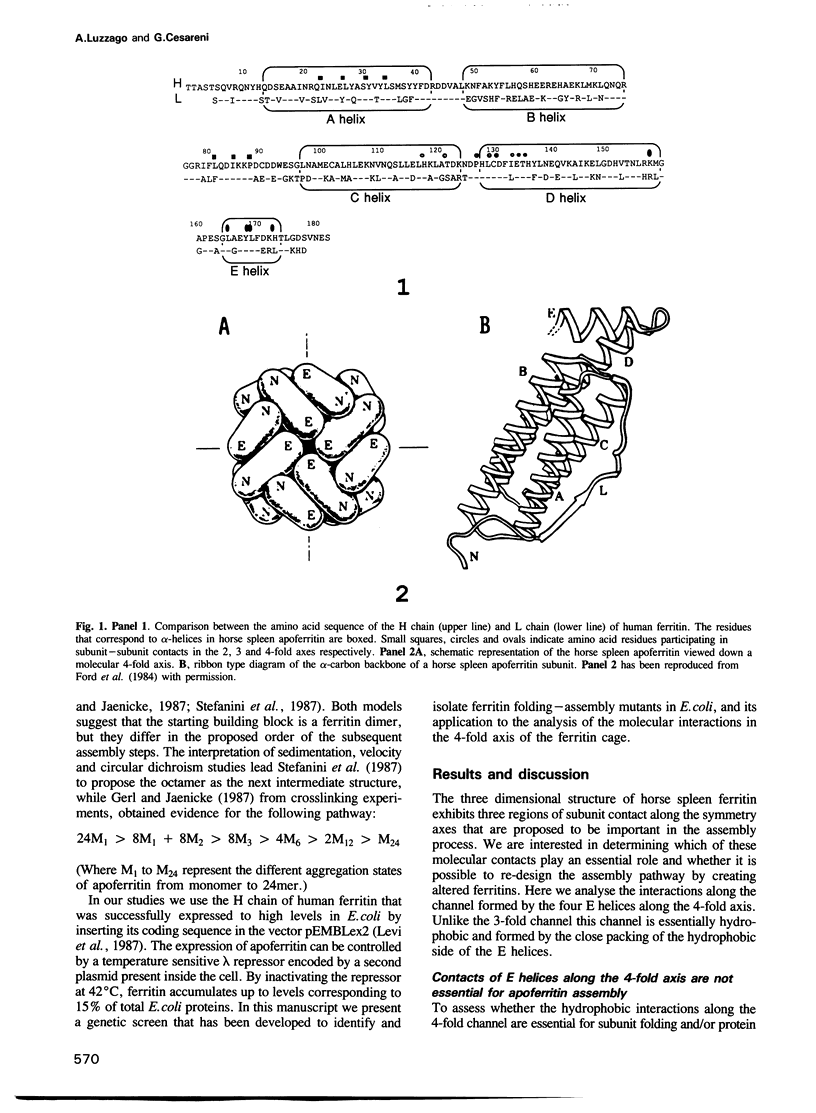
Abstract
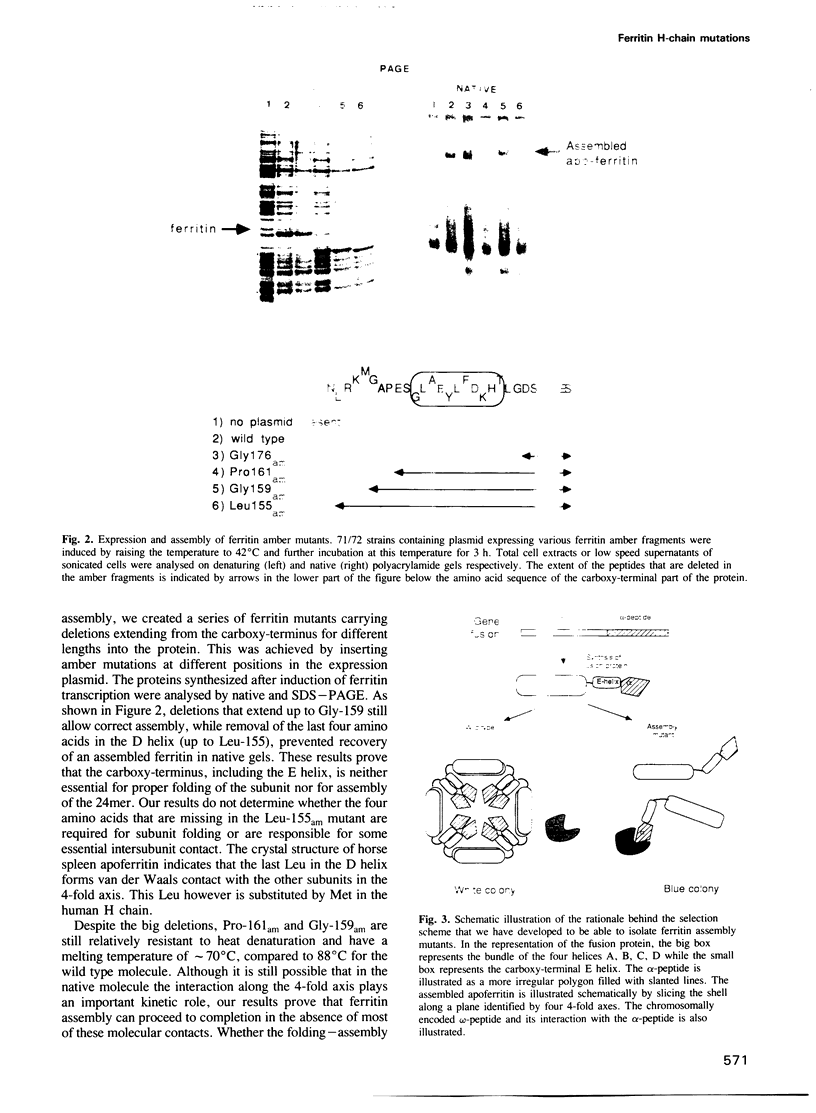
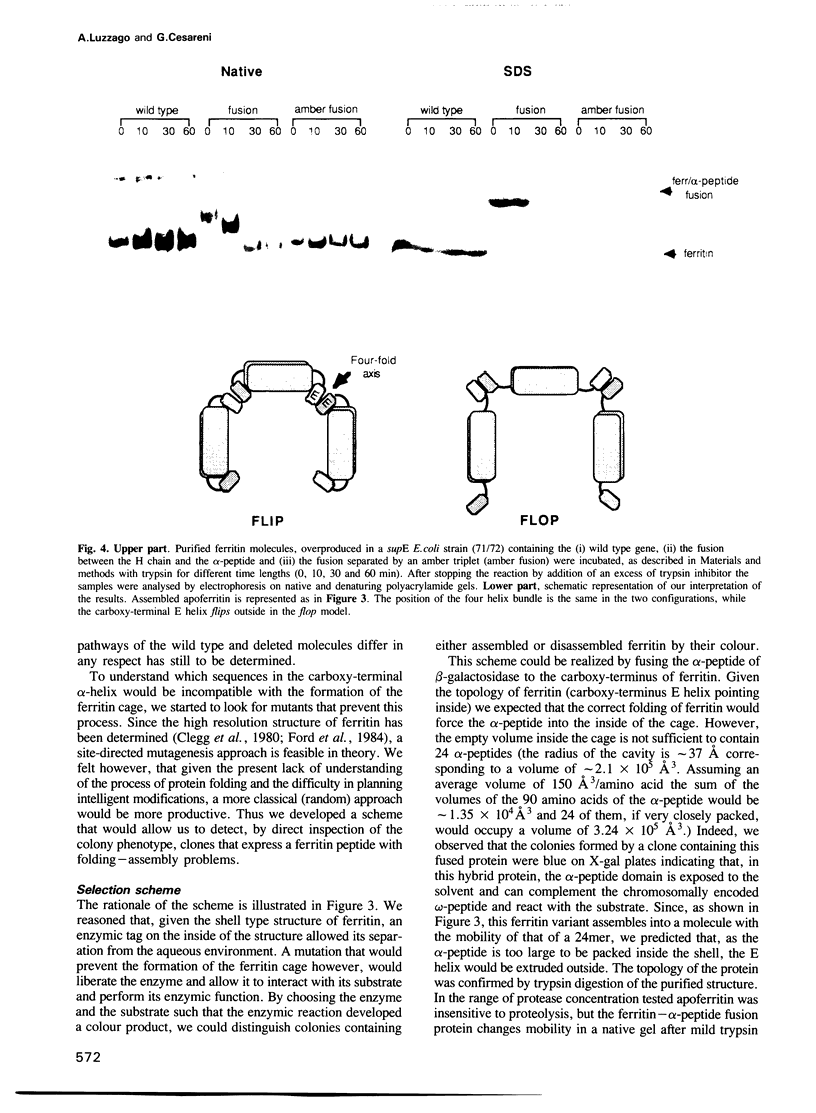
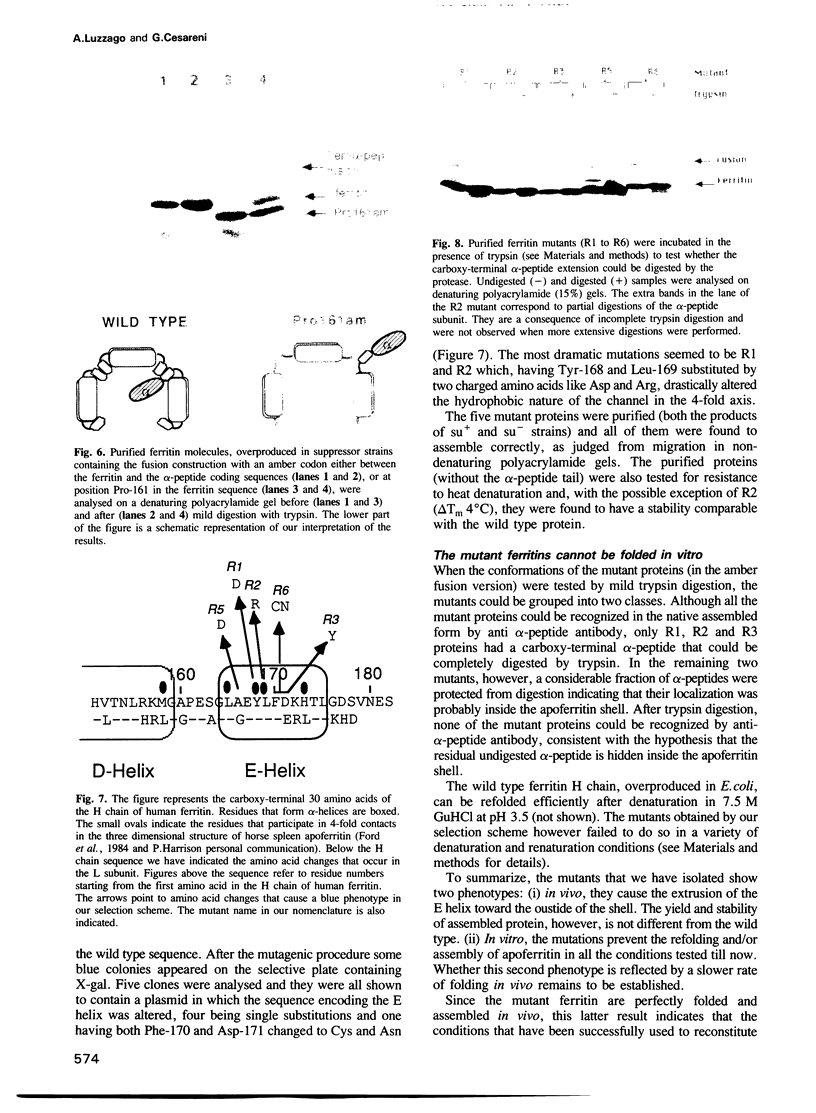
We have approached the problem of folding and assembly of the heavy (H) chain of human ferritin by isolating point mutations that affect this process. Apoferritin is an ideal model system to approach the problem of protein folding and assembly into multimeric structures. We have developed a recombinant hybrid molecule that allows us to select for ferritin mutants in which the folding-assembly process is altered or completely impaired. The selection procedure is based on a recombinant protein which consists of a fusion between the H chain of human ferritin and the alpha-peptide of beta-galactosidase. In the wild type situation, the alpha-peptide domain is segregated inside the apoferritin shell upon assembly and is unable to interact with the substrate and perform its enzymic function. We