Abstract
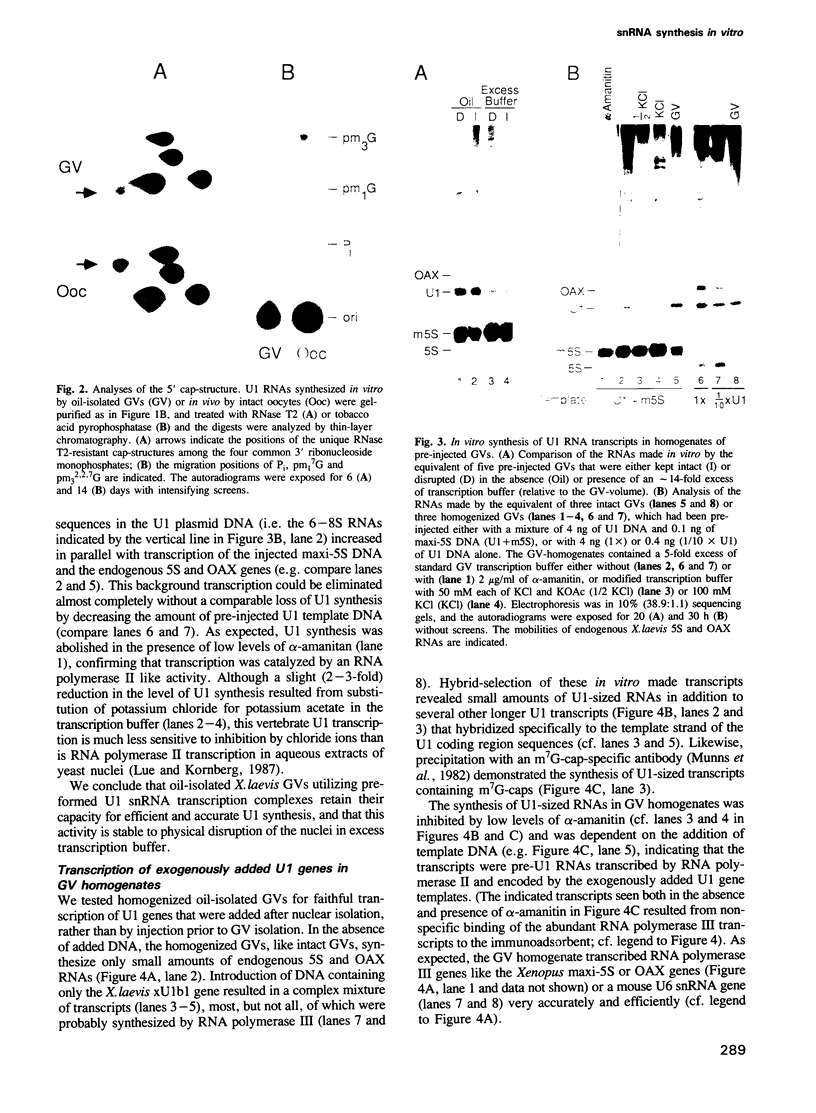
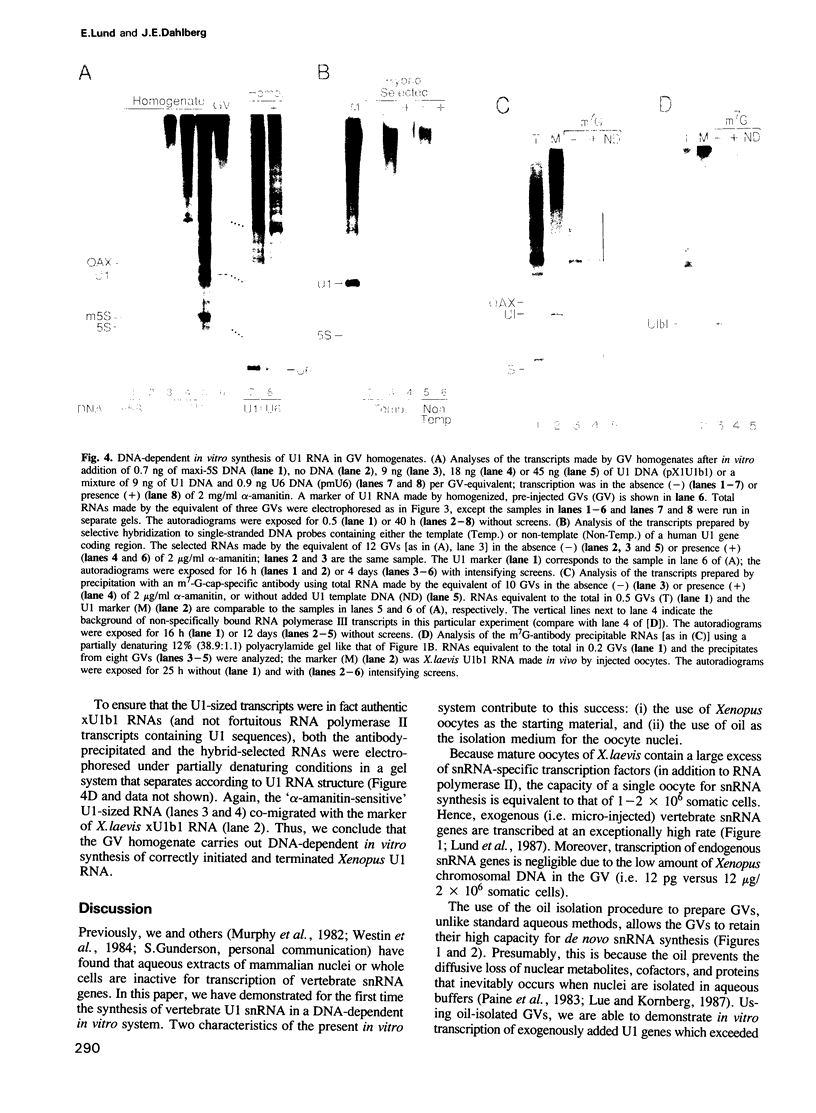
We have developed a DNA-dependent in vitro transcription system for vertebrate snRNA genes. By isolating the nuclei (germinal vesicles, GVs) of Xenopus laevis oocytes under oil to maintain the in vivo composition of their internal milieu, we are able to prepare nuclei that retain their ability to synthesize snRNAs efficiently. Homogenates of these GVs synthesize correctly initiated and terminated U1 snRNA using exogenous X.laevis U1 genes as templates. The templates may be either injected into the nucleus prior to its isolation or added to the nuclear homogenate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ares M., Jr, Mangin M., Weiner A. M. Orientation-dependent transcriptional activator upstream of a human U2 snRNA gene. Mol Cell Biol. 1985 Jul;5(7):1560–1570. doi: 10.1128/mcb.5.7.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier E. H., Brown D. D., Jordan E. A nuclear extract of Xenopus laevis oocytes that accurately transcribes 5S RNA genes. Cell. 1978 Nov;15(3):1077–1086. doi: 10.1016/0092-8674(78)90291-x. [DOI] [PubMed] [Google Scholar]
- Carbon P., Murgo S., Ebel J. P., Krol A., Tebb G., Mattaj L. W. A common octamer motif binding protein is involved in the transcription of U6 snRNA by RNA polymerase III and U2 snRNA by RNA polymerase II. Cell. 1987 Oct 9;51(1):71–79. doi: 10.1016/0092-8674(87)90011-0. [DOI] [PubMed] [Google Scholar]
- Ciliberto G., Palla F., Tebb G., Mattaj I. W., Philipson L. Properties of a U1 RNA enhancer-like sequence. Nucleic Acids Res. 1987 Mar 25;15(6):2403–2416. doi: 10.1093/nar/15.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark T. G., Merriam R. W. Diffusible and bound actin nuclei of Xenopus laevis oocytes. Cell. 1977 Dec;12(4):883–891. doi: 10.1016/0092-8674(77)90152-0. [DOI] [PubMed] [Google Scholar]
- Das G., Henning D., Reddy R. Structure, organization, and transcription of Drosophila U6 small nuclear RNA genes. J Biol Chem. 1987 Jan 25;262(3):1187–1193. [PubMed] [Google Scholar]
- Das G., Henning D., Wright D., Reddy R. Upstream regulatory elements are necessary and sufficient for transcription of a U6 RNA gene by RNA polymerase III. EMBO J. 1988 Feb;7(2):503–512. doi: 10.1002/j.1460-2075.1988.tb02838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri G. L. Formation of low molecular weight RNA species in HeLa cells. J Cell Physiol. 1980 Feb;102(2):199–207. doi: 10.1002/jcp.1041020211. [DOI] [PubMed] [Google Scholar]
- Eliceiri G. L., Sayavedra M. S. Small RNAs in the nucleus and cytoplasm of HeLa cells. Biochem Biophys Res Commun. 1976 Sep 20;72(2):507–512. doi: 10.1016/s0006-291x(76)80070-8. [DOI] [PubMed] [Google Scholar]
