Abstract
A novel dioxo-bridged dinuclear gold(III) complex with two 2,9-dimethylphenanthroline ligands was synthesized and thoroughly characterized. Its crystal structure was solved, and its solution behavior assessed. Remarkably, this compound revealed excellent antiproliferative properties in vitro against a wide panel of 36 cancer cell lines, combining a high cytotoxic potency to pronounced tumor selectivity. Very likely, these properties arise from an innovative mode of action (possibly involving histone deacetylase inhibition), as suggested by COMPARE analysis. In turn, electrospray ionization−mass spectrometry studies provided valuable insight into its molecular mechanisms of activation and of interaction with protein targets. Gold(III) reduction, dioxo bridge disruption, coordinative gold(I) binding to the protein, and concomitant release of the phenanthroline ligand were proposed to occur upon interaction with superoxide dismutase, used here as a model protein. Because of the reported results, this new gold(III) compound qualifies itself as an optimal candidate for further pharmacological testing.
Keywords: Cytotoxic gold compounds, COMPARE analysis, protein targets, anticancer agents
Cytotoxic metallodrugs form an important and established class within the current arsenal of anticancer agents; yet, they offer excellent and unexplored opportunities for further drug discovery. After the clinical success of cisplatin,1−3 a great number of metal-based compounds were synthesized and tested in search of new anticancer leads, taking advantage of the rich and diverse chemistry of the various metal centers (e.g., platinum, ruthenium, copper, tin, etc.); thus a large number of cytotoxic metal complexes were disclosed, often showing innovative modes of action.4−7
On the whole, gold compounds constitute a very promising family of cytotoxic and potentially anticancer agents. During the last years, we prepared and evaluated several gold(III) complexes and some organogold(III) compounds that revealed very attractive antiproliferative profiles in vitro.8 In the meantime, other research groups synthesized and characterized a few gold(III) porphyrins9,10 and gold(III) dithiocarbamates11 that manifested outstanding anticancer actions in animal models and are now undergoing advanced preclinical testing. Pairwise, gold(I) compounds have rapidly emerged as a group of potent antimitochondrial and pro-apoptotic agents.12 A systematic investigation describing the anticancer properties in vitro of a variegate panel of gold compounds against 36 cancer cell lines was recently reported, demonstrating that the observed cytotoxic effects are the result of various, and often unprecedented, molecular mechanisms.13
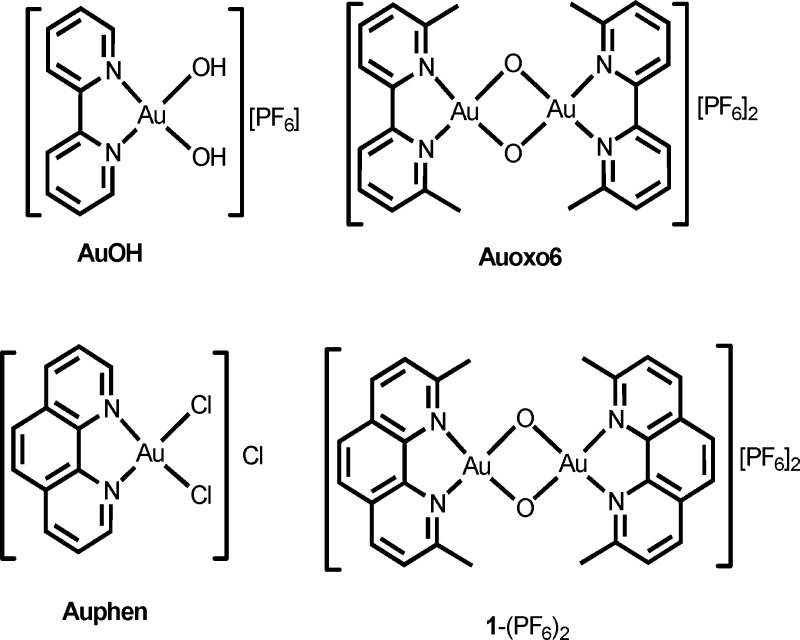
A mononuclear gold(III) derivative of 1,10-phenanthroline (phen), namely, [Au(phen)Cl2]Cl (Auphen), with pronounced cytotoxic properties, was reported by us a few years ago.14,15 More recently, we have characterized a series of dinuclear oxo-bridged gold(III) derivatives of substituted 2,2′-bipyridines (bipynR; n = none, 1, 2), namely [Au2(bipynR)2(μ-O)2][PF6]2 (a member of the series, Auoxo6, is reported in Chart 1), endowed with enhanced stability in solution and improved biological profiles in comparison with the related mononuclear species [Au(bipy)(OH)2][PF6] (AuOH).16 On the ground of those previous results and upon considering that multinuclearity may lead to innovative chemical and biological properties,17,18 we were prompted to prepare new dinuclear oxo-bridged gold(III) complexes using 2,9-dimethyl-1,10-phenathroline (phen2Me) as a ligand.