show that by selecting for mutations that restore beta-galactosidase activity we are able to identify ferritin mutations that affect the folding-assembly process. The selective procedure was applied to the analysis of the amino acid side chains that are important for the attainment of the correct conformation of the carboxy-terminal E helix in the 4-fold axis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anfinsen C. B. Principles that govern the folding of protein chains. Science. 1973 Jul 20;181(4096):223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- Arosio P., Adelman T. G., Drysdale J. W. On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem. 1978 Jun 25;253(12):4451–4458. [PubMed] [Google Scholar]
- Clegg G. A., Fitton J. E., Harrison P. M., Treffry A. Ferritin: molecular structure and iron-storage mechanisms. Prog Biophys Mol Biol. 1980;36(2-3):56–86. [PubMed] [Google Scholar]
- Dunnill P. Sequence similarities between hen egg-white and T4 phage lysozymes. Nature. 1967 Aug 5;215(5101):621–622. doi: 10.1038/215621a0. [DOI] [PubMed] [Google Scholar]
- Ford G. C., Harrison P. M., Rice D. W., Smith J. M., Treffry A., White J. L., Yariv J. Ferritin: design and formation of an iron-storage molecule. Philos Trans R Soc Lond B Biol Sci. 1984 Feb 13;304(1121):551–565. doi: 10.1098/rstb.1984.0046. [DOI] [PubMed] [Google Scholar]
- Gerl M., Jaenicke R. Mechanism of the self-assembly of apoferritin from horse spleen. Cross-linking and spectroscopic analysis. Eur Biophys J. 1987;15(2):103–109. doi: 10.1007/BF00257503. [DOI] [PubMed] [Google Scholar]
- Lang K., Schmid F. X., Fischer G. Catalysis of protein folding by prolyl isomerase. Nature. 1987 Sep 17;329(6136):268–270. doi: 10.1038/329268a0. [DOI] [PubMed] [Google Scholar]
- Levi S., Cesareni G., Arosio P., Lorenzetti R., Soria M., Sollazzo M., Albertini A., Cortese R. Characterization of human ferritin H chain synthetized in Escherichia coli. Gene. 1987;51(2-3):269–274. doi: 10.1016/0378-1119(87)90315-5. [DOI] [PubMed] [Google Scholar]
- Louvard D., Reggio H., Warren G. Antibodies to the Golgi complex and the rough endoplasmic reticulum. J Cell Biol. 1982 Jan;92(1):92–107. doi: 10.1083/jcb.92.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Albertini A. M. Effects of surrounding sequence on the suppression of nonsense codons. J Mol Biol. 1983 Feb 15;164(1):59–71. doi: 10.1016/0022-2836(83)90087-6. [DOI] [PubMed] [Google Scholar]
- Remaut E., Tsao H., Fiers W. Improved plasmid vectors with a thermoinducible expression and temperature-regulated runaway replication. Gene. 1983 Apr;22(1):103–113. doi: 10.1016/0378-1119(83)90069-0. [DOI] [PubMed] [Google Scholar]
- Stefanini S., Vecchini P., Chiancone E. On the mechanism of horse spleen apoferritin assembly: a sedimentation velocity and circular dichroism study. Biochemistry. 1987 Apr 7;26(7):1831–1837. doi: 10.1021/bi00381a007. [DOI] [PubMed] [Google Scholar]