Abstract
Chlamydia trachomatis urogenital serovars D–K are intracellular bacterial pathogens that replicate almost exclusively in human reproductive tract epithelium. In the C. muridarum mouse model for human Chlamydia genital tract infections CD4 T helper type 1 cell responses mediate protective immunity while CD8 T-cell responses have been associated with scarring and infertility. Scarring mediated by CD8 T cells requires production of tumour necrosis factor-α (TNF-α); however, TNF-α is associated with protective immunity mediated by CD4 T cells. The latter result suggests that TNF-α in-and-of itself may not be the sole determining factor in immunopathology. CD8 T cells mediating immunopathology presumably do something in addition to producing TNF-α that is detrimental during resolution of genital tract infections. To investigate the mechanism underlying CD8 immunopathology we attempted to isolate Chlamydia-specific CD8 T-cell clones from mice that self-cleared genital tract infections. They could not be derived with antigen-pulsed irradiated naive splenocytes; instead derivation required use of irradiated immune splenocyte antigen-presenting cells. The Chlamydia-specific CD8 T-cell clones had relatively low cell surface CD8 levels and the majority were not restricted by MHC class Ia molecules. They did not express Plac8, and had varying abilities to terminate Chlamydia replication in epithelial cells. Two of the five CD8 clones produced interleukin-13 (IL-13) in addition to IL-2, TNF-α, IL-10 and interferon-γ. IL-13-producing Chlamydia-specific CD8 T cells may contribute to immunopathology during C. muridarum genital tract infections based on known roles of TNF-α and IL-13 in scar formation.
Keywords: CD8, Chlamydia, interleukin-13
Introduction
Chlamydia trachomatis genital tract infections are a major public health problem throughout the world. In the western hemisphere the incidence of C. trachomatis infection in individuals of reproductive age is roughly 2–15% depending on population surveyed.1–3 The majority of infected individuals are asymptomatic and do not seek medical care. Aggressive public health test-and-treat programmes have reduced the incidence of infection-associated morbidities such as pelvic inflammatory disease, but not the incidence of infection.4 For that reason there is widespread interest in development of a C. trachomatis vaccine. However, early vaccination attempts with whole-inactivated C. trachomatis trachoma vaccines showed poor protection and exacerbated immunopathology upon infection or infectious challenge.5 The possibility of vaccine-enhanced immunopathology remains a major hurdle for Chlamydia vaccine development.
In women, C. trachomatis genital tract infections frequently ascend to damage the Fallopian tubes either acutely, presenting as pelvic inflammatory disease, or insidiously, presenting as infertility. Both scenarios carry risk for infection-associated scarring that can cause infertility and ectopic pregnancies. After-the-fact studies in women have shown infertility to be associated with peripheral blood mononuclear cell responses to Chlamydia antigens that are relatively deficient in interferon-γ (IFN-γ),6 or overly exuberant in production of interleukin-10 (IL-10)7,8 and tumour necrosis factor-α (TNF-α).8
The Chlamydia muridarum mouse model for genital tract infections reproduces all the major features of human infections including an ascending infection that reaches the oviducts to cause scarring, hydrosalpinx and infertility. Using this model others have elegantly shown that protective immunity is mediated by CD4 T cells with little apparent contribution from CD8 T cells.9,10 Conversely, immunopathology appears to be mediated by CD8 T cells. Adoptive transfer of T cells taken from mice that cleared previous infection leads to enhanced rates of infertility in recipient mice after infectious challenge, and mice with a CD8-dominant immune response (CD4 knockout mice) were more susceptible to infection-induced infertility than those with a CD4-dominant response (CD8 knockout mice).11 Murthy et al. have shown TNF-α, but not perforin, to be critical for CD8-mediated immunopathology.12,13 That result suggests that TNF-α causes detrimental tissue damage/remodelling during the course of genital tract infections. Conversely, nasal infection-induced14 and subunit vaccine-induced15,16 protective immunity are associated with a multifunctional CD4 T-cell phenotype that includes production of IFN-γ and TNF-α. Because vaccinated mice are protected from immunopathology, these data suggest that TNF-α in-and-of itself may not be sufficient for infection-associated scarring.
To date, no CD8 T-cell lines or clones have been derived from mice that self-cleared Chlamydia genital tract infections. Two Chlamydia-specific CD8 T-cell clones were derived from mice infected intravenously with C. muridarum; both were reported to be MHC-restricted.17 More extensive studies in humans with C. trachomatis genital tract infections have shown that the majority of Chlamydia-specific CD8 T-cell clones were not HLA-restricted.18,19 To investigate mechanisms underlying CD8 T-cell-associated immunopathology we attempted to derive CD8 T-cell clones from immune mice that had cleared a C. muridarum genital tract infection. Usual derivation protocols dependent on irradiated naive splenocytes pulsed with UV-inactivated C. muridarum were unsuccessful. We were able to derive Chlamydia-specific CD8 T-cell clones using antigen-pulsed irradiated immune splenocytes as feeder layers. We present the interesting results of studies with those CD8 T-cell clones here, including lack of MHC class Ia restriction and cytokine polarization patterns that include IL-13.
Materials and methods
Mice
Female C57BL/6 mice, 4–5 weeks old, were purchased from Harlan Labs (Indianapolis, IN). KbDb double knockout female mice (which lack MHC class Ia molecules) were purchased from Taconic (Hudson, NY). All mice were housed in a pathogen-free barrier animal facility in accordance with procedures and protocols approved by the Indiana University School of Medicine Animal Care & Use Committee (animal welfare assurance #A4091-01) and performed in accordance with the United States Animal Welfare Act and the National Research Council's Guide for the Care and Use of Laboratory Animals.
Epithelial cells and bacteria
C57epi.1 epithelial cells and McCoy fibroblasts were cultured as previously described.20 Mycoplasma-free C. muridarum (Nigg), previously known as C. trachomatis strain mouse pneumonitis (MoPn) (Nigg) was grown in McCoy cells as previously described.21 Elementary body (EB)-depleted Chlamydia antigen was prepared by infecting 175-cm2 flasks of McCoy cells with C. muridarum at 3 inclusion-forming units (IFU) per cell. Thirty-two hours after infection the monolayers were removed using sterile glass beads, sonicated for 60 seconds, spun at low speed (464 g for 10 min) to remove debris, then centrifuged at 19 000 g for 30 min to pellet EB; this gave ∼ 99·998% depletion. EB-depleted supernatants were collected, concentrated (4000 g for 30 min) in ultra-filtration centrifuge units with 30 000 molecular weight cut-off (Amicon Ultra-15; Millipore, Billerica MA), split into aliquots and stored at −80°.
