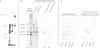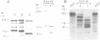Abstract
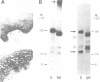
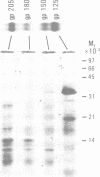
A new member of the integrin superfamily of adhesion receptors was isolated from human epithelial cells. Analogously to other integrins, this molecule is a heterodimer comprised of structurally unrelated subunits, both glycosylated. Unequivocal amino-acid sequence homologies were observed between these subunits and integrin alpha and beta chain sequences, indicating that this epithelial heterodimer is a novel integrin. No obvious serologic cross-reactivities were detected with other integrins. The beta chain of the epithelial integrin displayed a mol. wt significantly higher than other integrin beta chains, possibly due to a large sialic acid content. Integrin heterodimers are grouped into three families, based on which of three beta chains (beta 1, beta 2 and beta 3) they contain. Therefore, the epithelial integrin may represent the prototype of a fourth integrin family, because it contains a structurally distinct beta chain. The designation alpha E beta 4 is proposed for this novel human integrin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfors K. E., Lundberg C., Lindbom L., Lundberg K., Beatty P. G., Harlan J. M. A monoclonal antibody to the membrane glycoprotein complex CD18 inhibits polymorphonuclear leukocyte accumulation and plasma leakage in vivo. Blood. 1987 Jan;69(1):338–340. [PubMed] [Google Scholar]
- Argraves W. S., Suzuki S., Arai H., Thompson K., Pierschbacher M. D., Ruoslahti E. Amino acid sequence of the human fibronectin receptor. J Cell Biol. 1987 Sep;105(3):1183–1190. doi: 10.1083/jcb.105.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Horwitz A. F. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- Charo I. F., Fitzgerald L. A., Steiner B., Rall S. C., Jr, Bekeart L. S., Phillips D. R. Platelet glycoproteins IIb and IIIa: evidence for a family of immunologically and structurally related glycoproteins in mammalian cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8351–8355. doi: 10.1073/pnas.83.21.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Harper J. R. Arg-Gly-Asp recognition by a cell adhesion receptor requires its 130-kDa alpha subunit. J Biol Chem. 1987 Feb 5;262(4):1434–1437. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Damsky C. H., Knudsen K. A., Dorio R. J., Buck C. A. Manipulation of cell-cell and cell-substratum interactions in mouse mammary tumor epithelial cells using broad spectrum antisera. J Cell Biol. 1981 May;89(2):173–184. doi: 10.1083/jcb.89.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules. Science. 1983 Feb 4;219(4584):450–457. doi: 10.1126/science.6823544. [DOI] [PubMed] [Google Scholar]
- Ekblom P., Vestweber D., Kemler R. Cell-matrix interactions and cell adhesion during development. Annu Rev Cell Biol. 1986;2:27–47. doi: 10.1146/annurev.cb.02.110186.000331. [DOI] [PubMed] [Google Scholar]
- Fitzgerald L. A., Steiner B., Rall S. C., Jr, Lo S. S., Phillips D. R. Protein sequence of endothelial glycoprotein IIIa derived from a cDNA clone. Identity with platelet glycoprotein IIIa and similarity to "integrin". J Biol Chem. 1987 Mar 25;262(9):3936–3939. [PubMed] [Google Scholar]
- Giancotti F. G., Tarone G., Knudsen K., Damsky C., Comoglio P. M. Cleavage of a 135 kD cell surface glycoprotein correlates with loss of fibroblast adhesion to fibronectin. Exp Cell Res. 1985 Jan;156(1):182–190. doi: 10.1016/0014-4827(85)90272-1. [DOI] [PubMed] [Google Scholar]
- Hallenbeck P. C., Vimr E. R., Yu F., Bassler B., Troy F. A. Purification and properties of a bacteriophage-induced endo-N-acetylneuraminidase specific for poly-alpha-2,8-sialosyl carbohydrate units. J Biol Chem. 1987 Mar 15;262(8):3553–3561. [PubMed] [Google Scholar]
- Hemler M. E., Crouse C., Takada Y., Sonnenberg A. Multiple very late antigen (VLA) heterodimers on platelets. Evidence for distinct VLA-2, VLA-5 (fibronectin receptor), and VLA-6 structures. J Biol Chem. 1988 Jun 5;263(16):7660–7665. [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [PubMed] [Google Scholar]
