Abstract
Recent studies suggest that leukocytes and erythrocytes play a role in coagulation. However, whether leukocytes, erythrocytes and other hematologic variables are associated with risk of venous thrombosis is not well known. To study this, we used data from 2473 patients with venous thrombosis and 2935 controls. The variables assessed were: total leukocytes, granulocytes, lymphocytes, monocytes, hematocrit, hemoglobin, erythrocytes and red cell indices (mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration and red cell distribution width). We found a strong dose-response relation for higher red cell distribution width and monocyte count with risk of venous thrombosis, with odds ratios of 3.1 (95% confidence interval, 2.0–4.8) and 2.8 (95% confidence interval, 1.3–5.8), respectively, after adjustment for age, sex, C-reactive protein level, malignancy and co-morbidities. Monocyte count and red cell distribution width were associated with venous thrombosis even within reference ranges. A low monocyte count (<0.12×109/L) was associated with a lower risk of venous thrombosis after full adjustment (odds ratios 0.6; 95% confidence interval, 0.4–0.8). In summary, high red cell distribution width and blood monocyte count, two parameters that are inexpensive and easily obtainable, were clearly associated with an increased risk of venous thrombosis. Future studies should evaluate the underlying mechanism and the use of these variables in prediction models for first and recurrent thrombosis.
Introduction
Venous thrombosis is one of the leading causes of mortality. Its clinical presentation, pulmonary embolism and deep vein thrombosis, occurs in approximately 1–2 per 1000 persons per year.1 Although major progress has been made towards a better understanding of venous thrombosis, in 30%–50% of the cases it remains idiopathic.2 In the last decades, interest has focused on identifying new risk factors and building predictive models for venous thrombosis. For this, easily accessible and inexpensive variables should be targeted.
Recent studies have suggested that leukocytes and erythrocytes play a role in the process of coagulation3–10 and their presence is clearly observed in the anatomy of a venous clot, which consists of a laminar structure of erythrocytes and fibrin, permeated by large numbers of leukocytes.11 A recent study, using a mouse model of flow restriction-induced deep vein thrombosis without endothelium damage, showed that blood monocytes and neutrophils provide an initiating stimulus for development of deep vein thrombosis.7
Some studies have shown that leukocytosis is a predictor of venous thrombosis during the follow-up of patients with polycythemia vera and essential thrombocytemia.12,13 However, the effect of peripheral leukocytes on the risk of venous thrombosis in patients without such diseases has, to our knowledge, not been investigated. Furthermore, investigation of the role of erythrocytes and other blood cell variables in venous thrombosis is also scarce.14,15 We, therefore, investigated whether peripheral leukocytes, erythrocytes and other hematologic variables (hematocrit, hemoglobin and red cell indices) are associated with the risk of venous thrombosis. For this, we used data from the MEGA study, a large population-based case-control study into risk factors for venous thrombosis in the Netherlands.
Methods
Complete and detailed methods are provided in the Online Supplement. In brief, the MEGA study is a large population-based case-control study.16,17 Patients with a first venous thrombosis were between 18–70 years old. Control subjects were either patients’ partners or controls, identified by random digit dialing. All participants filled in a questionnaire that contained questions on recent thrombotic risk factors.
Blood was collected into trisodium citrate and processed within 4 hours. Except for the red cell distribution width (RDW), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) the final counts were multiplied by a factor of 1.1 in order to adjust the values due to the collection into sodium citrate. C-reactive protein (CRP) was measured on stored (at −80°C) and previously unthawed samples.
All participants provided informed consent and the study was approved by the Medical Ethics Committee of the Leiden University Medical Center, Leiden, the Netherlands.
Statistical analysis
Odds ratios and their 95% confidence intervals (95% CI) were cal culated for erythrocytes, leukocytes, hematocrit, hemoglobin and red cell indices and adjusted for age, sex, malignancy, co-morbidities and CRP. Additionally, since smoking is a risk factor for venous thrombosis and may affect hematocrit, hemoglobin and erythrocyte counts, these variables were further adjusted for smoking. Although anemia is not known to be a risk factor for venous thrombosis, it can act as a proxy for diseases that can affect the risk of venous thrombosis (e.g. malignancy). Thus, these variables were further adjusted for anemia. Cut-off points of variables were established at the 1st, 5th, 95th, 97.5th and 99th percentiles in the control subjects.