Genital tract infections
One week before infection, mice were treated with 2·5 μg of medroxyprogesterone delivered subcutaneously (Depo-Provera, Pfizer Pharmaceuticals, New York, NY). Lightly anaesthetized mice were infected vaginally with 5 × 104 IFU of C. muridarum biovar Nigg in 10 μl of sucrose phosphate glutamate buffer. Mice were swabbed 7 days after infection and IFU were quantified to document infection. Mice > 6 weeks post infection were considered immune mice.
Chlamydia-specific CD8 T cells
T-cell expansion cultures were performed in RPMI-1640–25 mm HEPES supplemented with 10% characterized fetal bovine serum (HyClone, Logan, UT), 2 mm l-alanyl-l-glutamine (Glutamax I; Gibco/Invitrogen, Carlsbad, CA), 25 μg/ml gentamicin (Sigma, St Louis, MO), and 5 × 10−5 m 2-mercaptoethanol (Sigma); referred to as RPMI CM. Immune splenocytes harvested from mice were plated at 12·5 × 106 cells per well in tissue culture treated 12-well plates, in RPMI CM containing murine recombinant IL-1α (2 ng/ml), IL-6 (2 ng/ml), IL-7 (3 ng/ml), IL-15 (4 ng/ml), human recombinant IL-2 (100 units/ml) (Chiron Corp. Emeryville, CA), 20% 2° mixed lymphocyte culture supernatant,22 and 20 μg of UV-inactivated C. muridarum (∼ 2·5 IFU equivalents per splenocyte) as previously described20 or with 20 µl per well EB-depleted C. muridarum antigen. Subsequent passages in 24-well plates used 2·5 × 105 T cells and 5 × 106 γ-irradiated (1200 rads) immune splenocyte antigen-presenting cells (APC) and the same conditions as in the primary culture. CD4 T cells were depleted from polyclonal populations by magnetic bead separation per the manufacturer's protocol (Miltenyi Biotec, Auburn, CA). The resulting polyclonal CD8 T-cell populations were cloned by limiting dilution and passed weekly as above. Recombinant mouse cytokines were purchased from R&D Systems (Minneapolis, MN).
Flow cytometry
T-cell clones were stained for 20 min on ice in RPMI CM with: unconjugated 145-2c11 (CD3), FITC-coupled phycoerythrin (PE)-coupled 53-5.8 (CD8β), PE-coupled 53-6.7 (CD8α) (BD Biosciences, San Jose CA), PE-coupled YTS191.1 (CD4) (Cedarlane Laboratories, Burlington, NC), FITC-coupled mouse IgG2a (control antibody), PE-coupled rat IgG2b (control antibody) (Ebioscience, San Diego, CA), FITC-coupled goat anti-Armenian hamster immunoglobulin (Jackson Immunoresearch Laboratories, West Grove, PA). Cells were fixed with 1% paraformaldehyde after staining and analysed on a FACSCalibur cytometer (BD Biosciences).
T-cell proliferation assays
For proliferation assay, 2·5 × 104 T cells were added to 2·5 × 105 γ-irradiated immune splenocytes (2000 rads) without antigen (only sucrose phosphate glutamate buffer) and with antigen (UV-C. muridarum or soluble antigen) in 96-well U-bottom plates; wells pulsed with 0·5 μCi/well [3H]thymidine (ICN, Costa Mesa, CA) for 12 hr at 36–48 hr of the culture cycle. [3H]Thymidine incorporation was measured with a TopCount β-counter.
T-cell cytokine ELISAs
For the specificity experiment in Fig. 2 the conditions were the same as for the proliferation assay detailed above. For the MHC mapping experiments (Fig. 3) the conditions were the same except immune and naive splenocytes were more lightly irradiated with 1000 rads (better antigen presentation capabilities;23) and all wells contained 1 ng/ml recombinant IL-7 to support T-cell viability over the 72-hr experiment. For cytokine polarization determination T cells were activated by immobilized anti-CD3 antibody (145-2C11; NA/LE BD Pharmingen, San Jose, CA). Flat-bottom 96-well tissue culture plates were prepared by incubating 50 μl of 0·5 μg/ml 145-2C11 in PBS overnight at 4°. Wells were washed once with medium before use. Then, 5 × 104 T cells were added to each well and supernatants were collected at 24 hr. Relative levels of IL-2, IL-10, IL-13, IL-17, IFN-γ and TNF-α determined by ELISA using capture and biotinylated monoclonal antibody pairs with recombinant murine standards according to the manufacturer's protocols: IL-2 ELISA: 1A12 and 5H4, IFN-γ: XMG1 2 (ThermoScientific, Rockford, IL); TNF-α: TN3-19.12/C1150-14; IL-10: JES5-2A5/SXC-1 (BD Biosciences, San Jose, CA); IL-13: Ebio13a/Ebio1316H (Ebioscience). Recombinant murine IL-2, IL-10 (ThermoScientific), IFN-γ (R&D Systems), IL-13 (Ebioscience) and IL-17a (Biolegend, San Diego, CA) were used as standards. Detection was accomplished with streptavidin-horseradish peroxidase (BD Biosciences) and tetramethylbenzidine substrate (Sigma).
Figure 2.
