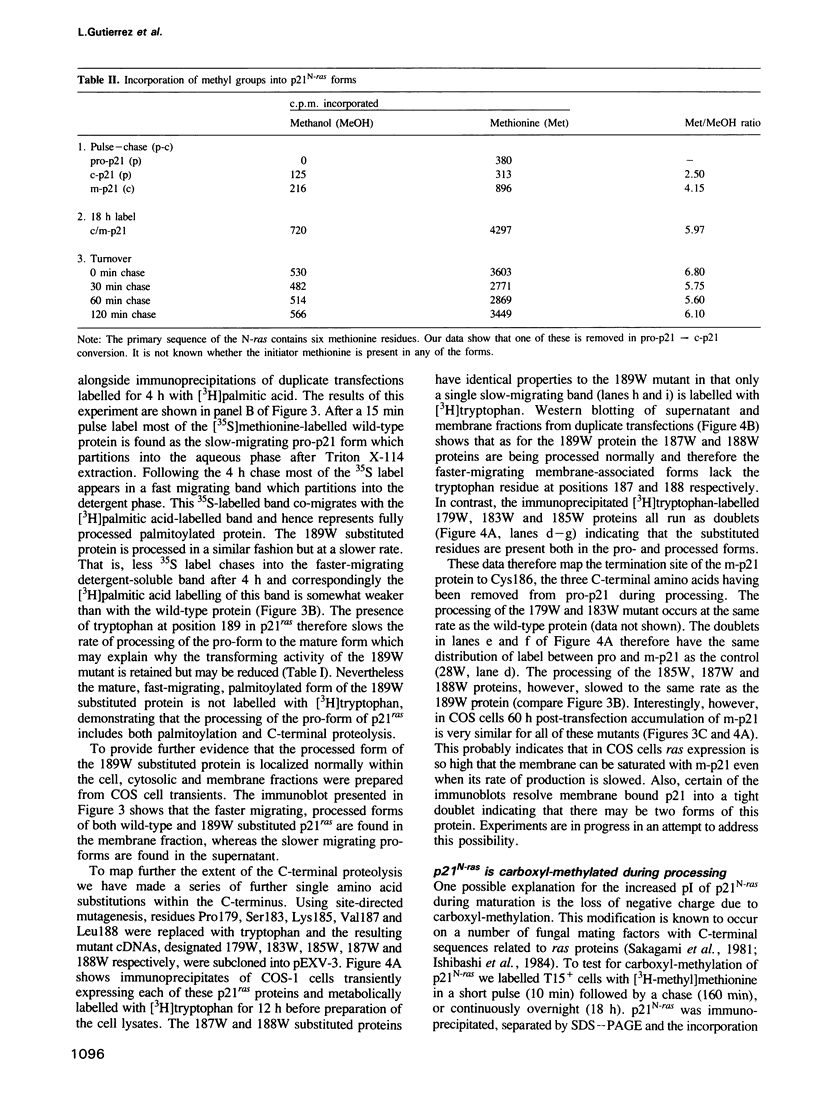
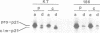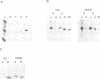Abstract
We have studied the post-translational processing of p21ras proteins. The primary translation product pro-p21 is cytosolic and is rapidly converted to a cytosolic form (c-p21) of higher mobility on SDS-PAGE. c-p21 is converted in turn to the membrane-bound mature palmitoylated form (m-p21) of slightly higher mobility. These processing steps are accompanied by increases in isoelectric point and in hydrophobicity as judged by Triton X-114 partitioning. Although the increases in electrophoretic mobility and hydrophobicity precede acylation we show that mutation of Cys186, which has been shown to block acylation, also abolishes the pro-p21 to c-p21 conversion. Thus the Cys186 residue is involved in the processing steps prior to acylation. We have identified two processing events which contribute to the pro-p21 conversion. Site-directed mutagenesis to insert tryptophan, which is not present in the wild type, followed by metabolic labelling with [3H]tryptophan has allowed us to map a proteolytic processing event which removes the three C-terminal residues. In addition, both the c-p21 and m-p21 forms are carboxyl-methylated. Approximately one methyl group is incorporated per molecule of p21 at steady state, which can partially account for the increase in isoelectric point. Unlike palmitate, methyl group turnover is not observed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Betz R., Crabb J. W., Meyer H. E., Wittig R., Duntze W. Amino acid sequences of a-factor mating peptides from Saccharomyces cerevisiae. J Biol Chem. 1987 Jan 15;262(2):546–548. [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chelsky D., Gutterson N. I., Koshland D. E., Jr A diffusion assay for detection and quantitation of methyl-esterified proteins on polyacrylamide gels. Anal Biochem. 1984 Aug 15;141(1):143–148. doi: 10.1016/0003-2697(84)90437-8. [DOI] [PubMed] [Google Scholar]
- Chelsky D., Ruskin B., Koshland D. E., Jr Methyl-esterified proteins in a mammalian cell line. Biochemistry. 1985 Nov 5;24(23):6651–6658. doi: 10.1021/bi00344a053. [DOI] [PubMed] [Google Scholar]
- Chen Z. Q., Ulsh L. S., DuBois G., Shih T. Y. Posttranslational processing of p21 ras proteins involves palmitylation of the C-terminal tetrapeptide containing cysteine-186. J Virol. 1985 Nov;56(2):607–612. doi: 10.1128/jvi.56.2.607-612.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S., Vogel J. P., Deschenes R. J., Stock J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4643–4647. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes R. J., Broach J. R. Fatty acylation is important but not essential for Saccharomyces cerevisiae RAS function. Mol Cell Biol. 1987 Jul;7(7):2344–2351. doi: 10.1128/mcb.7.7.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein N., Ali I. U. Comparative analysis of p21 proteins from various cell types by two-dimensional gel electrophoresis. J Cell Biochem. 1985;29(3):253–263. doi: 10.1002/jcb.240290309. [DOI] [PubMed] [Google Scholar]