Results
Patients and control subjects
The clinical characteristics of the participants are shown in Online Supplementary Table S1. In total, there were 5388 participants, of whom 47% were men. The median age at onset of the first episode of venous thrombosis was 50 years (range, 18–70). At the time of venipuncture, 288 patients (12%) and 30 controls (1%) were on anticoagulant treatment. As expected, classical venous thrombosis risk factors and malignancy were more commonly present in patients than in controls (Online Supplementary Table S1). Current smoking, alcohol consumption and other co-morbidities were also more common in patients than in controls.
White blood cells
In Table 1, the risk of venous thrombosis is presented for percentiles of total leukocytes, granulocytes, lymphocytes and monocytes. For the leukocytes, only very low levels (below the 1st percentile) were associated with a risk of venous thrombosis with an odds ratio of 1.9 (95% CI, 1.1–3.3) after adjustment for age, sex, CRP, malignancy and co-morbidities, in comparison with the reference value.
Table 1.
Risk of venous thrombosis for strata of white blood cell counts for cases and controls.
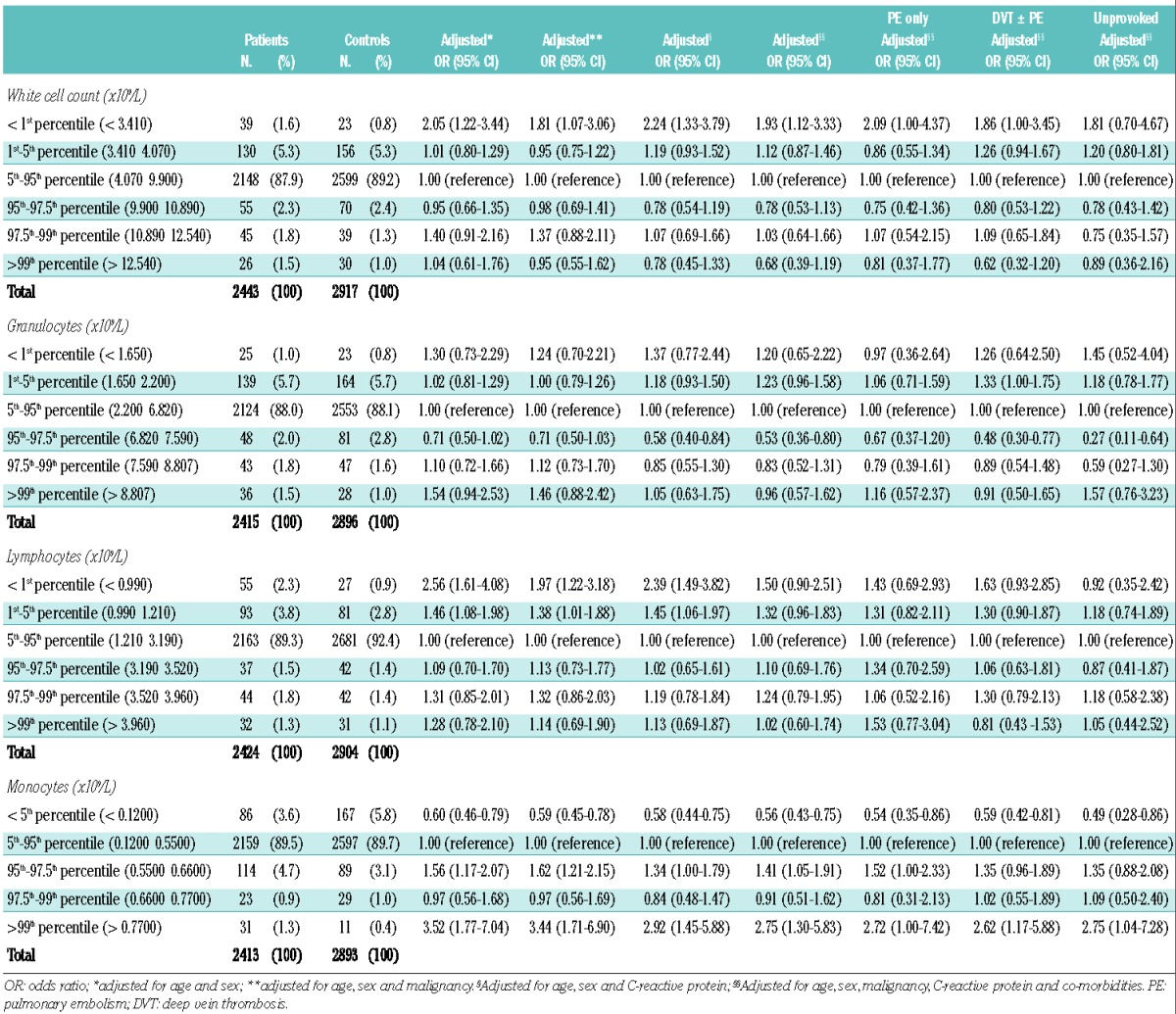
For lymphocytes, we observed an association for the lowest level with an odds ratio of 2.4 (95% CI, 1.5–3.8) for below the 1st percentile and an odds ratio of 1.5 (95% CI, 1.1–2.0) for between the 1st and 5th percentiles, after adjustment for age, sex, malignancy and CRP. However, this was attenuated when the analyses were further adjusted for co-morbidities, resulting in odds ratios of 1.5 (95% CI, 0.9–2.5) and 1.3 (95% CI, 1.0–1.8), respectively, versus the reference value (Table 1).