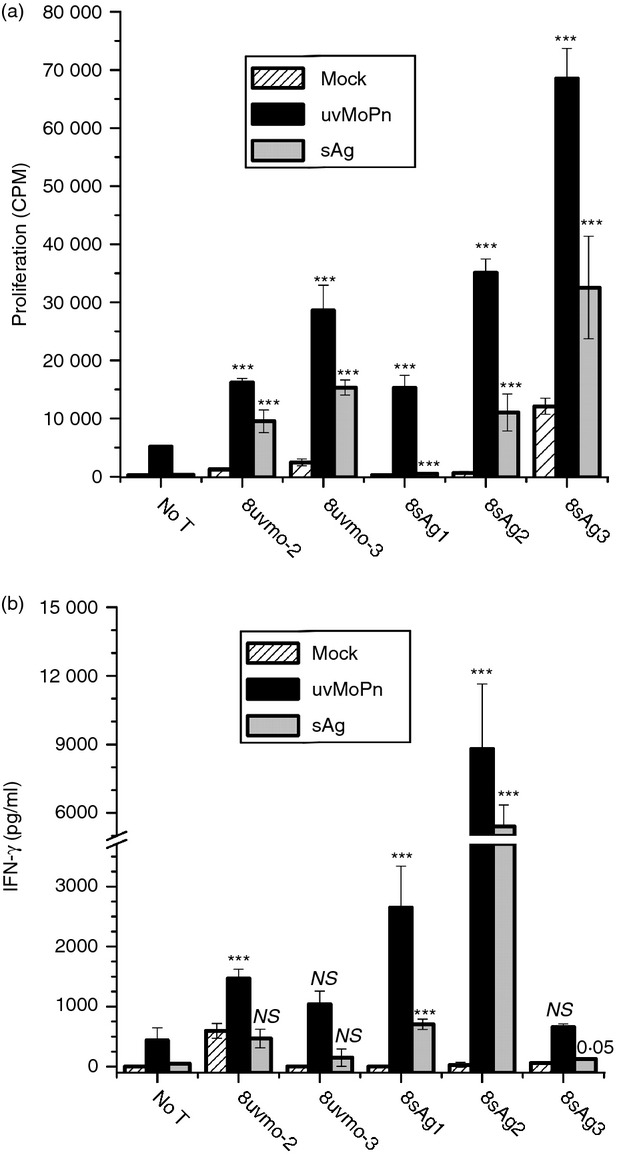
Specificity of CD8 T-cell clones. Each T-cell clone was activated with irradiated (2000 rads) immune splenocytes mock-pulsed, UV-inactivated Chlamydia muridarum (uvMoPn)-pulsed, and elementary bodies-depleted antigen (sAg)-pulsed. At 36 hr culture supernatants were harvested and [3H]thymidine was added; wells were harvested at 48 hr to score proliferation. (a) Proliferation in counts per minute (CPM). (b) Interferon-γ (IFN-γ) production as determined by ELISA. Data are means and SD for one experiment performed as quadruplicates. For each T-cell clone the experimental wells were compared with their mock-pulsed control and with the antigen-presenting-cells-only control (No T) for the relevant antigen. The higher P-value of those two comparisons (the least significant) was assigned and graphed. **P < 0·005; ***P < 0·0005.
Figure 3.
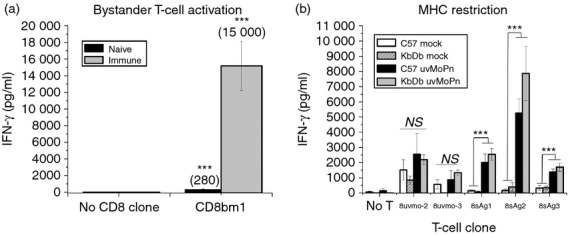
Mapping the restriction element for the CD8 T-cell clones. (a) Irradiated (1000 rads) immune and naive splenocytes from C57BL/6 mice were pulsed with UV-inactivated Chlamydia muridarum (uvMoPn) and co-cultured without T-cell clone (No CD8 clone) and with non-specific bystander CD8bm1 T cells in quadruplicate. Supernatants were collected at 72 hr and levels of interferon-γ (IFN-γ) determined by ELISA. CD8bm1 experimental wells were compared with the relevant ‘No CD8 clone’ control wells; mean IFN-γ levels shown in parentheses. (b) CD8 T-cell clones were mock-activated and uvMoPn-activated with irradiated (1000 rads) naive C57BL/6 and KbDb knockout (KO) splenocytes antigen-presenting cells. Supernatants were collected at 72 hr and levels of IFN-γ were determined by ELISA. Data presented are aggregate data from two independent experiments. IFN-γ released by 8sAg1, 8sAg2 and 8sAg3 in response to uvMoPn-pulsed irradiated splenocytes was at least fivefold higher than the bystander effect shown in (a). **P < 0·005; ***P < 0·0005
Chlamydia replication experiments
C57epi.1 cell monolayers in 48-well plates were untreated or treated with IFN-γ (10 µg/ml) for 14 hr pre-infection. Wells were infected with 3 IFU/cell. After addition of C. muridarum, the plates were spun at 1200 rpm (300 g) for 30 min. Mock-infected wells received an equivalent volume of sucrose glutamate phosphate buffer lacking C. muridarum. Four hours after infection, the inoculums were removed, and 1·5 × 105 CD4 T-cell clone cells were added per well. After 28 hr (32 hr post-infection) wells were scraped, harvested in sucrose glutamate phosphate buffer, and stored at − 80° until titres could be determined on McCoy cell monolayers.
RT-PCR
T-cell clones at the end of their culture cycle were purified by Ficoll-Hypaque and cultured for 3 days in RPMI CM supplemented with 3 µg/ml recombinant IL-7. Total RNA was isolated from each T-cell clone using a protocol that included an RNase-free DNase I treatment step (RNAeasy; Qiagen, Valencia, CA). Specific mRNA gene reverse transcription and amplification were performed using AMV reverse transcriptase/Tfl DNA polymerase in a one-step system (AccessQuick RT-PCR; Promega, Madison, WI). Amplification conditions were (i) 48° for 45 min; (ii) 95° for 2 min; (iii) 95° for 30 seconds; (iv) 56° for 20 seconds; (v) 72° for 30 seconds; (vi) go to step 3 for 37 times; (vii) 72° for 7 min; and (viii) hold at 4° using an MJ Research J200 PCR machine. Primer pairs used were β-actin (5′-CCTGACGGCCAGGTCATCACTATT-3′, 5′-ACTCCTGCTTGCTGATCCACAT3-′; 358 bp product); perforin (5′-TGGATGTGAACCCTAGGCCAGAG3-′, 5′-AAGTACTTCGACGTGACGCTCAC-3′; 515 bp product), and Plac8 (5′-ATGGCTCAGGCACCAACAGTTA-3′, 5′-GAAAGCGTTCATGGCTCTCCTC-3′; 336 bp product).
Statistical analysis
Summary figures for each experimental investigation are presented as ‘pooled’ means and with their associated standard error of the mean (SEM). Figure legends indicate the number of independent experiments pooled to generate each figure. Student's two-tailed t-tests and Wilcoxon non-parametric tests, depending on data distribution, were used to assess significance of experimental data. Homogeneity of variances was assessed using a folded F-test. All data were verified to meet analytical assumptions. Analyses were performed using SAS 9·3 (SAS Institute, Cary, NC). P-values < 0·05 were considered statistically significant.
Results
Derivation of Chlamydia-specific CD8 T cells required immune splenocyte APC
We previously found derivation of Chlamydia-specific CD4 T-cell clones from immune mice to be relatively straightforward.20 However, those protocols based on irradiated naive splenocytes pulsed with UV-inactivated C muridarum did not expand Chlamydia-specific CD8 T cells (unpublished observations and ref. 24). We therefore investigated culture conditions using alternative APC feeder layers and Chlamydia antigens.
