Abstract
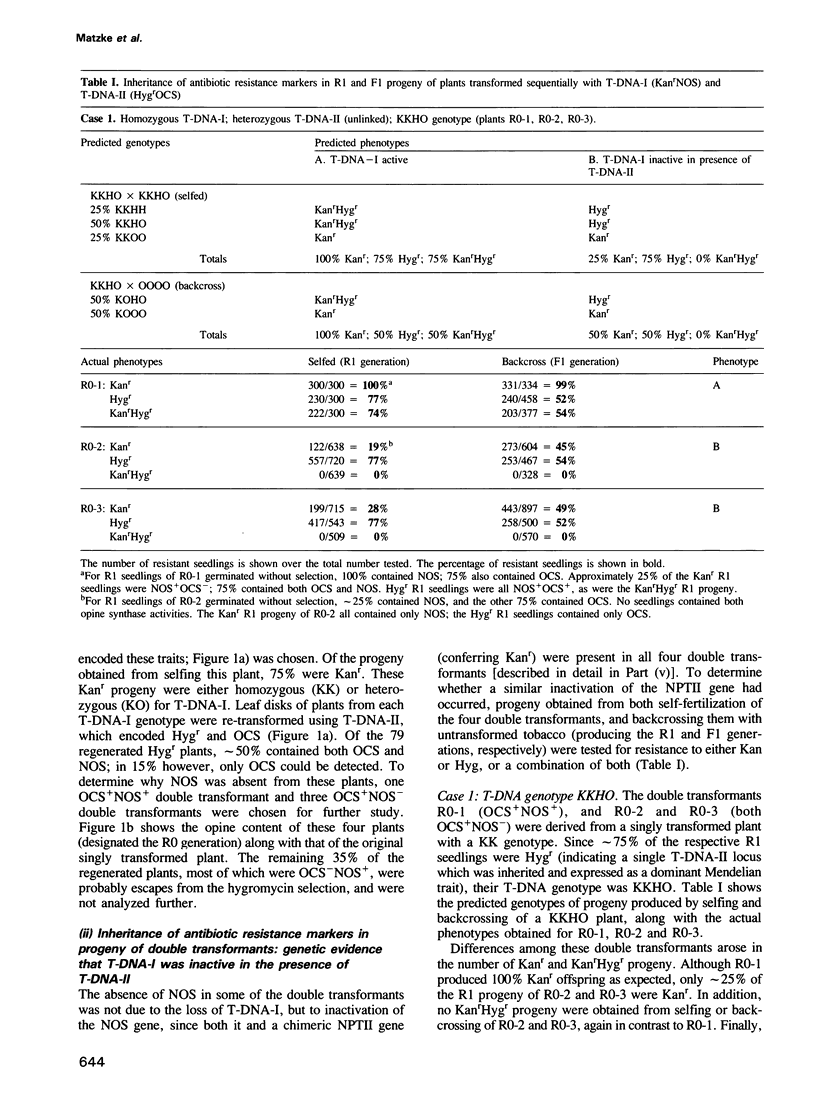
Doubly transformed tobacco plants were obtained following sequential transformation steps using two T-DNAs encoding different selection and screening markers: T-DNA-I encoded kanamycin resistance and nopaline synthase; T-DNA-II encoded hygromycin resistance and octopine synthase. A genetic analysis of the inheritance of the selection and screening marker genes in progeny of the doubly tranformed plants revealed that the expression of T-DNA-I genes was often suppressed. This suppression could be correlated with methylation in the promoters of these genes. Surprisingly, both the methylation and inactivation of T-DNA-I genes occurred only in plants containing both T-DNAs: when self-fertilization or backcrossing produced progeny containing only T-DNA-I, expression of the genes on this T-DNA was restored and the corresponding promoters were partially or completely demethylated. These results indicated that the presence of one T-DNA could affect the state of methylation and expression of genes on a second, unlinked T-DNA in the same genome.