The only clear association found, which was only marginally affected by any adjustment, was for the monocyte count. There was a graded effect with higher risks at higher counts (‘dose-response’), reaching the highest risk at and above the 99th percentile with an odds ratio of 2.8 (95% CI, 1.3–5.8) after full adjustment, in comparison with the reference category. The association was also present for pulmonary embolism only, deep vein thrombosis with or without pulmonary embolism and unprovoked venous thrombosis. Interestingly, the risk was reduced relative to the reference group for monocyte counts below the 5th percentile (< 0.12×109/L) after full adjustment with an odds ratio of 0.6 (95% CI, 0.4–0.8).
Red cell indices
Table 2 shows the risk of venous thrombosis presented for percentiles of MCV, MCH, MCHC and RDW. MCV and MCH showed an U-shaped response, with higher odds ratios in the two lowest (below 1st and between 1st and 5th) and highest (above 99th) percentiles.
Table 2.
Risk of venous thrombosis for strata of red blood cell indices for cases and controls.
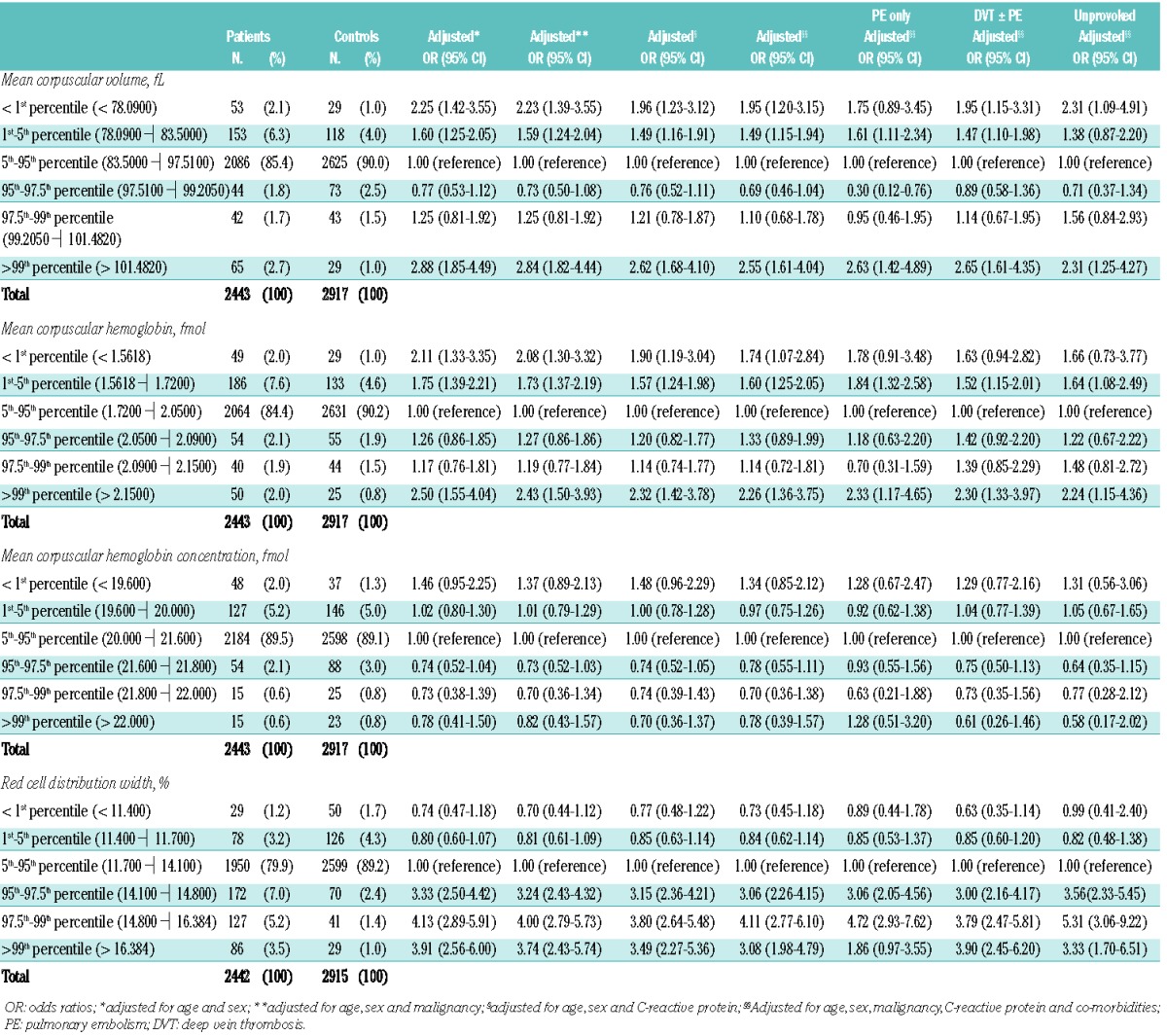
For the below 1st, between 1st and 5th and above the 99th percentiles of the MCV, the odds ratios were 2.0 (95% CI 1.2–3.2), 1.5 (95% CI 1.2–1.9) and 2.6 (95% CI 1.6–4.0), respectively, after full adjustment. Further adjustment for anemia led to marginal changes.
For the below 1st, between 1st and 5th and above the 99th percentiles of the MCH, the odds ratios were 1.7 (95% CI 1.1–2.8), 1.6 (95% CI 1.3–2.1) and 2.3 (95% CI 1.4–3.8), respectively, after full adjustment. We further adjusted the analysis for anemia, which also resulted in a marginal effect.
We found a strong and consistent association with a dose-response effect for the RDW with increasing odds ratios above the 95th percentile (Table 2). This corresponded to a RDW above 14.1%, which is still within the reference range. After full adjustment, the odds ratios at the 95th to 97.5th, 97.5th to 99th and above 99th percentiles were 3.1 (95% CI 2.3–4.2), 4.1 (95% CI 2.8–6.1) and 3.1 (95% CI 2.0–4.8), respectively. Adjustment for anemia changed the results only slightly. For all percentiles above the 95th, a higher risk was also observed within all subgroups, i.e., for pulmonary embolism only, deep vein thrombosis with or without pulmonary embolism and unprovoked venous thrombosis.