- Fleischman L. F., Chahwala S. B., Cantley L. ras-transformed cells: altered levels of phosphatidylinositol-4,5-bisphosphate and catabolites. Science. 1986 Jan 24;231(4736):407–410. doi: 10.1126/science.3001936. [DOI] [PubMed] [Google Scholar]
- Fuhrer J. P., DeBiasi F., Cooper H. L., Schlom J. Analysis of ras oncogene products by two-dimensional gel electrophoresis: evidence for protein families with distinctive molecular forms. Biochim Biophys Acta. 1986 May 5;866(4):204–215. doi: 10.1016/0167-4781(86)90045-x. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B., Schaber M. D., Allard W. J., Sigal I. S., Scolnick E. M. Purification of ras GTPase activating protein from bovine brain. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5026–5030. doi: 10.1073/pnas.85.14.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grand R. J., Smith K. J., Gallimore P. H. Purification and characterisation of the protein encoded by the activated human N-ras gene and its membrane localisation. Oncogene. 1987;1(3):305–314. [PubMed] [Google Scholar]
- Hancock J. F., Marshall C. J., McKay I. A., Gardner S., Houslay M. D., Hall A., Wakelam M. J. Mutant but not normal p21 ras elevates inositol phospholipid breakdown in two different cell systems. Oncogene. 1988 Aug;3(2):187–193. [PubMed] [Google Scholar]
- Magee A. I., Gutierrez L., McKay I. A., Marshall C. J., Hall A. Dynamic fatty acylation of p21N-ras. EMBO J. 1987 Nov;6(11):3353–3357. doi: 10.1002/j.1460-2075.1987.tb02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee T., Hanley M. Protein modification. Sticky fingers and CAAX boxes. Nature. 1988 Sep 8;335(6186):114–115. doi: 10.1038/335114a0. [DOI] [PubMed] [Google Scholar]
- Marshall C. J., Hall A., Weiss R. A. A transforming gene present in human sarcoma cell lines. Nature. 1982 Sep 9;299(5879):171–173. doi: 10.1038/299171a0. [DOI] [PubMed] [Google Scholar]
- McKay I. A., Marshall C. J., Calés C., Hall A. Transformation and stimulation of DNA synthesis in NIH-3T3 cells are a titratable function of normal p21N-ras expression. EMBO J. 1986 Oct;5(10):2617–2621. doi: 10.1002/j.1460-2075.1986.tb04542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Germain R. N. Efficient cell surface expression of class II MHC molecules in the absence of associated invariant chain. J Exp Med. 1986 Nov 1;164(5):1478–1489. doi: 10.1084/jem.164.5.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar C. M., Prange R., Gallwitz D. A carboxyl-terminal cysteine residue is required for palmitic acid binding and biological activity of the ras-related yeast YPT1 protein. EMBO J. 1988 Apr;7(4):971–976. doi: 10.1002/j.1460-2075.1988.tb02903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonis V. R., Anderson G. R., Doyle D. Two-dimensional polyacrylamide gel electrophoresis analysis of phosphorylated, membrane-localized ras p21 proteins. Arch Biochem Biophys. 1987 May 1;254(2):541–546. doi: 10.1016/0003-9861(87)90135-4. [DOI] [PubMed] [Google Scholar]