Chart 1. Mononuclear and Dinuclear Gold(III) Compounds.
The novel complex [Au2(phen2Me)2(μ-O)2](PF6)2, 1-(PF6)2, described here (Chart 1), was obtained by reaction of [Au(phen2Me)Cl3]19 with NaOAc and excess KPF6 in H2O-MeCN (see the Supporting Information for details). X-ray quality crystals were obtained for the [BAr′4]-salt 1-(BAr′4)2 (BAr′4 = B{C6H3(CF3)2}4) by slow diffusion of diethyl ether into a dichloromethane solution of the complex.
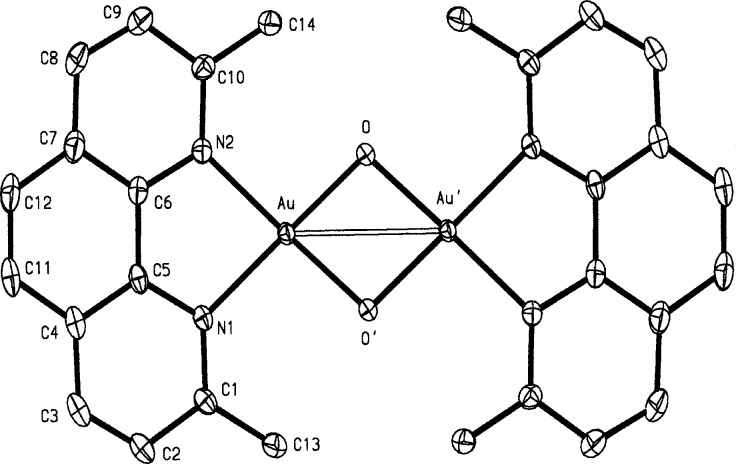
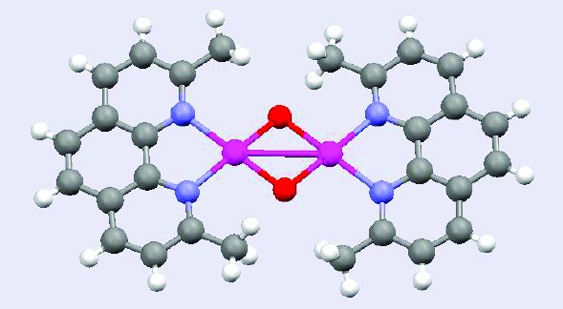
The crystal structure of the complex20 is shown in Figure 1, with principal bond lengths and angles reported in the caption. The structure consists of the packing of 12+ cations and [B{C6H3(CF3)2}4]− anions, in a molar ratio of 1:2, in the triclinic space group P1̅, with two interionic C−H···F hydrogen bonds (see the Supporting Information).
Figure 1.
ORTEP diagram of the dication in 1-(BAr′4)2. Ellipsoids are shown at the 35% probability level. The cation lies on a crystallographic inversion center. Principal bond parameters are as follows: Au···Au′, 3.019(1); O···O′, 2.493(2); Au−O, 1.960(2); Au−O′, 1.956(2); Au−N(1), 2.071(2); and Au−N(2), 2.057(2) Å; and Au−O−Au′, 100.88(8); O−Au−O′, 79.12(6); O−Au−N(1), 176.89(5); O−Au−N(2), 99.38(7); O′−Au−N(1), 100.30(6); O′−Au−N(2), 176.65(7); and N(1)−Au-N(2), 81.04(7)°.