We performed an extra analysis in which we repeated all age- and sex-adjusted analyses after exclusion of all patients and controls with malignancy. This did not alter the results (data not shown).
Erythrocytes, hematocrit and hemoglobin
The analyses of erythrocytes, hematocrit and hemoglobin were stratified by sex due to higher levels of these variables in men. For hematocrit, the relative risk was higher between the 95th and 97.5th percentiles with odds ratio of 2.0 (95% CI, 1.2–3.3) after full adjustment. For erythrocytes, the odds ratios were higher below the 1st and above the 99th percentiles, i.e. 2.3 (95% CI, 1.2–4.6) and 3.6 (95% CI 1.6–8.1), respectively, after full adjustment. For hemoglobin, the effect was higher between the 97.5th and 99th percentiles with an odds ratio of 1.8 (95% CI, 1.0–3.1) after full adjustment (data not shown).
In women, for hematocrit, the effect was increased at the percentiles below the 1st and between the 1st and 5th, with odds ratios of 2.3 (95% CI 1.2–4.3) and 1.5 (95% CI 1.0–2.2), respectively, after full adjustment. For hemoglobin, there was an increased effect at the lowest percentile (below the 1st percentile) with an odds ratio of 3.4 (95% CI 1.7–6.7) after full adjustment (data not shown).
Influence of time between the thrombotic event and blood sampling
To quantify the possibility of a post hoc phenomenon in which levels of hematologic variables gradually diminish after the onset of venous thrombosis, boxplots were constructed for hematologic variables within the group of patients at 3–6 months, 6–9 months, 9–12 months and >12 months after venous thrombosis had occurred to see if there were any differences (Figure 1). As shown by the figure, hematologic variables were not influenced by time between the thrombotic event and blood sampling. For example, the mean level of the RDW measured between 3–6 months after the event took place was 13.6%, measured between 6–9 months it was 13.2%, between 9–12 months 13.2% and >12 months 13.3% (Figure 1).