Splenocytes from mice that had previously self-cleared genital tract infections were harvested and stimulated in vitro with UV-inactivated C. muridarum (usual protocol) or with EB-depleted (∼ 99·998%) C. muridarum antigen prepared by depleting infected cell lysates of EB by centrifugation (see Materials and methods). For the secondary stimulation and subsequent passages the T-cell populations were activated with irradiated immune splenocytes pulsed with UV-inactivated C. muridarum or EB-depleted antigen as per the primary culture. Under these conditions there was significant expansion of a CD8+ T-cell population (Fig. 1a,b). Polyclonal cultures activated with UV-inactivated C muridarum had a minority CD8 T-cell population of ∼ 3%, whereas cultures activated with EB-depleted antigen had a minority CD8 T-cell population of ∼ 10%. Those bulk T-cell populations were depleted of CD4 T cells using magnetic bead separation, then cloned by limiting dilution on irradiated immune splenocytes pulsed with the relevant antigen. Five CD8 T-cell clones were kept for further study. The clones were stained for CD8β chain and compared with a typical alloreactive CD8 T-cell clone CD8bm125 (Fig. 1c). The Chlamydia-specific CD8 T-cell clones had significantly lower levels of cell surface CD8 than the alloreactive CD8 T-cell clone CD8bm1; all clones were CD3+ CD8αβ T cells (data not shown). We tested their specificity by proliferation and IFN-γ production when activated by irradiated immune splenocytes mock-pulsed or pulsed with UV-inactivated C. muridarum or EB-depleted Chlamydia antigen (Fig. 2). All the clones recognized immune splenocytes pulsed with UV-inactivated C. muridarum or EB-depleted antigen to greater or lesser degrees. All clones recognized their original antigen by proliferation and IFN-γ production with one exception; 8uvmo-3 production of IFN-γ was not statistically greater than IFN-γ production by antigen-pulsed immune splenocytes. None of the clones recognized immune splenocytes pulsed with freeze–thawed McCoy cells, ruling out cross-presentation of McCoy alloantigens (see Supporting information, Fig. S1).
Figure 1.
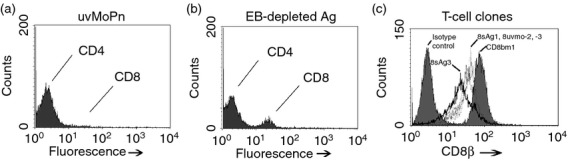
Derivation of Chlamydia-specific CD8 T-cell clones. CD4 and CD8 staining of polyclonal T-cell populations expanded from immune mice using irradiated immune splenocytes with (a) UV-inactivated Chlamydia muridarum (uvMoPn), (b) Elementary bodies-depleted Chlamydia antigen. (c) CD8β staining of CD8 T-cell clones 8uvmo-2, 8uvmo-3, 8sAg1 and 8sAg3 compared with alloreactive CD8 T-cell clone CD8bm1.
Three Chlamydia-specific CD8 T-cell clones are not restricted by MHC class Ia molecules
In two previous independent human studies the majority of Chlamydia-specific CD8 T cells isolated from individuals with C. trachomatis genital tract infections were not restricted by HLA class Ia molecules.18,19 For that reason we were interested in determining the restriction element for the five murine CD8 T-cell clones. Irradiated immune splenocytes produce cytokines capable of supporting antigen-independent proliferation and IFN-γ production making them ill-suited for MHC restriction mapping. However, irradiated naive splenocytes pulsed with Chlamydia antigens do not produce measurable cytokines and did not support meaningful antigen-independent IFN-γ production by bystander T cells (Fig. 3a). We tested whether the CD8 T-cell clones were MHC class Ia restricted by comparing activation of mock-pulsed and UV-inactivated C. muridarum-pulsed irradiated naive splenocytes from C57BL/6 mice (wild-type H-2b) and KbDb dual knockout mice (H-2b; no MHC class Ia molecules) (Fig. 3b). KbDb knockout splenocytes lacking MHC class Ia molecules were as competent as wild-type splenocytes for activating three of the CD8 T-cell clones, 8sAg1, 8sAg2, 8sAg3; whereas 8uvmo-2 and 8uvmo-3 were not significantly activated by antigen-pulsed naive splenocytes. The majority of CD8 T-cell clones (three of five) were not restricted by MHC class Ia.
The CD8 clones have varying abilities to terminate Chlamydia replication in epithelial cells
We have previously shown that Chlamydia-specific CD4 T-cell clones had varying abilities to terminate Chlamydia replication in epithelial cells.20 We similarly tested the ability of the CD8 T-cell clones to terminate Chlamydia replication in epithelial cells. C57epi.1 cells, untreated or pre-treated with IFN-γ, were infected with C. muridarum at 3 IFU/cell. Four hours later the inocula were removed, monolayers were washed, and T-cell clones were added to each well at an effector to target ratio of ∼ 0·75 : 1. Wells were harvested at 32 hr after infection and recovered IFU were quantified on McCoy monolayers (Fig. 4a,b). 8uvmo-2 and 8uvmo-3 were potent terminators of C. muridarum replication, with or without IFN-γ pre-treatment of the epithelial monolayer. 8sAg1, 8sAg2, and 8sAg3 were entirely unable to control replication, and IFN-γ pre-treatment improved the efficiency of only 8sAg3, and that only modestly. Relative ability to terminate C. muridarum replication did not correlate cleanly with IFN-γ produced during interaction with infected epithelial cells (Fig. 4c). For the Chlamydia-specific CD4 T-cell clones previously studied, potent termination of Chlamydia replication and relative independence from IFN-γ pre-treatment of epithelial monolayers correlated with T-cell expression of Plac8. Total mRNA was isolated from the CD8 T-cell clones, and from CD4 T-cell clones uvmo-2 and spl4-10, serving as positive and negative controls, respectively. All the CD8 clones had mRNA levels of perforin comparable with that of the alloreactive T-cell clone CD8bm1; none of the CD8 T-cell clones had detectable mRNA for Plac8 (Fig. 5).
Figure 4.