- Feldherr C. M., Richmond P. A. Manual enucleation of Xenopus oocytes. Methods Cell Biol. 1978;17:75–79. doi: 10.1016/s0091-679x(08)61135-8. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Brown D. D. The transcription of 5 S DNA injected into Xenopus oocytes. Dev Biol. 1978 Dec;67(2):346–356. doi: 10.1016/0012-1606(78)90205-1. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Melton D. A. Gene transfer in amphibian eggs and oocytes. Annu Rev Genet. 1981;15:189–218. doi: 10.1146/annurev.ge.15.120181.001201. [DOI] [PubMed] [Google Scholar]
- Hoffman M. L., Korf G. M., McNamara K. J., Stumph W. E. Structural and functional analysis of chicken U4 small nuclear RNA genes. Mol Cell Biol. 1986 Nov;6(11):3910–3919. doi: 10.1128/mcb.6.11.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol A., Carbon P., Ebel J. P., Appel B. Xenopus tropicalis U6 snRNA genes transcribed by Pol III contain the upstream promoter elements used by Pol II dependent U snRNA genes. Nucleic Acids Res. 1987 Mar 25;15(6):2463–2478. doi: 10.1093/nar/15.6.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol A., Lund E., Dahlberg J. E. The two embryonic U1 RNA genes of Xenopus laevis have both common and gene-specific transcription signals. EMBO J. 1985 Jun;4(6):1529–1535. doi: 10.1002/j.1460-2075.1985.tb03813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel G. R., Pederson T. Transcription boundaries of U1 small nuclear RNA. Mol Cell Biol. 1985 Sep;5(9):2332–2340. doi: 10.1128/mcb.5.9.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel G. R., Pederson T. Upstream elements required for efficient transcription of a human U6 RNA gene resemble those of U1 and U2 genes even though a different polymerase is used. Genes Dev. 1988 Feb;2(2):196–204. doi: 10.1101/gad.2.2.196. [DOI] [PubMed] [Google Scholar]
- Lobo S. M., Marzluff W. F. Synthesis of U1 RNA in isolated mouse cell nuclei: initiation and 3'-end formation. Mol Cell Biol. 1987 Dec;7(12):4290–4296. doi: 10.1128/mcb.7.12.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue N. F., Kornberg R. D. Accurate initiation at RNA polymerase II promoters in extracts from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8839–8843. doi: 10.1073/pnas.84.24.8839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Bostock C. J., Dahlberg J. E. The transcription of Xenopus laevis embryonic U1 snRNA genes changes when oocytes mature into eggs. Genes Dev. 1987 Mar;1(1):47–56. doi: 10.1101/gad.1.1.47. [DOI] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E., Forbes D. J. The two embryonic U1 small nuclear RNAs of Xenopus laevis are encoded by a major family of tandemly repeated genes. Mol Cell Biol. 1984 Dec;4(12):2580–2586. doi: 10.1128/mcb.4.12.