining an operational channel, resulting in a phenotype more akin to spherocytosis.
Acknowledgments
The authors would like to thank George Lutfalla for providing the MPEG-mCherry transgenic line. AF is supported by a Fondation pour la Recherche Médicale (FRM) postdoctoral fellowship. This work was supported by grants from INSERM ATIP-AVENIR (to CJ), the Agence Nationale pour la Recherche (to KK), and the Languedoc-Roussillon Region Chercheur d’Avenir (to KK and CJ), and grant ANR-2010-BLAN-1128-01 (to MEM). AF, JN, MEM and CJ are members of the Laboratory of Excellence «Ion Channel Science and Therapeutics» supported by a grant from ANR.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10(1):63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81(2):685–740 [DOI] [PubMed] [Google Scholar]
- 3.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science (New York, NY). 2010;330(6000):55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb PA, Sachs F. Piezo1: properties of a cation selective mechanical channel. Channels (Austin). 2012;6(4):214–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vollrath MA, Kwan KY, Corey DP. The micromachinery of mechanotransduction in hair cells. Annu Rev Neurosci. 2007;30:339–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lammerding J, Kamm RD, Lee RT. Mechanotransduction in cardiac myocytes. Ann N Y Acad Sci. 2004;1015:53–70 [DOI] [PubMed] [Google Scholar]
- 7.Patel A, Honore E. Polycystins and renovascular mechanosensory transduction. Nat Rev Nephrol. 2010;6(9):530–8 [DOI] [PubMed] [Google Scholar]
- 8.Nilius B, Honore E. Sensing pressure with ion channels. Trends Neurosci. 2012;35(8):477–86 [DOI] [PubMed] [Google Scholar]
- 9.Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012; 483(7388):209–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarychanski R, Schulz VP, Houston BL, Maksimova Y, Houston DS, Smith B, et al. Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood. 2012;120(9):1908–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203(3):253–310 [DOI] [PubMed] [Google Scholar]
- 12.Lin HF, Traver D, Zhu H, Dooley K, Paw BH, Zon LI, et al. Analysis of thrombocyte development in CD41-GFP transgenic zebrafish. Blood. 2005;106(12):3803–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellett F, Pase L, Hayman JW, Andrianopoulos A, Lieschke GJ. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood. 2011;117(4):e49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu H, Traver D, Davidson AJ, Dibiase A, Thisse C, Thisse B, et al. Regulation of the lmo2 promoter during hematopoietic and vascular development in zebrafish. Dev Biol. 2005;281(2):256–69 [DOI] [PubMed] [Google Scholar]
- 15.Shafizadeh E, Paw BH, Foott H, Liao EC, Barut BA, Cope JJ, et al. Characterization of zebrafish merlot/chablis as non-mammalian vertebrate models for severe congenital anemia due to protein 4.1 deficiency. Development. 2002; 129(18):4359–70 [DOI] [PubMed] [Google Scholar]
- 16.Raya A, Koth CM, Buscher D, Kawakami Y, Itoh T, Raya RM, et al. Activation of Notch signaling pathway precedes heart regeneration in zebrafish. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11889–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hultman KA, Bahary N, Zon LI, Johnson SL. Gene duplication of the zebrafish kit ligand and partitioning of melanocyte development functions to kit ligand a. PLoS Genet. 2007;3(1):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monteiro R, Pouget C, Patient R. The gata1/pu.1 lineage fate paradigm varies between blood populations and is modulated by tif1gamma. EMBO J. 2011;30(6):1093–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pase L, Layton JE, Kloosterman WP, Carradice D, Waterhouse PM, Lieschke GJ. miR-451 regulates zebrafish erythroid maturation in vivo via its target gata2. Blood. 2009;113(8):1794–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian F, Zhen F, Xu J, Huang M, Li W, Wen Z. Distinct functions for different scl isoforms in zebrafish primitive and definitive hematopoiesis. PLoS Biol. 2007;5(5):e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kissa K, Murayama E, Zapata A, Cortes A, Perret E, Machu C, et al. Live imaging of emerging hematopoietic stem cells and early thymus colonization. Blood. 2008;111(3):1147–56 [DOI] [PubMed] [Google Scholar]
- 22.Le Guyader D, Redd MJ, Colucci-Guyon E, Murayama E, Kissa K, Briolat V, et al. Origins and unconventional behavior of neutrophils in developing zebrafish. Blood. 2008;111(1):132–41 [DOI] [PubMed] [Google Scholar]
- 23.de Jong JL, Zon LI. Use of the zebrafish system to study primitive and definitive hematopoiesis. Annu Rev Genet. 2005;39:481–501 [DOI] [PubMed] [Google Scholar]
- 24.Liao EC, Paw BH, Peters LL, Zapata A, Pratt SJ, Do CP, et al. Hereditary spherocytosis in zebrafish riesling illustrates evolution of erythroid beta-spectrin structure, and function in red cell morphogenesis and membrane stability. Development. 2000;127(23):5123–32 [DOI] [PubMed] [Google Scholar]
- 25.An X, Mohandas N. Disorders of red cell membrane. Br J Haematol. 2008;141(3):367–75 [DOI] [PubMed] [Google Scholar]
- 26.Lang E, Qadri SM, Lang F. Killing me softly -suicidal erythrocyte death. Int J Biochem Cell Biol. 2012;44(8):1236–43 [DOI] [PubMed] [Google Scholar]
- 27.Hua SZ, Gottlieb PA, Heo J, Sachs F. A mechanosensitive ion channel regulating cell volume. Am J Physiol Cell Physiol. 2010;298(6):C1424–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauviat MP, Boydron-Le Garrec R, Masson JB, Lewis RL, Vernoux JP, Molgo J, et al. Mechanisms involved in the swelling of erythrocytes caused by Pacific and Caribbean ciguatoxins. Blood Cells Mol Dis. 2006;36(1):1–9 [DOI] [PubMed] [Google Scholar]
- 29.Andolfo I, Alper SL, De Franceschi L, Auriemma C, Russo R, De Falco L, et al. Multiple clinical forms of dehydrated hereditary stomatocytosis arise from mutations in PIEZO1. Blood. 2013;121(19):3925–35 [DOI] [PubMed] [Google Scholar]
- 30.Jokinen CH, Swaim WR, Nuttall FQ. A case of hereditary xerocytosis diagnosed as a result of suspected hypoglycemia and observed low glycohemoglobin. J Lab Clin Med. 2004;144(1):27–30 [DOI] [PubMed] [Google Scholar]
- 31.Bruce LJ. Hereditary stomatocytosis and cation-leaky red cells--recent developments. Blood Cells Mol Dis. 2009;42(3):216–22 [DOI] [PubMed] [Google Scholar]
- 32.Gallagher PG. Disorders of red cell volume regulation. Curr Opin Hematol. 2013;20(3):201–7 [DOI] [PubMed] [Google Scholar]
- 33.Bae C, Gnanasambandam R, Nicolai C, Sachs F, Gottlieb PA. Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc Natl Acad Sci USA. 2013;110(12):E1162–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albuisson J, Murthy SE, Bandell M, Coste B, Louis-Dit-Picard H, Mathur J, et al. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat Commun. 2013;4:1884. [DOI] [PMC free article] [PubMed] [Google Scholar]


