Abstract
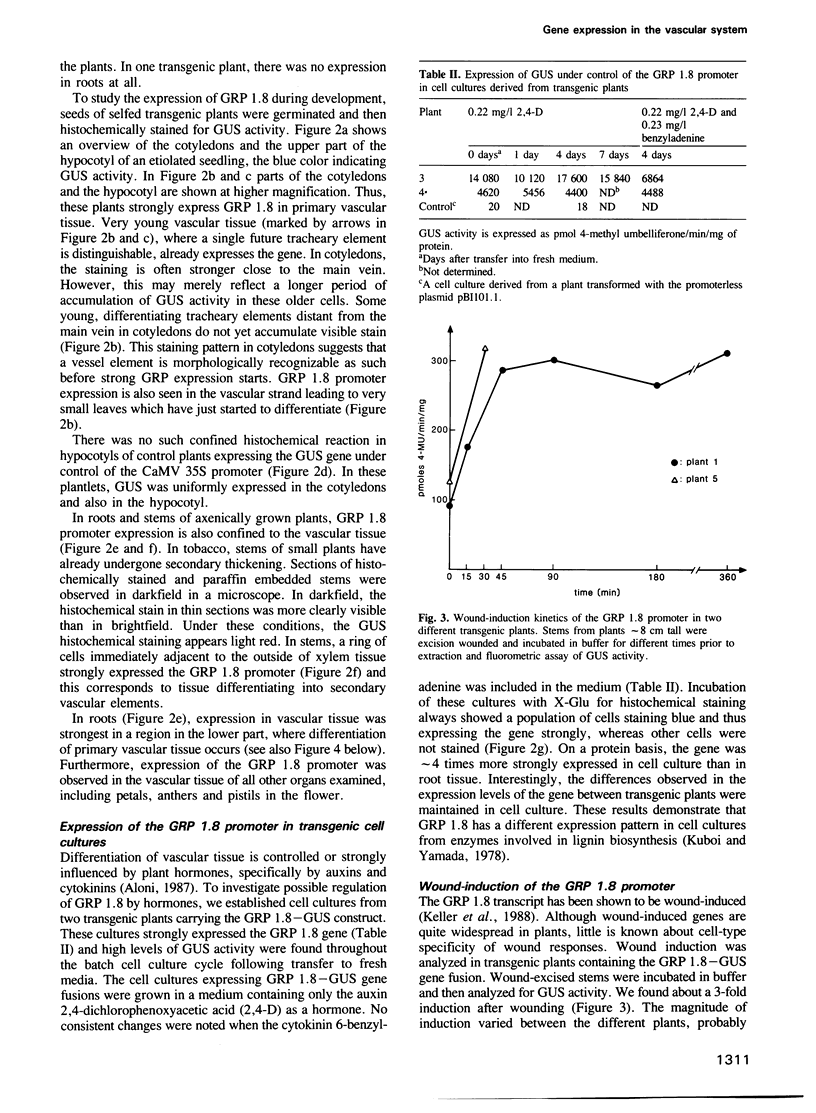
In French bean (Phaseolus vulgaris L.), the glycine-rich wall protein GRP 1.8 is specifically synthesized in protoxylem tracheary elements of the vascular system. A 494 bp upstream promoter fragment of the gene encoding GRP 1.8 was isolated and translationally fused to the β-glucuronidase reporter gene. Transgenic tobacco plants containing this construct expressed the gene in vascular tissue of roots, stems, leaves and flowers. The gene was developmentally expressed during differentiation of both primary and secondary vascular tissue and was also rapidly induced (in < 30 min) after excision-wounding of young stems. This wound response is more rapid than in bean hypocotyls, indicating possible differences between the activation mechanism for glycine-rich protein gene expression in wounded bean and tobacco. Only a subset of cells were found to participate in the wound response. In young stems, the GRP wound induction was localized in pith parenchyma cells adjacent to the wound surface, where vessel regeneration is known to occur. Thus, a promoter fragment of 494 bp, including 427 bp upstream from the transcription start site, contains information for tissue-specific and wound-induced gene regulation. The cell-type specificity of expression suggests that the GRP 1.8 promoter is regulated by very specific developmental and environmental signals.