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore S. J., Wieben E. D., Pederson T. Intracellular site of U1 small nuclear RNA processing and ribonucleoprotein assembly. J Cell Biol. 1984 Jan;98(1):188–192. doi: 10.1083/jcb.98.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Sharp P. A., Gefter M. L. RNA synthesis in isolated nuclei: in vitro initiation of adenovirus 2 major late mRNA precursor. Proc Natl Acad Sci U S A. 1979 Jan;76(1):160–164. doi: 10.1073/pnas.76.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I. W. Cap trimethylation of U snRNA is cytoplasmic and dependent on U snRNP protein binding. Cell. 1986 Sep 12;46(6):905–911. doi: 10.1016/0092-8674(86)90072-3. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W., Lienhard S., Jiricny J., De Robertis E. M. An enhancer-like sequence within the Xenopus U2 gene promoter facilitates the formation of stable transcription complexes. Nature. 1985 Jul 11;316(6024):163–167. doi: 10.1038/316163a0. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W., Zeller R. Xenopus laevis U2 snRNA genes: tandemly repeated transcription units sharing 5' and 3' flanking homology with other RNA polymerase II transcribed genes. EMBO J. 1983;2(11):1883–1891. doi: 10.1002/j.1460-2075.1983.tb01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. F., Price D. H., Marzluff W. F. Synthesis of U1 RNA in a DNA-dependent system from sea urchin embryos. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3674–3678. doi: 10.1073/pnas.83.11.3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns T. W., Liszewski M. K., Tellam J. T., Sims H. F., Rhoads R. E. Antibody-nucleic acid complexes. Immunospecific retention of globin messenger ribonucleic acid with antibodies specific for 7-methylguanosine. Biochemistry. 1982 Jun 8;21(12):2922–2928. doi: 10.1021/bi00541a018. [DOI] [PubMed] [Google Scholar]
- Murphy J. T., Burgess R. R., Dahlberg J. E., Lund E. Transcription of a gene for human U1 small nuclear RNA. Cell. 1982 May;29(1):265–274. doi: 10.1016/0092-8674(82)90111-8. [DOI] [PubMed] [Google Scholar]
- Murphy J. T., Skuzeski J. T., Lund E., Steinberg T. H., Burgess R. R., Dahlberg J. E. Functional elements of the human U1 RNA promoter. Identification of five separate regions required for efficient transcription and template competition. J Biol Chem. 1987 Feb 5;262(4):1795–1803. [PubMed] [Google Scholar]