Figure 1.
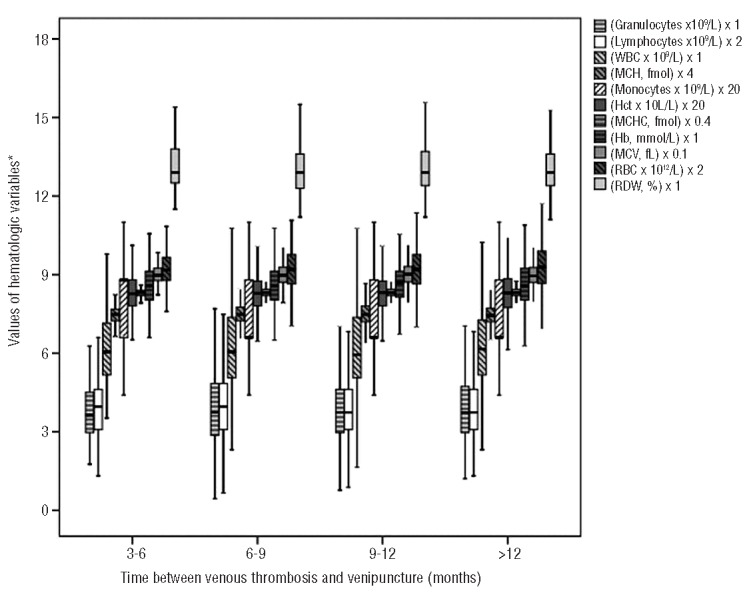
Time elapsed between venous thrombosis and blood collection for different hematologic variables. The values of hematologic variables are, in some cases, multiplied to make it possible to present all outcomes in one figure (see figure legend for multiplication numbers). Hct hematocrit; WBC: white blood count; RBC: red blood cell; Hb: hemoglobin; RDW: red cell distribution width; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration.
Discussion
Blood cell count is a widely available, easy to perform and inexpensive test. We aimed to investigate the role of peripheral blood leukocytes, erythrocytes and red cell indices in the risk of venous thrombosis. In a large case-control study with 2453 patients and 2935 controls, we found a dose-related increased risk of venous thrombosis with higher blood monocyte count and RDW.
Automated cell counters can estimate erythrocyte volume cell by cell, sampling millions of erythrocytes in the process. In addition to the calculation of the MCV, they also determine the dispersion of values about this mean. The latter value, also defined as RDW, is the coefficient of variation (expressed as a percent), which is the product of the standard deviation of red blood cell size divided by the MCV. The RDW is a semi-quantitative measure of erythrocyte anisocytosis. There is no condition which regularly yields a RDW less than normal,18 so, in clinical practice, the RDW is either normal or elevated, with higher values reflecting greater heterogeneity in cell size. RDW is currently mainly used as an auxiliary index in the differential diagnosis of microcytic anemia, in which iron deficiency anemia is associated with a high RDW and thalassemia syndromes with a normal RDW, due to a more homogenous size of erythocytes. An increased RDW is commonly found when there is a nutritional deficiency, such as iron, folate, or vitamin B12 deficiency.
Several studies and a meta-analysis have recently shown that a high RDW is not only associated with hematologic disorders.19–25,30 Even within the normal reference range, it has been shown to be associated with total mortality and cardiovascular disease in middle-aged and older adults without major age-associated disorders.19–25 These cardiovascular disorders included heart failure,19 coronary artery disease,26 conditions requiring percutaneous coronary intervention27 and a first acute cerebral infarction.28 In a study by Poludasu et al., the association was independent of the level of hemoglobin and was present despite the fact that RDW was within the normal range in 88% of the subjects.27 In a study by Tonelli et al., a higher RDW was a strong and independent predictor of increased long-term mortality in patients undergoing percutaneous coronary intervention who were not anemic at baseline.26 Kim et al. studied 847 consecutive patients with a first acute cerebral infarction and reported that a higher RDW was independently associated with poor functional outcome and increased risk of all-cause mortality.28
Moreover, an elevated RDW was reported to be associated with an increased risk of all-cause mortality, including death from chronic lower respiratory tract disease and cancer-related death.21,23,29 The study by Patel et al. showed that for every 1% increase in RDW, all-cause mortality risk increased by 22%.21 The relationship persisted even when analyses were performed in non-anemic subjects or in those with RDW levels within the normal range (11%–15%) without deficiency of iron, folate or vitamin B12.21 A recently published study has also reported an independent association between high RDW and increased risk of acute pulmonary embolism-related early mortality.30
In our study, RDW was associated with an increased risk of venous thrombosis, even when the RDW was still within the usual reference range (above 14.1%). This association remained after adjustment for age, sex, CRP, malignancy and co-morbidities (OR 3.1; 95% CI 2.0–4.8 for above the 99th percentile) and for all subgroup analyses. Further adjustments for anemia did not reduce the odds ratio. To our knowledge, this is the first study to report an association between venous thrombosis and higher RDW.