Both gold atoms display a classical square-planar coordination, with just a slight square-pyramidal distortion. The phenanthroline moiety, taken as a whole, shows a good degree of planarity. The dihedral angle between the planes of the Au2O2 moiety, which is rigorously planar by symmetry, and the least-squares plane of the phenanthroline ligand, is 15.4(2)°. As a result, the whole 12+ cation is definitely nonplanar.
Cyclic voltammetry measurements carried out on 1-(PF6)2 show that this compound features an electrochemical behavior strictly similar to that of the Auoxo derivatives, previously described,16 implying that the phenatroline ligand greatly stabilizes the +3 gold oxidation state (unpublished results). The solution behavior of 1-(PF6)2 was then investigated. This complex is very soluble in organic solvents but only moderately soluble in aqueous solutions. Freshly prepared solutions of 1-(PF6)2 in 50 mM phosphate buffer (pH 7.4) show a main absorption band with two close maxima at 272 and 280 nm.
Time−course studies revealed small but significant spectral changes upon 24 h of monitoring consisting of slight band shape modifications and of a continuous intensity increase between 6 and 24 h (Figure S3 in the Supporting Information). These spectral changes are suggestive of the progressive breaking of the two oxo-bridges. However, as this process is relatively slow, 1-(PF6)2 can be considered acceptably stable in phosphate buffer and appropriate for the biological tests.
The in vitro antiproliferative properties of 1-(PF6)2 were assayed according to the procedure developed at Oncotest21 and described in previous papers. The new compound was challenged against a representative panel containing 36 different human tumor cell lines. Results are synoptically shown in Figure S4 in the Supporting Information and compared, in Table 1, with those of Auphen and Auoxo6 (bearing bipynR = 6,6′-dimethyl-2,2′-bipyridine as ligand), previously reported.13
Table 1. In Vitro Anticancer Potency, Tumor Selectivity, and COMPARE Analysis Results for the Described Gold(III) Compounds.
| compound | mean IC50 (μg/mL) | mean IC70 (μg/mL) | tumor selectivity ratinga | proposed target by COMPARE analysis |
|---|---|---|---|---|
| 1-(PF6)2 | 0.036 | 0.245 | +++ | HDAC |
| Auoxo6 | 0.572 | 4.438 | +++ | HDAC |
| Auphen | 0.370 | 1.019 | + | CDK |
Selective activity (individual IC70 < 1/3 of mean IC70 value) in + (4−10% of cell lines), ++ (10−20% of cell lines), and +++ (more than 20% of cell lines).
Remarkably, 1-(PF6)2 turned out to be a potent antiproliferative agent (mean IC70 value = 0.245 μg/mL), being about 10 times more effective than Auphen and also displaying a significantly different cytotoxicity profile.131-(PF6)2 was found to be particularly active against a selected lung cancer line (1121 L), a few prostate cancer lines (22RV1, LNCAP, DU145, and PC3M), and an ovarian cancer line (1619 L), while being far less effective toward a few renal cancer cell lines. Even more importantly, 1-(PF6)2 evidenced a marked selectivity in its cytotoxic actions with an overall scoring of 44% versus a value of only 6% for Auphen (see Table 1).
The COMPARE algorithm22,23 was applied to the analysis of the growth inhibition data of 1-(PF6)2 to obtain clues regarding its possible mechanism of action. The IC50/IC70 values were correlated to the corresponding IC50/IC70 values of 110 standard agents determined against the same cell lines (see Table S2 in the Supporting Information). These standard agents represent the main molecular mechanisms of action for established anticancer drugs. Similarities between the sensitivity pattern of a test compound and the sensitivity pattern of standard drugs are expressed quantitatively as Spearman correlation coefficients (ρ).24 High level correlations between the sensitivity patterns of two compounds are strongly suggestive of a similar mode of action. In the case of 1-(PF6)2, COMPARE analysis revealed striking similarities between this complex and various histone deacetylase (HDAC) inhibitors, that is, ρ = 0.72 for both benzamide acetyldinaline and the cyclic peptide apicidin and ρ = 0.61 for suberic bishydroxamate, suggesting that HDACs might constitute a likely target for this gold(III) compound. Notably, HDACs are nuclear proteins involved in histone regulation; a severe inhibition of their activities may induce growth arrest and promote cell differentiation.25 Further experiments have been planned to validate this hypothesis on a series of HDAC enzymes.