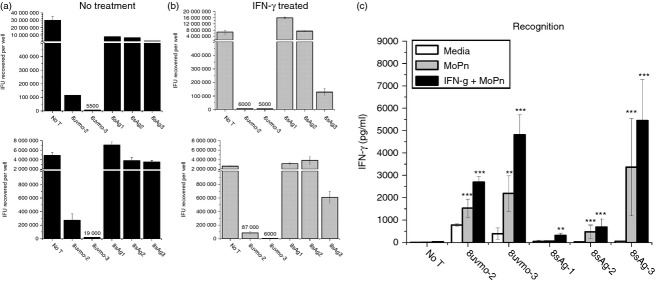
CD8 T clone recognition and termination of Chlamydia muridarum replication in epithelial cells. (a) C57ep.1 epithelial cells, untreated (a) or treated with IFN-γ (10 ng/ml for 10 hr) (b), were infected with three inclusion forming units (IFU) C. muridarum per cell. After 4 hr the inocula were removed, monolayers were washed then T cells were added. Wells were harvested 32 hr after infection; recovered IFU were quantified on McCoy monolayers. Top panels of (a) and (b) = experiment #1; bottom panels = experiment #2. For IFU values below 100 000, actual recovered IFU are shown immediately above the data bars. (c) Supernatants in experiments #1 and #2 were collected immediately before harvesting monolayers; interferon-γ (IFN-γ) levels were determined by ELISA. Aggregate data from experiments 1 and 2. **P < 0·005; ***P < 0·0005.
Figure 5.
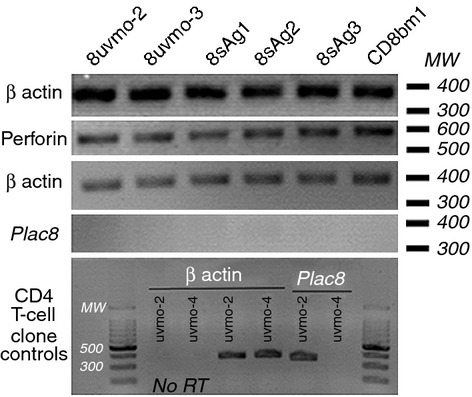
Chlamydia-specific CD8 T-cell clones express perforin but not Plac8. Total mRNA was isolated from each of the T-cell clones and tested for presence of β-actin, perforin and Plac8. In the bottom panel, previously published Chlamydia-specific CD4 T-cell clones uvmo-2 and uvmo-4 were included as positive and negative controls, respectively, for the Plac8 RT-PCR. No RT, no reverse transcriptase control. 2% agarose gel stained with ethidium bromide; image inverted for presentation.
The CD8 clones have multifunctional cytokine profiles including production of IL-13 by two clones
We investigated the cytokine profiles of the CD8 T-cell clones using wells coated with anti-CD3 antibody. Supernatants were collected at 24 hr and analysed for levels of IL-2, IL-10, IL-13, IL-17, IFN-γ and TNF-α by ELISA (Fig. 6a). All the clones produced significant amounts of IFN-γ and TNF-α, with varying amounts of IL-2; three clones produced large amounts of IL-10, and two significant amounts of IL-13; 8uvmo-2 made a trace amount of IL-17. These multifunctional cytokine patterns do not fall neatly into existing paradigms for CD8 T-cell cytokine polarization.
Figure 6.
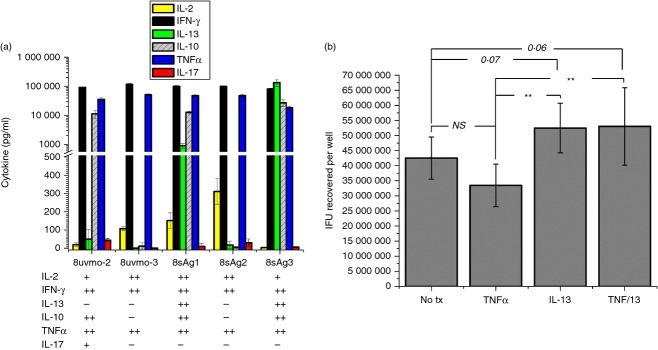
Cytokine profiles of the CD8 T-cell clones. (a) CD8 T-cell clones were activated with immobilized anti-CD3 antibody. Supernatants were collected at 24 hr and indicated cytokine levels were determined by ELISA. Aggregate data from two independent experiments. (b) Interleukin-12 (IL-13) facilitates Chlamydia muridarum replication in epithelial cells. C57epi.1 epithelial cells untreated, and treated with tumour necrosis factor-α (TNF-α (5 µg/ml), IL-13 (30 µg/ml) and TNF-α/IL-13 (5/30 ηg/ml) for 10 hr then infected with C. muridarum at 3 inclusion forming units (IFU) per cell. Monolayers were harvested 25 hr later; recovered IFU were quantified on McCoy monolayers. Aggregate data from six independent experiments; **P < 0·005.
Interleukin-13 has been shown to be detrimental to clearance of C. muridarum as determined by genome copy number in mouse lungs and genital tract.26 Because epithelial cells including C57epi.1 express IL-13 receptors (data not shown), we investigated whether IL-13 had a direct effect on Chlamydia replication. C57epi.1 epithelial monolayers untreated and treated with TNF-α, IL-13 and TNF-α plus IL-13, for 10 hr were infected with C. muridarum at 3 IFU/cell. After 25 hr the wells were harvested and recovered IFU were quantified on McCoy monolayers (Fig. 6b). Interleukin-13 modestly promoted C. muridarum replication in epithelial cells, and abrogated the negative effect of TNF-α on replication.
Discussion
The role of CD8 T-cell subsets in protection and immunopathology during Chlamydia genital tract infections is unclear. Experiments using adoptive transfer of Chlamydia-specific CD8 T-cell lines and clones into chronically infected nude mice demonstrated that CD8 T cells are capable of clearing genital tract infections.17,27 Subsequent research using knockout mice revealed that MHC class II (class II knockout) was critical while MHC class I (β2 microglobulin knockout) and CD4 (CD4 knockout) were non-essential for clearing the genital tract.10 A follow-up study using CD4 and CD8 depletions before secondary infectious challenge in B-cell-deficient mice confirmed the importance of CD4 T cells and identified a role for B cells in clearing C. muridarum from the genital tract, while failing to show any protective effect of the Chlamydia-specific CD8 T cells that were presumably generated during the primary infection.28 Those studies did not exclude the possibility that development of protective CD8 T-cell immunity is dependent on both CD4 T cells (MHC class II knockout mice) and antibody (T-cell depletions in B-cell-deficient mice). An important technical hurdle for derivation of CD8 T-cell clones in this study was a requirement for immune splenocyte presentation of Chlamydia antigens; the two CD8 clones that efficiently terminated C. muridarum replication in epithelial cells recognized immune splenocytes but not naive splenocytes pulsed with C. muridarum.