The physiological mechanisms underlying the association between elevated RDW and increased cardiovascular disease, all-cause mortality and now venous thrombosis are unknown, although oxidative stress, poor pulmonary function and inflammation have been suggested.31–33 In the case of venous thrombosis, we hypothesize that a greater heterogeneity in cell size (and maybe lower erythrocyte deformability and higher aggregation) could increase viscosity and impair blood flow, leading to stasis, one of the main risk factors for venous thrombosis. This is supported by a recent study that showed that an increase in erythrocyte aggregation promotes thrombosis in femoral veins in a rabbit model.9 Furthermore, erythrocytes have been shown to participate in thrombin generation in tissue factor-activated blood8 and to form adhesive interactions under low flow conditions.34
We found an association between high MCV (above 101.5 fL) and high MCH (above 2.15 fmol) and venous thrombosis, which did not attenuate after adjustment for age, sex, malignancy, CRP, co-morbidities and anemia. A recent study did not find an association between venous thrombosis and MCV.14 Although this study included more than 20,000 participants, blood samples were collected many years before the thrombotic event, which is likely to have led to a dilution of the effect of MCV.
Additionally, we found that a higher peripheral blood monocyte count, even within the reference range (i.e., above 0.55×109/L), was associated with venous thrombosis in a dose-response manner. It reached its maximum effect above 0.77×109/L with an odds ratio of 2.8 (95% CI 1.3–5.8) after full adjustment. Interestingly, a low monocyte count (below 0.12×109/L) was associated with a lower risk of venous thrombosis after full adjustment, which was also the case in all subgroup analyses. To our knowledge, this is the first study to report an association between venous thrombosis and peripheral blood monocyte count.
Monocytes are known to express tissue factor and represent about 30% of the leukocytes in a venous thrombus after 48 hours of flow restriction.7 According to a recent mouse model of deep vein thrombosis, monocytes are the type of leukocyte that predominantly contribute to tissue factor-driven coagulation.7 Furthermore, monocytes are the most important leukocyte involved in the modulation of venous thrombus resolution.35–37 In atherosclerosis, monocytes are the central drivers of vascular inflammation and experimental studies have proven a causative role of monocytes in atherogenesis.38 A recent study showed that a subset of monocytes, CD14++CD16+, independently predicted cardiovascular events in participants referred for elective coronary angiography.39
Some methodological issues in this study need to be addressed. First, our study is a classical case-control study in which blood samples were collected after the thrombotic event. We cannot, therefore, exclude the possibility that alterations of the hematologic variables were the result of the thrombotic event itself. However, blood was collected at least 3 months after discontinuation of oral anticoagulation, and it is unlikely that the event itself caused persistent abnormalities in the hematologic variables. Second, the hematologic variables were measured only once after the thrombotic event; which did not allow an analysis of potential changes of the variables over time. Third, a question that could not be answered by this study is whether the association between high RDW, high monocyte count and venous thrombosis is causal or whether it can be explained by the presence of other diseases or conditions (for example, hemoglobinopathies, use of medication) that could lead to altered blood cell counts and venous thrombosis and for which we were not able to adjust. However, we adjusted the analysis for the most important conditions that can influence these hematologic parameters, such as age, sex, inflammation, malignancy and other co-morbidities. Fourth, because of low numbers due to stratification by sex, we could not determine the role of hematocrit, hemoglobin and erythrocytes on the risk of venous thrombosis in this study. Fifth, there was an overall low prevalence of major illnesses (such as cardiovascular diseases) in MEGA, which is likely due to the age criterion (maximum age 70 years, mean age 50 years). These data were self-reported. However, since these are major diseases with a large impact, we expect that both patients and controls reported their illnesses to a similar extent, thus limiting recall bias. Lastly, as mentioned in the Methods section, blood counts as well as red cell indices were obtained from blood collected into tubes containing citrate, while in routine care these blood parameters are normally obtained in tubes with EDTA. Whether our results apply to routine care should, therefore, be interpreted with caution.