- Hunkapiller M. W., Hewick R. M., Dreyer W. J., Hood L. E. High-sensitivity sequencing with a gas-phase sequenator. Methods Enzymol. 1983;91:399–413. doi: 10.1016/s0076-6879(83)91038-8. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Kajiji S. M., Davceva B., Quaranta V. Six monoclonal antibodies to human pancreatic cancer antigens. Cancer Res. 1987 Mar 1;47(5):1367–1376. [PubMed] [Google Scholar]
- Kishimoto T. K., O'Connor K., Lee A., Roberts T. M., Springer T. A. Cloning of the beta subunit of the leukocyte adhesion proteins: homology to an extracellular matrix receptor defines a novel supergene family. Cell. 1987 Feb 27;48(4):681–690. doi: 10.1016/0092-8674(87)90246-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Miller L. J., Wiebe M., Springer T. A. Purification and alpha subunit N-terminal sequences of human Mac-1 and p150,95 leukocyte adhesion proteins. J Immunol. 1987 Apr 15;138(8):2381–2383. [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pischel K. D., Bluestein H. G., Woods V. L., Jr Platelet glycoproteins Ia, Ic, and IIa are physicochemically indistinguishable from the very late activation antigens adhesion-related proteins of lymphocytes and other cell types. J Clin Invest. 1988 Feb;81(2):505–513. doi: 10.1172/JCI113348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischel K. D., Hemler M. E., Huang C., Bluestein H. G., Woods V. L., Jr Use of the monoclonal antibody 12F1 to characterize the differentiation antigen VLA-2. J Immunol. 1987 Jan 1;138(1):226–233. [PubMed] [Google Scholar]
- Poncz M., Eisman R., Heidenreich R., Silver S. M., Vilaire G., Surrey S., Schwartz E., Bennett J. S. Structure of the platelet membrane glycoprotein IIb. Homology to the alpha subunits of the vitronectin and fibronectin membrane receptors. J Biol Chem. 1987 Jun 25;262(18):8476–8482. [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Hogervorst F., Osterop A., Veltman F. E. Identification and characterization of a novel antigen complex on mouse mammary tumor cells using a monoclonal antibody against platelet glycoprotein Ic. J Biol Chem. 1988 Oct 5;263(28):14030–14038. [PubMed] [Google Scholar]
- Sonnenberg A., Janssen H., Hogervorst F., Calafat J., Hilgers J. A complex of platelet glycoproteins Ic and IIa identified by a rat monoclonal antibody. J Biol Chem. 1987 Jul 25;262(21):10376–10383. [PubMed] [Google Scholar]
- Springer T. A., Teplow D. B., Dreyer W. J. Sequence homology of the LFA-1 and Mac-1 leukocyte adhesion glycoproteins and unexpected relation to leukocyte interferon. Nature. 1985 Apr 11;314(6011):540–542. doi: 10.1038/314540a0. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Argraves W. S., Pytela R., Arai H., Krusius T., Pierschbacher M. D., Ruoslahti E. cDNA and amino acid sequences of the cell adhesion protein receptor recognizing vitronectin reveal a transmembrane domain and homologies with other adhesion protein receptors. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8614–8618. doi: 10.1073/pnas.83.22.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Huang C., Hemler M. E. Fibronectin receptor structures in the VLA family of heterodimers. Nature. 1987 Apr 9;326(6113):607–609. doi: 10.1038/326607a0. [DOI] [PubMed] [Google Scholar]
- Takada Y., Strominger J. L., Hemler M. E. The very late antigen family of heterodimers is part of a superfamily of molecules involved in adhesion and embryogenesis. Proc Natl Acad Sci U S A. 1987 May;84(10):3239–3243. doi: 10.1073/pnas.84.10.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamkun J. W., DeSimone D. W., Fonda D., Patel R. S., Buck C., Horwitz A. F., Hynes R. O. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell. 1986 Jul 18;46(2):271–282. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki N. M., Reisfeld R. A., Walker L. E. Antigens associated with a human lung adenocarcinoma defined by monoclonal antibodies. Cancer Res. 1984 Feb;44(2):681–687. [PubMed] [Google Scholar]
- Weinberger C., Hollenberg S. M., Ong E. S., Harmon J. M., Brower S. T., Cidlowski J., Thompson E. B., Rosenfeld M. G., Evans R. M. Identification of human glucocorticoid receptor complementary DNA clones by epitope selection. Science. 1985 May 10;228(4700):740–742. doi: 10.1126/science.2581314. [DOI] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]