Although the mechanism underlying the immune splenocytes requirement remains to be determined, it probably involves one of two known antigen presentation mechanisms. The first is an antigen concentrating mechanism wherein specific antibody efficiently binds and delivers soluble antigen to the pH-dependent endosome/lysosome exogenous pathway for MHC class II presentation. Rock et al. showed that B cells specific for the hapten 2,4,6-trinitrophenyl (TNP) could activate an MHC class II-restricted T-cell hybridoma with 1000-fold less TNP-haptenated cognate antigen than could naive B cells.29 Similarly, IgG specific for tetanus toxoid (TT) improved the efficiency of monocyte-derived dendritic cell activation of TT-specific human CD4 T-cell clones by 100-fold.30 The second mechanism involves delivery of exogenous antigen into an otherwise inaccessible MHC class I presentation pathway. Murine dendritic cells could not cross-present physiological concentrations of soluble antigen to an MHC class I-restricted T-cell hybridoma, but could cross-present IgG-complexed antigen via Fc receptors to the same hybridoma; demonstrating a role for IgG and Fc receptors in directing soluble antigen into an otherwise inaccessible MHC class I presentation pathway.31 Fc receptors and Chlamydia specific antibodies have been shown to play a role in Chlamydia pathogenesis. Chlamydia-specific antibodies increase the ability of naive splenocyte APC to activate purified immune T cells to make IFN-γ, and mice deficient in Fc receptors lose their protection from secondary infection.32 Whether the defect in secondary immunity is due to less efficient antigen delivery into the exogenous MHC class II antigen presentation pathway, or loss of an MHC class I cross-presentation pathway, or both, or other, is not known.
The majority of Chlamydia specific CD8 T cells in this report were not restricted by MHC class Ia molecules. Because 8uvmo-2 and 8uvmo-3 were not activated by naive splenocytes pulsed with UV-inactivated C. muridarum we were unable to determine their MHC restriction elements. However, 8sAg1, 8sAg2 and 8sAg3 were sufficiently activated by antigen-pulsed naive C57BL/6 and KbDb knockout splenocytes to draw the conclusion that they are not restricted by MHC class Ia molecules. This finding in the mouse model is consistent with the finding that the majority of Chlamydia-specific CD8 T-cell clones isolated from humans with C. trachomatis infections were not restricted by HLA class Ia molecules.18,19
Relatively little is known about Chlamydia antigens recognized by CD8 T cells. Gervassi et al.33 showed that a human class Ia-restricted CD8 clone recognized OmcB, and there is evidence for a human CD8 major outer membrane protein epitope.34 In mice Cap1,CrpA and PmpI were identified as containing CD8 epitopes using mice infected intravenously or intraperitoneally with C. trachomatis serovar L2.35–37 A subset of murine CD8 T cells specific for C. pneumonia recognized formylated bacterial peptides in the context of H2-M3, an MHC class Ib molecule.38 Interestingly, a proteomic approach toward identifying T-cell epitopes identified only a single C. muridarum peptide from amino acid permease (TC_0653) associated with MHC class Ia molecules, while multiple peptide epitopes were extracted from MHC class II molecules.39 There is no information regarding C. trachomatis or C. muridarum antigens recognized by non-class Ia restricted CD8 T cells in humans or mice.
The CD8 T-cell clones in this study had widely varying abilities to terminate C. muridarum replication in epithelial cells. 8uvmo-2 and 8uvmo-3 were as potent as our most potent Chlamydia-specific CD4 T-cell clones in a previous study20, while 8sAg1, 8sAg2, and 8sAg3 were unable or inefficient at terminating replication, even when the epithelial monolayers were pre-treated with IFN-γ. The greater efficiency of 8uvmo-2 and 8uvmo-3 did not correlate cleanly with their relative production of IFN-γ. For Chlamydia-specific CD4 T-cell clones, potent termination of Chlamydia replication correlated with high Plac8 and low perforin mRNA levels.40 In this study all of the CD8 clones expressed perforin and none had detectable Plac8 mRNA. Efficient termination of Chlamydia replication by 8uvmo-2 and 8uvmo-3 is probably due either to the previously described iNOS-dependent mechanism41,42 or to cytolysis of infected epithelial cells in the non-infectious reticulate body stage of infection/replication (< 18 hr after infection for C. muridarum).
A surprising finding in this study was that two of the five CD8 T-cell clones produced significant amounts of IL-13 upon activation, along with IL-2, IFN-γ, IL-10 and TNF-α. Interleukin-13 has been shown to be detrimental to C. muridarum clearance from lung and genital tract as IL-13 knockout mice clear infections more quickly than wild-type mice.26 Interleukin-10 is similarly detrimental to C. muridarum clearance from lung, and is associated with increased residual scarring.43,44 CD8 T cells producing IL-10, IL-13 and TNF-α are interesting with respect to immunopathology because in addition to a role for IL-10 in scarring, the combination of TNF-α and IL-13 has been shown to induce expression of transforming growth factor-β1 in some cell types; this cytokine combination is the underlying mechanism for bleomycin-induced pulmonary fibrosis45 and trinitrobenzene sulphonic acid-induced colonic fibrosis46 in mouse models. Interleukin-13 expressing CD8 T cells are thought to mediate immunopathology in systemic sclerosis, a rheumatological disorder that manifests as progressive scarring of skin.47 It is reasonable to hypothesize that Chlamydia-specific CD8 T cells making IL-10, IL-13 and TNF-α contribute to the CD8-mediated immunopathology observed in experimental murine genital tract infections.
Previous studies in the C. muridarum mouse model have shown that CD8 T cells are capable of mediating bacterial clearance from the genital tract; however, on the whole, CD8 T cells play a greater role in immunopathology than in protection. This investigation using Chlamydia-specific CD8 T-cell clones revealed potential for protection and immunopathology. CD8 T cells that may mediate protection, represented by 8uvmo-2 and 8uvmo-3, terminated C. muridarum replication in epithelial cells and had a multifunctional cytokine profile that included IFN-γ and TNF-α. CD8 T cells that may contribute to immunopathology, represented by 8sAg1 and 8sAg3, were not restricted by MHC class Ia, were relatively ineffective at terminating C. muridarum replication in epithelial cells, and had a cytokine profile including IL-10, TNF-α and IL-13. Production of IL-13 did not cleanly identify poor terminators of Chlamydia replication in epithelial cells as IL-13neg 8sAg2 was also ineffective. In this study IL-13 modestly facilitated C. muridarum replication in epithelial cells, consistent with published data showing that IL-13 prolongs C. muridarum genital tract infections.26 More importantly, CD8/TNF-α immunopathology has been shown to be a cytokine-mediated event unrelated to bacterial clearance.12 Because IL-13 and TNF-α synergize to induce expression of the scarring-associated cytokine transforming growth factor-β in some cell types, IL-13 may play a role in CD8/TNF-α-mediated Chlamydia immunopathology. A better understanding of CD8 T cells producing IL-13 in the genital tract may provide useful insights into Chlamydia-associated immunopathology.