In summary, we have shown that high RDW and blood monocyte counts, two parameters that are inexpensive and easily obtainable, were dose-responsively and independently associated with an increased risk of venous thrombosis. Whether this association is causal or not requires further investigation. Future studies should also target the evaluation of these variables as predictors of a recurrent event, which could assist in future decisions regarding prophylaxis.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
The Multiple Environmental and Genetic Assessment of Risk Factors for Venous Thrombosis study was supported by grant NHS 98.113 from the Netherlands Heart Foundation, grant RUL99/1992 from the Dutch Cancer Foundation, and grant 912-03-033l 2003 from the Netherlands Organization for Scientific Research. SMR was funded by grant number 6796-11-3 from CAPES/NUFFIC and by Universidade Federal de Minas Gerais. The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review or approval of the manuscript. The authors thank Camila Caram Deelder for her help with the statistical analysis.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrom J. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost. 2007;5(4):692–9 [DOI] [PubMed] [Google Scholar]
- 2.Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;37(9828):1835–46 [DOI] [PubMed] [Google Scholar]
- 3.Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32(8):1777–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kageyama K, Nakajima Y, Shibasaki M, Hashimoto S, Mizobe T. Increased platelet, leukocyte, and endothelial cell activity are associated with increased coagulability in patients after total knee arthroplasty. J Thromb Haemost. 2007;5(4):738–45 [DOI] [PubMed] [Google Scholar]
- 5.LoPresti R, Ferrara F, Canino B, Montana M, Caimi G. Deep venous thrombosis: leukocyte rheology at baseline and after in vitro activation. Haemostasis. 2000;30(4):168–73 [DOI] [PubMed] [Google Scholar]
- 6.Vaya A, Suescun M. Hemorheological parameters as independent predictors of venous thromboembolism. Clin Hemorheol Microcirc. 2013;53(1–2):131–41 [DOI] [PubMed] [Google Scholar]
- 7.von Brühl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209(4):819–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whelihan MF, Zachary V, Orfeo T, Mann KG. Prothrombin activation in blood coagulation: the erythrocyte contribution to thrombin generation. Blood. 2012;120(18):3837–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu FT, Armstrong JK, Tripette J, Meiselman HJ, Cloutier G. A local increase in red blood cell aggregation can trigger deep vein thrombosis: evidence based on quantitative cellular ultrasound imaging. J Thromb Haemost. 2011;9(3):481–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman M, Monroe DM., 3rd A cell-based model of hemostasis. Thromb Haemost. 2001;85(6):958–65 [PubMed] [Google Scholar]
- 11.Bovill EG, van der Vliet A. Venous valvular stasis-associated hypoxia and thrombosis: What is the link? Annu Rev Physiol. 2011;73:527–45 [DOI] [PubMed] [Google Scholar]
- 12.Barbui T, Carobbio A, Rambaldi A, Finazzi G. Perspectives on thrombosis in essential thrombocythemia and polycythemia vera: is leukocytosis a causative factor? Blood. 2009;114(4):759–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gangat N, Strand J, Li CY, Wu W, Pardanani A, Tefferi A. Leucocytosis in poly-cythaemia vera predicts both inferior survival and leukaemic transformation. Br J Haematol. 2007;138(3):354–8 [DOI] [PubMed] [Google Scholar]
- 14.Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Hansen JB. Hematocrit and risk of venous thromboembolism in a general population. The Tromso study. Haematologica. 2010;95(2):270–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eischer L, Tscholl V, Heinze G, Traby L, Kyrle PA, Eichinger S. Hematocrit and the risk of recurrent venous thrombosis: a prospective cohort study. PLoS One. 2012;7(6):e38705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Stralen KJ, Rosendaal FR, Doggen CJ. Minor injuries as a risk factor for venous thrombosis. Arch Intern Med. 2008;168(1):21–6 [DOI] [PubMed] [Google Scholar]
- 17.Pomp ER, le Cessie S, Rosendaal FR, Doggen CJ. Risk of venous thrombosis: obesity and its joint effect with oral contraceptive use and prothrombotic mutations. Br J Haematol. 2007;139(2):289–96 [DOI] [PubMed] [Google Scholar]