Notably, cisplatin, oxaliplatin, carboplatin, and tetraplatin were included in the list of reference compounds; however, COMPARE analysis did not indicate any significant (ρ > 0.6) correlation of any of the gold compounds to the conventional platinum anticancer compounds. The COMPARE profile of 1-(PF6)2 closely resembles that of Auoxo6, previously described (see Table 1); this is not surprising given the pronounced structural analogy existing between the two compounds. However, 1-(PF6)2 is, in general, found to be superior to Auoxo6 in terms of both average cytotoxic potency and tumor selectivity (see Table 1). Interestingly, the same kind of analysis revealed that mononuclear Auphen possibly acts by cyclin-dependent kinase (CDK) inhibition, with CDK playing an important role in cell cycle regulation.26
To gain further mechanistic insight into its mode of interaction with likely protein targets, the reactions of 1-(PF6)2 with three model proteins, that is, ubiquitin, cytochrome c, and superoxide dismutase (SOD), were explored through high-resolution electrospray ionization−mass spectrometry (ESI-MS), according to an experimental protocol recently developed in our laboratory.27
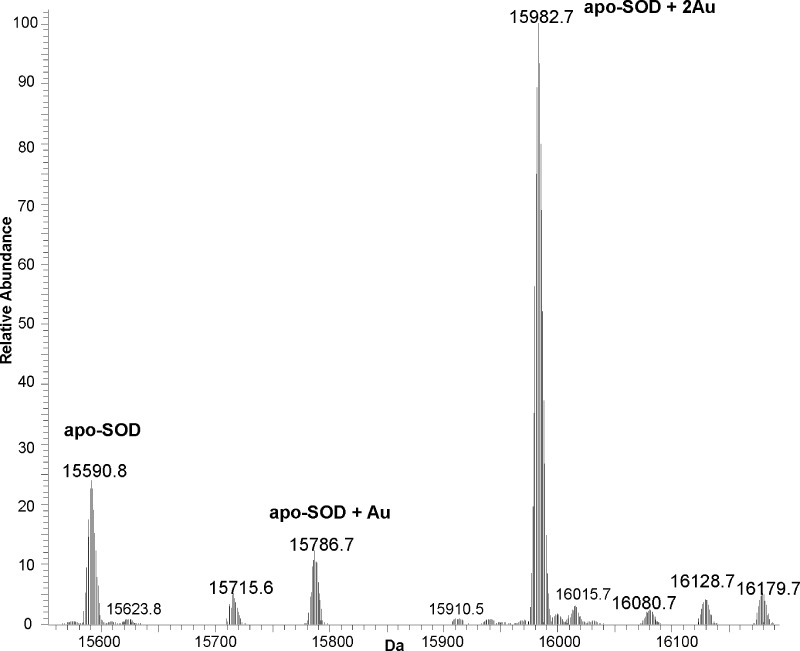
On the basis of this protocol, the reactivity of 1-(PF6)2 with a mixture of the three mentioned proteins was probed without any separation step prior to MS analysis. The selectivity of the gold(III) complex in binding proteins was assessed using this approach. Notably, ESI-MS data evidenced a high reactivity of 1-(PF6)2 with SOD, whereas adduct formation with the other two proteins was very limited. Figure 2 shows the detail of the deconvoluted ESI-MS spectrum focusing on the SOD adducts with 1-(PF6)2 and on the proposed peak assignments. Notably, the peak of the major gold adduct at 15982.7 Da, which corresponds to two Au ions bound to SOD, is far more intense than the peak of the unmodified protein (at 15590.8 Da), suggesting that a high level of protein metalation was afforded. On the ground of previous results for similar systems and of chemical considerations, it is very likely that the Au ions bound to SOD are in the oxidation state +1.16 This hypothesis was confirmed by an independent ESI-MS experiment. Indeed, the reaction of SOD with a gold(I) disaccharinate complex, Au(sac)2−, known to deliver Au+ ions (Maiore et al, submitted), originated an intense peak at 15982.7 Da identical to that found for SOD/1-(PF6)2 .Overall, these observations point out that gold(III) reduction occurs upon 1-(PF6)2 binding to SOD, the two phen2Me ligands being concomitantly released. This kind of reactivity (“activation by reduction”) might be conserved—and even enhanced—in the reducing intracellular milieu of cancer cells.