Acknowledgments
This work was supported by NIH grant R01AI070514 (RMJ). RMJ designed the experiments. RMJ and MSK performed them. JES did the statistical analysis. RMJ wrote the manuscript and all authors participated in its revision.
Glossary
- APC
antigen-presenting cells
- CM
complete medium
- EB
elementary bodies
- IFN-γ
interferon-γ
- IFU
inclusion forming units
- IL-10
interleukin-10
- TNF-α
tumour necrosis factor-α
Disclosures
RMJ and the Indiana University School of Medicine have a provisional patent related to work presented in this manuscript. MSK and JES have no conflicts of interest related to this manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
CD8 T-cell clones do not recognize McCoy cell alloantigens.
References
- 1.Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187–93. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Benitez C, Mejuto-Lopez P, Otero-Guerra L, Margolles-Martins MJ, Suarez-Leiva P, Vazquez F Chlamydial Primary Care Group. Prevalence of genital Chlamydia trachomatis infection among young men and women in Spain. BMC Infect Dis. 2013;13:388. doi: 10.1186/1471-2334-13-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Control. ECfDPa. Annual Epidemiological Report 2012. Reporting on 2010 surveillance data and 2011 epidemic intelligence data. Stockholm: ECDC, 2013.
- 4.Brunham RC, Pourbohloul B, Mak S, White R, Rekart ML. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J Infect Dis. 2005;192:1836–44. doi: 10.1086/497341. [DOI] [PubMed] [Google Scholar]
- 5.Schachter J. Overview of Chlamydia trachomatis infection and the requirements for a vaccine. Rev Infect Dis. 1985;7:713–6. doi: 10.1093/clinids/7.6.713. [DOI] [PubMed] [Google Scholar]
- 6.Debattista J, Timms P, Allan J. Reduced levels of γ-interferon secretion in response to chlamydial 60 kDa heat shock protein amongst women with pelvic inflammatory disease and a history of repeated Chlamydia trachomatis infections. Immunol Lett. 2002;81:205–10. doi: 10.1016/s0165-2478(02)00036-6. [DOI] [PubMed] [Google Scholar]
- 7.Kinnunen A, Surcel HM, Halttunen M, et al. Chlamydia trachomatis heat shock protein-60 induced interferon-γ and interleukin-10 production in infertile women. Clin Exp Immunol. 2003;131:299–303. doi: 10.1046/j.1365-2249.2003.02048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava P, Jha R, Bas S, Salhan S, Mittal A. In infertile women, cells from Chlamydia trachomatis infected sites release higher levels of interferon-γ, interleukin-10 and tumor necrosis factor-α upon heat-shock-protein stimulation than fertile women. Reprod Biol Endocrinol. 2008;6:20. doi: 10.1186/1477-7827-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70:2741–51. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–8. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igietseme JU, He Q, Joseph K, et al. Role of T lymphocytes in the pathogenesis of Chlamydia disease. J Infect Dis. 2009;200:926–34. doi: 10.1086/605411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murthy AK, Li W, Chaganty BK, et al. Tumor necrosis factor α production from CD8+ T cells mediates oviduct pathological sequelae following primary genital Chlamydia muridarum infection. Infect Immun. 2011;79:2928–35. doi: 10.1128/IAI.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manam S, Nicholson BJ, Murthy AK. OT-1 mice display minimal upper genital tract pathology following primary intravaginal Chlamydia muridarum infection. Pathog Dis. 2013;67:221–4. doi: 10.1111/2049-632X.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu H, Karunakaran KP, Kelly I, Shen C, Jiang X, Foster LJ, Brunham RC. Immunization with live and dead Chlamydia muridarum induces different levels of protective immunity in a murine genital tract model: correlation with MHC class II peptide presentation and multifunctional Th1 cells. J Immunol. 2011;186:3615–21. doi: 10.4049/jimmunol.1002952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu H, Jiang X, Shen C, Karunakaran KP, Jiang J, Rosin NL, Brunham RC. Chlamydia muridarum T-cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection that correlates with a high frequency of γ interferon (IFN-γ)/tumor necrosis factor α and IFN-γ/interleukin-17 double-positive CD4+ T cells. Infect Immun. 2010;78:2272–82. doi: 10.1128/IAI.01374-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu H, Karunakaran KP, Jiang X, Shen C, Andersen P, Brunham RC. Chlamydia muridarum T cell antigens and adjuvants that induce protective immunity in mice. Infect Immun. 2012;80:1510–8. doi: 10.1128/IAI.06338-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igietseme JU, Magee DM, Williams DM, Rank RG. Role for CD8+ T cells in antichlamydial immunity defined by Chlamydia-specific T-lymphocyte clones. Infect Immun. 1994;62:5195–7. doi: 10.1128/iai.62.11.5195-5197.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gervassi AL, Probst P, Stamm WE, Marrazzo J, Grabstein KH, Alderson MR. Functional characterization of class Ia-and non-class Ia-restricted Chlamydia-reactive CD8+ T cell responses in humans. J Immunol. 2003;171:4278–86. doi: 10.4049/jimmunol.171.8.4278. [DOI] [PubMed] [Google Scholar]
- 19.Matyszak MK, Gaston JS. Chlamydia trachomatis-specific human CD8+ T cells show two patterns of antigen recognition. Infect Immun. 2004;72:4357–67. doi: 10.1128/IAI.72.8.4357-4367.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayarapu K, Kerr MS, Katschke A, Johnson RM. Chlamydia muridarum-specific CD4 T-cell clones recognize infected reproductive tract epithelial cells in an interferon-dependent fashion. Infect Immun. 2009;77:4469–79. doi: 10.1128/IAI.00491-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson RM. Murine oviduct epithelial cell cytokine responses to Chlamydia muridarum infection include interleukin-12-p70 secretion. Infect Immun. 2004;72:3951–60. doi: 10.1128/IAI.72.7.3951-3960.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson RM, Lancki DW, Fitch FW, Spear PG. Herpes simplex virus glycoprotein D is recognized as antigen by CD4+ and CD8+ T lymphocytes from infected mice. Characterization of T cell clones. J Immunol. 1990;145:702–10. [PubMed] [Google Scholar]