- 18.Schrier SL, Landaw SA. Mean corpuscular volume. In: Basow DS, ed. UpToDate®. Waltham: Wolters Kluwer Health; 2012 [Google Scholar]
- 19.Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007;50(1):40–7 [DOI] [PubMed] [Google Scholar]
- 20.Forhecz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohaszka Z, Janoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009;158(4):659–66 [DOI] [PubMed] [Google Scholar]
- 21.Patel KV, Ferrucci L, Ershler WB, Longo DL, Guralnik JM. Red blood cell distribution width and the risk of death in middle–aged and older adults. Arch Intern Med. 2009;169(5):515–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel KV, Semba RD, Ferrucci L, Newman AB, Fried LP, Wallace RB, et al. Red cell distribution width and mortality in older adults: a meta–analysis. J Gerontol A Biol Sci Med Sci. 2010;65(3):258–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med. 2009;169(6):588–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhodes CJ, Wharton J, Howard LS, Gibbs JS, Wilkins MR. Red cell distribution width outperforms other potential circulating bio-markers in predicting survival in idiopathic pulmonary arterial hypertension. Heart. 2011;97(13):1054–60 [DOI] [PubMed] [Google Scholar]
- 25.Zalawadiya SK, Veeranna V, Niraj A, Pradhan J, Afonso L. Red cell distribution width and risk of coronary heart disease events. Am J Cardiol. 2010;106(7):988–93 [DOI] [PubMed] [Google Scholar]
- 26.Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer MCholesterol and Recurrent Events (CARE) Trial Investigators. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008;117(2):163–8 [DOI] [PubMed] [Google Scholar]
- 27.Poludasu S, Marmur JD, Weedon J, Khan W, Cavusoglu E. Red cell distribution width (RDW) as a predictor of long-term mortality in patients undergoing percutaneous coronary intervention. Thromb Haemost. 2009;102(3):581–7 [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Kim YD, Song TJ, Park JH, Lee HS, Nam CM, et al. Red blood cell distribution width is associated with poor clinical outcome in acute cerebral infarction. Thromb Haemost. 2012;108(2):349–56 [DOI] [PubMed] [Google Scholar]
- 29.Horne BD, May HT, Muhlestein JB, Ronnow BS, Lappé DL, Renlund DG, et al. Exceptional mortality prediction by risk scores from common laboratory tests. Am J Med. 2009;122(6):550–8 [DOI] [PubMed] [Google Scholar]
- 30.Zorlu A, Bektasoglu G, Guven FM, Dogan OT, Gucuk E, Ege MR, et al. Usefulness of admission red cell distribution width as a predictor of early mortality in patients with acute pulmonary embolism. Am J Cardiol. 2012;109(1):128–34 [DOI] [PubMed] [Google Scholar]
- 31.Garcez ME, Peres W, Salvador M. Oxidative stress and hematologic and biochemical parameters in individuals with Down syndrome. Mayo Clin Proc. 2005; 80(12):1607–11 [DOI] [PubMed] [Google Scholar]
- 32.Grant BJ, Kudalkar DP, Muti P, McCann SE, Trevisan M, Freudenheim JL, et al. Relation between lung function and RBC distribution width in a population-based study. Chest. 2003;124(2):494–500 [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi S, Moriya H, Aso K, Ohtake T. Vitamin E-bonded hemodialyzer improves atherosclerosis associated with a rheological improvement of circulating red blood cells. Kidney Int. 2003;63(5):1881–7 [DOI] [PubMed] [Google Scholar]
- 34.Goel MS, Diamond SL. Adhesion of normal erythrocytes at depressed venous shear rates to activated neutrophils, activated platelets, and fibrin polymerized from plasma. Blood. 2002;100(10):3797–803 [DOI] [PubMed] [Google Scholar]
- 35.Henke PK, Pearce CG, Moaveni DM, Moore AJ, Lynch EM, Longo C, et al. Targeted deletion of CCR2 impairs deep vein thombosis resolution in a mouse model. J Immunol. 2006;177(5):3388–97 [DOI] [PubMed] [Google Scholar]
- 36.Henke PK, Varga A, De S, Deatrick CB, Eliason J, Arenberg DA, et al. Deep vein thrombosis resolution is modulated by monocyte CXCR2-mediated activity in a mouse model. Arterioscler Thromb Vasc Biol. 2004;24(6):1130–7 [DOI] [PubMed] [Google Scholar]
- 37.Ali T, Humphries J, Burnand K, Sawyer B, Bursill C, Channon K, et al. Monocyte recruitment in venous thrombus resolution. J Vasc Surg. 2006;43(3):601–8 [DOI] [PubMed] [Google Scholar]
- 38.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7(2):77–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, et al. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol. 2012;60(16):1512–20 [DOI] [PubMed] [Google Scholar]