Figure 2.
LTQ-Orbitrap ESI-MS spectrum of SOD treated with 1-(PF6)2 (3:1, metal:protein ratio) incubated in buffer TMeAmAc (pH 7.4) for 24 h.
In conclusion, we have shown here that a novel dioxo-bridged binuclear gold(III) complex bearing two 2,9-dimethylphenanthroline ligands displays excellent biological features in vitro against a wide panel of cancer cells; these results make the present compound far superior to several other cytotoxic gold(III) compounds previously tested according to the same protocol.13 The importance of multinuclearity28 in modulating and enhancing the biological actions of anticancer metallodrugs is further proved. In particular, the new compound manifests a very high selectivity in its cytotoxic actions making it particularly attractive for further preclinical development. Differences in the biological profile as compared to Auphen are ascribed to the greater stability of the dimetallic scaffold and to the occurrence of some cooperativity between the two nearby gold(III) centers. In turn, COMPARE analysis suggests that the mode of action of 1-(PF6)2 strictly resembles that of Auoxo6 and might involve HDAC inhibition. Moreover, we have shown here that 1-(PF6)2, similarly to several related cytotoxic gold(III) compounds, behaves as a classical prodrug. Indeed, upon reaction with SOD, taken here as a model protein, the dimetallic complex breaks down, gold(III) reduction occurs, and two gold(I) ions are eventually found associated to the protein while the phen2Me ligands are released. In view of the favorable biological results and, in particular, of its impressive tumor selectivity, this novel dinuclear gold(III) complex qualifies itself as an optimal candidate for further pharmacological testing.
Acknowledgments
We thank COST D39 action for fruitful discussions.
Supporting Information Available
Experimental procedures including information on the synthesis of Au(III) complexes, X-ray data collection and structure determination, full crystallographic data, UV−visible studies and ESI-MS experimental details, description of the cytotoxicity assay, and anticancer activity profile for compound 1-(PF6)2. This material is available free of charge via the Internet at http://pubs.acs.org.
This paper is dedicated to Professor Giovanni Minghetti on the occasion of his retirement.
A.C. gratefully acknowledges the Swiss National Science Foundation (AMBIZIONE Project no. PZ00P2-121933) and the Swiss Confederation (Action COST D39—Accord de recherche—SER Project No. C09.0027) for financial support. M.A.C. is grateful to the University of Sassari for financial support (FAR). L.M. and C.G. sincerely acknowledge financial support from Beneficentia Stiftung.
Supplementary Material
References
- Rosenberg B.; Vancamp L.; Krigas T. Nature 1965, 205, 698. [DOI] [PubMed] [Google Scholar]
- Wang D.; Lippard S. J. Nature Rev. Drug Discovery 2005, 4, 307–320. [DOI] [PubMed] [Google Scholar]
- Cohen S. M.; Lippard S. J. Cisplatin: From DNA damage to cancer chemotherapy. Prog. Nucleic Acid Res. Mol. Biol. 2001, 67, 93–130. [DOI] [PubMed] [Google Scholar]
- Barnes K. R.; Lippard S. J.. Cisplatin and related anticancer drugs: recent advances and insights. In Metal Ions in Biological Systems; Sigel, A. and Sigel, H., Eds; Marcel Dekker: New York, 2004; Vol. 42, pp 143−177. [PubMed] [Google Scholar]