- 23.Ashwell JD, DeFranco AL, Paul WE, Schwartz RH. Antigen presentation by resting B cells. Radiosensitivity of the antigen-presentation function and two distinct pathways of T cell activation. J Exp Med. 1984;159:881–905. doi: 10.1084/jem.159.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson RM, Kerr MS, Slaven JE. Perforin is detrimental to controlling C. muridarum replication in vitro, but not in vivo. PLoS ONE. 2013;8:e63340. doi: 10.1371/journal.pone.0063340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jayarapu K, Kerr M, Ofner S, Johnson RM. Chlamydia-specific CD4 T cell clones control Chlamydia muridarum replication in epithelial cells by nitric oxide-dependent and-independent mechanisms. J Immunol. 2010;185:6911–20. doi: 10.4049/jimmunol.1002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asquith KL, Horvat JC, Kaiko GE, Carey AJ, Beagley KW, Hansbro PM, Foster PS. Interleukin-13 promotes susceptibility to chlamydial infection of the respiratory and genital tracts. PLoS Pathog. 2011;7:e1001339. doi: 10.1371/journal.ppat.1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsey KH, Rank RG. Resolution of chlamydial genital infection with antigen-specific T-lymphocyte lines. Infect Immun. 1991;59:925–31. doi: 10.1128/iai.59.3.925-931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect Immun. 2000;68:6979–87. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rock KL, Benacerraf B, Abbas AK. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J Exp Med. 1984;160:1102–13. doi: 10.1084/jem.160.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regnault A, Lankar D, Lacabanne V, et al. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–80. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore T, Ekworomadu CO, Eko FO, et al. Fc receptor-mediated antibody regulation of T cell immunity against intracellular pathogens. J Infect Dis. 2003;188:617–24. doi: 10.1086/377134. [DOI] [PubMed] [Google Scholar]
- 33.Gervassi AL, Grabstein KH, Probst P, Hess B, Alderson MR, Fling SP. Human CD8+ T cells recognize the 60-kDa cysteine-rich outer membrane protein from Chlamydia trachomatis. J Immunol. 2004;173:6905–13. doi: 10.4049/jimmunol.173.11.6905. [DOI] [PubMed] [Google Scholar]
- 34.Kim SK, Devine L, Angevine M, DeMars R, Kavathas PB. Direct detection and magnetic isolation of Chlamydia trachomatis major outer membrane protein-specific CD8+ CTLs with HLA class I tetramers. J Immunol. 2000;165:7285–92. doi: 10.4049/jimmunol.165.12.7285. [DOI] [PubMed] [Google Scholar]
- 35.Fling SP, Sutherland RA, Steele LN, et al. CD8+ T cells recognize an inclusion membrane-associated protein from the vacuolar pathogen Chlamydia trachomatis. Proc Natl Acad Sci U S A. 2001;98:1160–5. doi: 10.1073/pnas.98.3.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starnbach MN, Loomis WP, Ovendale P, Regan D, Hess B, Alderson MR, Fling SP. An inclusion membrane protein from Chlamydia trachomatis enters the MHC class I pathway and stimulates a CD8+ T cell response. J Immunol. 2003;171:4742–9. doi: 10.4049/jimmunol.171.9.4742. [DOI] [PubMed] [Google Scholar]
- 37.Grotenbreg GM, Roan NR, Guillen E, Meijers R, Wang JH, Bell GW, Starnbach MN, Ploegh HL. Discovery of CD8+ T cell epitopes in Chlamydia trachomatis infection through use of caged class I MHC tetramers. Proc Natl Acad Sci U S A. 2008;105:3831–6. doi: 10.1073/pnas.0711504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tvinnereim A, Wizel B. CD8+ T cell protective immunity against Chlamydia pneumoniae includes an H2-M3-restricted response that is largely CD4+ T cell-independent. J Immunol. 2007;179:3947–57. doi: 10.4049/jimmunol.179.6.3947. [DOI] [PubMed] [Google Scholar]
- 39.Karunakaran KP, Rey-Ladino J, Stoynov N, et al. Immunoproteomic discovery of novel T cell antigens from the obligate intracellular pathogen Chlamydia. J Immunol. 2008;180:2459–65. doi: 10.4049/jimmunol.180.4.2459. [DOI] [PubMed] [Google Scholar]
- 40.Johnson RM, Kerr MS, Slaven JE. Plac8-dependent and inducible NO synthase-dependent mechanisms clear Chlamydia muridarum infections from the genital tract. J Immunol. 2012;188:1896–904. doi: 10.4049/jimmunol.1102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Igietseme JU. The molecular mechanism of T-cell control of Chlamydia in mice: role of nitric oxide. Immunology. 1996;87:1–8. [PMC free article] [PubMed] [Google Scholar]
- 42.Igietseme JU, Uriri IM, Hawkins R, Rank RG. Integrin-mediated epithelial-T cell interaction enhances nitric oxide production and increased intracellular inhibition of Chlamydia. J Leukoc Biol. 1996;59:656–62. doi: 10.1002/jlb.59.5.656. [DOI] [PubMed] [Google Scholar]
- 43.Yang X, HayGlass KT, Brunham RC. Genetically determined differences in IL-10 and IFN-γ responses correlate with clearance of Chlamydia trachomatis mouse pneumonitis infection. J Immunol. 1996;156:4338–44. [PubMed] [Google Scholar]
- 44.Yang X, Gartner J, Zhu L, Wang S, Brunham RC. IL-10 gene knockout mice show enhanced Th1-like protective immunity and absent granuloma formation following Chlamydia trachomatis lung infection. J Immunol. 1999;162:1010–7. [PubMed] [Google Scholar]
- 45.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13α2 receptor is involved in induction of TGF-β1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 46.Fichtner-Feigl S, Fuss IJ, Young CA, Watanabe T, Geissler EK, Schlitt HJ, Kitani A, Strober W. Induction of IL-13 triggers TGF-β1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol. 2007;178:5859–70. doi: 10.4049/jimmunol.178.9.5859. [DOI] [PubMed] [Google Scholar]
- 47.Fuschiotti P, Larregina AT, Ho J, Feghali-Bostwick C, Medsger TA., Jr Interleukin-13-producing CD8+ T cells mediate dermal fibrosis in patients with systemic sclerosis. Arthritis Rheum. 2013;65:236–46. doi: 10.1002/art.37706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CD8 T-cell clones do not recognize McCoy cell alloantigens.