Keywords: β-glucuronidase, cell wall, glycine-rich protein, Phaseolus vulgaris, xylem
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachy R. N., Chen Z. L., Horsch R. B., Rogers S. G., Hoffmann N. J., Fraley R. T. Accumulation and assembly of soybean beta-conglycinin in seeds of transformed petunia plants. EMBO J. 1985 Dec 1;4(12):3047–3053. doi: 10.1002/j.1460-2075.1985.tb04044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. N., Ryder T. B., Wingate V. P., Bailey J. A., Lamb C. J. Differential accumulation of plant defense gene transcripts in a compatible and an incompatible plant-pathogen interaction. Mol Cell Biol. 1986 May;6(5):1615–1623. doi: 10.1128/mcb.6.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlmann H., Clausen S., Behnke S., Giese H., Hiller C., Reimann-Philipp U., Schrader G., Barkholt V., Apel K. Leaf-specific thionins of barley-a novel class of cell wall proteins toxic to plant-pathogenic fungi and possibly involved in the defence mechanism of plants. EMBO J. 1988 Jun;7(6):1559–1565. doi: 10.1002/j.1460-2075.1988.tb02980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Varner J. E. An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO J. 1985 Sep;4(9):2145–2151. doi: 10.1002/j.1460-2075.1985.tb03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit C. M., Meagher R. B. Expression of a gene encoding a glycine-rich protein in petunia. Mol Cell Biol. 1987 Dec;7(12):4273–4279. doi: 10.1128/mcb.7.12.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K., Cramer C. L., Bolwell G. P., Dixon R. A., Schuch W., Lamb C. J. Rapid transient induction of phenylalanine ammonia-lyase mRNA in elicitor-treated bean cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6731–6735. doi: 10.1073/pnas.82.20.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. S., Pearce G., Merryweather J., Titani K., Ericsson L., Ryan C. A. Wound-induced proteinase inhibitors from tomato leaves. I. The cDNA-deduced primary structure of pre-inhibitor I and its post-translational processing. J Biol Chem. 1985 Jun 10;260(11):6555–6560. [PubMed] [Google Scholar]
- Horsch R. B., Fraley R. T., Rogers S. G., Sanders P. R., Lloyd A., Hoffmann N. Inheritance of functional foreign genes in plants. Science. 1984 Feb 3;223(4635):496–498. doi: 10.1126/science.223.4635.496. [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller B., Sauer N., Lamb C. J. Glycine-rich cell wall proteins in bean: gene structure and association of the protein with the vascular system. EMBO J. 1988 Dec 1;7(12):3625–3633. doi: 10.1002/j.1460-2075.1988.tb03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller B., Templeton M. D., Lamb C. J. Specific localization of a plant cell wall glycine-rich protein in protoxylem cells of the vascular system. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1529–1533. doi: 10.1073/pnas.86.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboi T., Yamada Y. Regulation of the enzyme activities related to lignin synthesis in cell aggregates of tobacco cell culture. Biochim Biophys Acta. 1978 Aug 17;542(2):181–190. doi: 10.1016/0304-4165(78)90014-4. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Schell J. Transgenic plants as tools to study the molecular organization of plant genes. Science. 1987 Sep 4;237(4819):1176–1183. doi: 10.1126/science.237.4819.1176. [DOI] [PubMed] [Google Scholar]
- Thornburg R. W., An G., Cleveland T. E., Johnson R., Ryan C. A. Wound-inducible expression of a potato inhibitor II-chloramphenicol acetyltransferase gene fusion in transgenic tobacco plants. Proc Natl Acad Sci U S A. 1987 Feb;84(3):744–748. doi: 10.1073/pnas.84.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmitzer L. The use of transgenic plants to study plant gene expression. Trends Genet. 1988 Jan;4(1):13–18. doi: 10.1016/0168-9525(88)90122-9. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]