- Hartinger C. G.; Dyson P. J. Chem. Soc. Rev. 2009, 38, 391. [DOI] [PubMed] [Google Scholar]
- Nobili S.; Mini E.; Landini I.; Gabbiani C.; Casini A.; Messori L. Med. Res. Rev. 2010, 30, 550–580. [DOI] [PubMed] [Google Scholar]
- Reedijk J. Eur. J. Inorg. Chem. 2009, 1303. [Google Scholar]
- Casini A.; Hartinger C.; Gabbiani C.; Mini E.; Dyson P. J.; Keppler B. K.; Messori L. J. Inorg. Biochem. 2008, 102, 564. [DOI] [PubMed] [Google Scholar]
- Sun R. W.-Y.; K.-Lei C.; Ma D.-L.; Yan J. J.; Lok C.-N.; Leung C.-H.; Zhu N.; Che C.-M. Chem. Eur. J. 2010, 16, 3097–3113. [DOI] [PubMed] [Google Scholar]
- Sun R. W. Y.; Che C. M. Coord. Chem. Rev. 2009, 253, 1682–1691. [Google Scholar]
- Fregona D.; Ronconi L.; Aldinucci D. Drug Discovery Today 2009, 14, 1075. [Google Scholar]
- Berners-Price S. J.; Filipovska A. Aust. J. Chem. 2008, 61, 661–668. [Google Scholar]
- Casini A.; Kelter G.; Gabbiani C.; Cinellu M. A.; Minghetti G.; Fregona D.; Fiebig H. H.; Messori L. J. Biol. Inorg. Chem. 2009, 14, 1139. [DOI] [PubMed] [Google Scholar]
- Messori L.; Abbate F.; Marcon G.; Orioli P.; Fontani M.; Mini E.; Mazzei T.; Carotti S.; O'Connell T.; Zanello P. J. Med. Chem. 2000, 43, 3541. [DOI] [PubMed] [Google Scholar]
- Abbate F.; Orioli P.; Bruni B.; Marcon G.; Messori L. Inorg. Chim. Acta 2000, 311. [Google Scholar]
- Casini A.; Cinellu M. A.; Minghetti G.; Gabbiani C.; Coronnello M.; Mini E.; Messori L. J. Med. Chem. 2006, 49, 5524. [DOI] [PubMed] [Google Scholar]
- Farrell N. Met. Ions Biol. Syst. 2004, 42, 251–296. [PubMed] [Google Scholar]
- Mendoza-Ferri M. G.; Hartinger C. G.; Mendoza M. A.; Groessl M.; Egger A. E.; Eichinger R. E.; Mangrum J. B.; Farrell N. P.; Maruszak M.; Bednarski P. J.; Klein F.; Jakupec M. A.; Nazarov A. A.; Severin K.; Keppler B. K. J. Med. Chem. 2009, 52, 916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor C. J.; Sinn E. Inorg. Chem. 1978, 17, 2067–2071. [Google Scholar]
- The Supporting Information contains the full crystallographic table and the crystallographic experimental part for this structure. The CCDC file 770565 contains other crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc-cam.ac.uk/data_requested/cif.
- www.oncotest.de.
- Huang R. L.; Wallqvist A.; Covell D. G. Biochem. Pharmacol. 2005, 69, 1009. [DOI] [PubMed] [Google Scholar]
- Paull K. D.; Shoemaker R. H.; Hodes L.; Monks A.; Scudiero D. A.; Rubinstein L.; Plowman J.; Boyd M. R. J. Natl. Cancer Inst. 1989, 81, 1088. [DOI] [PubMed] [Google Scholar]
- Fang L.; Shao L.; Zhang H.; Wang S. M. J. Chem. Inf. Comput. Sci. 2004, 44, 249. [DOI] [PubMed] [Google Scholar]
- Horn D. Drug Targets Kinetoplastid Parasites 2008, 625, 81. [PubMed] [Google Scholar]
- Sharma P. S.; Sharma R.; Tyagi R. Curr. Cancer Drug Targets 2008, 8, 53. [DOI] [PubMed] [Google Scholar]
- Casini A.; Gabbiani C.; Michelucci E.; Pieraccini G.; Moneti G.; Dyson P. J.; Messori L. J. Biol. Inorg. Chem. 2009, 14, 761. [DOI] [PubMed] [Google Scholar]
- Mendoza-Ferri M. G.; Hartinger C. G.; Mendoza M. A.; Groessl M.; Egger A. E.; Eichinger R. E.; Mangrum J. B.; Farrell N. P.; Maruszak M.; Bednarski P. J.; Klein F.; Jakupec M. A.; Nazarov A. A.; Severin K.; Keppler B. K. J. Med. Chem. 2009, 52, 916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.