- Ohshima Y., Okada N., Tani T., Itoh Y., Itoh M. Nucleotide sequences of mouse genomic loci including a gene or pseudogene for U6 (4.8S) nuclear RNA. Nucleic Acids Res. 1981 Oct 10;9(19):5145–5158. doi: 10.1093/nar/9.19.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine P. L., Austerberry C. F., Desjarlais L. J., Horowitz S. B. Protein loss during nuclear isolation. J Cell Biol. 1983 Oct;97(4):1240–1242. doi: 10.1083/jcb.97.4.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Millstein L., Eversole-Cire P., Gottesfeld J. M., Varshavsky A. Transcriptionally inactive oocyte-type 5S RNA genes of Xenopus laevis are complexed with TFIIIA in vitro. Mol Cell Biol. 1987 Oct;7(10):3503–3510. doi: 10.1128/mcb.7.10.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder R. G. Multiple forms of deoxyribonucleic acid-dependent ribonucleic acid polymerase in Xenopus laevis. Levels of activity during oocyte and embryonic development. J Biol Chem. 1974 Jan 10;249(1):249–256. [PubMed] [Google Scholar]
- Saba J. A., Busch H., Wright D., Reddy R. Isolation and characterization of two putative full-length Drosophila U4 small nuclear RNA genes. J Biol Chem. 1986 Jul 5;261(19):8750–8753. [PubMed] [Google Scholar]
- Skuzeski J. M., Lund E., Murphy J. T., Steinberg T. H., Burgess R. R., Dahlberg J. E. Synthesis of human U1 RNA. II. Identification of two regions of the promoter essential for transcription initiation at position +1. J Biol Chem. 1984 Jul 10;259(13):8345–8352. [PubMed] [Google Scholar]
- Strub K., Birnstiel M. L. Genetic complementation in the Xenopus oocyte: co-expression of sea urchin histone and U7 RNAs restores 3' processing of H3 pre-mRNA in the oocyte. EMBO J. 1986 Jul;5(7):1675–1682. doi: 10.1002/j.1460-2075.1986.tb04411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin G., Lund E., Murphy J. T., Pettersson U., Dahlberg J. E. Human U2 and U1 RNA genes use similar transcription signals. EMBO J. 1984 Dec 20;3(13):3295–3301. doi: 10.1002/j.1460-2075.1984.tb02293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormington W. M., Bogenhagen D. F., Jordan E., Brown D. D. A quantitative assay for Xenopus 5S RNA gene transcription in vitro. Cell. 1981 Jun;24(3):809–817. doi: 10.1016/0092-8674(81)90106-9. [DOI] [PubMed] [Google Scholar]
- Yuo C. Y., Ares M., Jr, Weiner A. M. Sequences required for 3' end formation of human U2 small nuclear RNA. Cell. 1985 Aug;42(1):193–202. doi: 10.1016/s0092-8674(85)80115-x. [DOI] [PubMed] [Google Scholar]
- de Vegvar H. E., Lund E., Dahlberg J. E. 3' end formation of U1 snRNA precursors is coupled to transcription from snRNA promoters. Cell. 1986 Oct 24;47(2):259–266. doi: 10.1016/0092-8674(86)90448-4. [DOI] [PubMed] [Google Scholar]