Keywords: gene inactivation, promoter methylation, sequential transformation, T-DNA, transgenic plants
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Amasino R. M., Powell A. L., Gordon M. P. Changes in T-DNA methylation and expression are associated with phenotypic variation and plant regeneration in a crown gall tumor line. Mol Gen Genet. 1984;197(3):437–446. doi: 10.1007/BF00329940. [DOI] [PubMed] [Google Scholar]
- Ambros P. F., Matzke A. J. M., Matzke M. A. Localization of Agrobacterium rhizogenes T-DNA in plant chromosomes by in situ hybridization. EMBO J. 1986 Sep;5(9):2073–2077. doi: 10.1002/j.1460-2075.1986.tb04468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler V. L., Talbert L. E., Raymond F. Sequence, genomic distribution and DNA modification of a Mu1 element from non-mutator maize stocks. Genetics. 1988 Aug;119(4):951–958. doi: 10.1093/genetics/119.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler V. L., Walbot V. DNA modification of a maize transposable element correlates with loss of activity. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1767–1771. doi: 10.1073/pnas.83.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomet P. S., Wessler S., Dellaporta S. L. Inactivation of the maize transposable element Activator (Ac) is associated with its DNA modification. EMBO J. 1987 Feb;6(2):295–302. doi: 10.1002/j.1460-2075.1987.tb04753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Greve H., Dhaese P., Seurinck J., Lemmers M., Van Montagu M., Schell J. Nucleotide sequence and transcript map of the Agrobacterium tumefaciens Ti plasmid-encoded octopine synthase gene. J Mol Appl Genet. 1982;1(6):499–511. [PubMed] [Google Scholar]
- Delauney A. J., Tabaeizadeh Z., Verma D. P. A stable bifunctional antisense transcript inhibiting gene expression in transgenic plants. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4300–4304. doi: 10.1073/pnas.85.12.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depicker A., Stachel S., Dhaese P., Zambryski P., Goodman H. M. Nopaline synthase: transcript mapping and DNA sequence. J Mol Appl Genet. 1982;1(6):561–573. [PubMed] [Google Scholar]
- Gelvin S. B., Karcher S. J., DiRita V. J. Methylation of the T-DNA in Agrobacterium tumefaciens and in several crown gall tumors. Nucleic Acids Res. 1983 Jan 11;11(1):159–174. doi: 10.1093/nar/11.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritz L., Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983 Nov;25(2-3):179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Hepburn A. G., Clarke L. E., Pearson L., White J. The role of cytosine methylation in the control of nopaline synthase gene expression in a plant tumor. J Mol Appl Genet. 1983;2(3):315–329. [PubMed] [Google Scholar]
- Monk M. Genomic imprinting. Genes Dev. 1988 Aug;2(8):921–925. doi: 10.1101/gad.2.8.921. [DOI] [PubMed] [Google Scholar]
- Otten L. A., Schilperoort R. A. A rapid micro scale method for the detection of lysopine and nopaline dehydrogenase activities. Biochim Biophys Acta. 1978 Dec 8;527(2):497–500. doi: 10.1016/0005-2744(78)90363-7. [DOI] [PubMed] [Google Scholar]
- Pietrzak M., Shillito R. D., Hohn T., Potrykus I. Expression in plants of two bacterial antibiotic resistance genes after protoplast transformation with a new plant expression vector. Nucleic Acids Res. 1986 Jul 25;14(14):5857–5868. doi: 10.1093/nar/14.14.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrykus I., Paszkowski J., Saul M. W., Petruska J., Shillito R. D. Molecular and general genetics of a hybrid foreign gene introduced into tobacco by direct gene transfer. Mol Gen Genet. 1985;199(2):169–177. doi: 10.1007/BF00330255. [DOI] [PubMed] [Google Scholar]
- Schernthaner J. P., Matzke M. A., Matzke A. J. Endosperm-specific activity of a zein gene promoter in transgenic tobacco plants. EMBO J. 1988 May;7(5):1249–1255. doi: 10.1002/j.1460-2075.1988.tb02938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Schell J. Transgenic plants as tools to study the molecular organization of plant genes. Science. 1987 Sep 4;237(4819):1176–1183. doi: 10.1126/science.237.4819.1176. [DOI] [PubMed] [Google Scholar]
- Watson J. C., Kaufman L. S., Thompson W. F. Developmental regulation of cytosine methylation in the nuclear ribosomal RNA genes of Pisum sativum. J Mol Biol. 1987 Jan 5;193(1):15–26. doi: 10.1016/0022-2836(87)90622-x. [DOI] [PubMed] [Google Scholar]
- Willmitzer L. The use of transgenic plants to study plant gene expression. Trends Genet. 1988 Jan;4(1):13–18. doi: 10.1016/0168-9525(88)90122-9. [DOI] [PubMed] [Google Scholar]