- Powers S., Michaelis S., Broek D., Santa Anna S., Field J., Herskowitz I., Wigler M. RAM, a gene of yeast required for a functional modification of RAS proteins and for production of mating pheromone a-factor. Cell. 1986 Nov 7;47(3):413–422. doi: 10.1016/0092-8674(86)90598-2. [DOI] [PubMed] [Google Scholar]
- Sakagami Y., Yoshida M., Isogai A., Suzuki A. Peptidal Sex Hormones Inducing Conjugation Tube Formation in Compatible Mating-Type Cells of Tremella mesenterica. Science. 1981 Jun 26;212(4502):1525–1527. doi: 10.1126/science.212.4502.1525. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Trowbridge I. S., Cooper J. A., Scolnick E. M. The transforming proteins of Rous sarcoma virus, Harvey sarcoma virus and Abelson virus contain tightly bound lipid. Cell. 1982 Dec;31(2 Pt 1):465–474. doi: 10.1016/0092-8674(82)90139-8. [DOI] [PubMed] [Google Scholar]
- Shen W. P., Aldrich T. H., Venta-Perez G., Franza B. R., Jr, Furth M. E. Expression of normal and mutant ras proteins in human acute leukemia. Oncogene. 1987 May;1(2):157–165. [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Gruss P., Dhar R., Oroszlan S., Scolnick E. M. Identification of a precursor in the biosynthesis of the p21 transforming protein of harvey murine sarcoma virus. J Virol. 1982 Apr;42(1):253–261. doi: 10.1128/jvi.42.1.253-261.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamanoi F., Hsueh E. C., Goodman L. E., Cobitz A. R., Detrick R. J., Brown W. R., Fujiyama A. Posttranslational modification of ras proteins: detection of a modification prior to fatty acid acylation and cloning of a gene responsible for the modification. J Cell Biochem. 1988 Mar;36(3):261–273. doi: 10.1002/jcb.240360307. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Trahey M., McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987 Oct 23;238(4826):542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J., Davies S. A., Houslay M. D., McKay I., Marshall C. J., Hall A. Normal p21N-ras couples bombesin and other growth factor receptors to inositol phosphate production. Nature. 1986 Sep 11;323(6084):173–176. doi: 10.1038/323173a0. [DOI] [PubMed] [Google Scholar]
- Willumsen B. M., Norris K., Papageorge A. G., Hubbert N. L., Lowy D. R. Harvey murine sarcoma virus p21 ras protein: biological and biochemical significance of the cysteine nearest the carboxy terminus. EMBO J. 1984 Nov;3(11):2581–2585. doi: 10.1002/j.1460-2075.1984.tb02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfman A., Macara I. G. Elevated levels of diacylglycerol and decreased phorbol ester sensitivity in ras-transformed fibroblasts. Nature. 1987 Jan 22;325(6102):359–361. doi: 10.1038/325359a0. [DOI] [PubMed] [Google Scholar]