Abstract
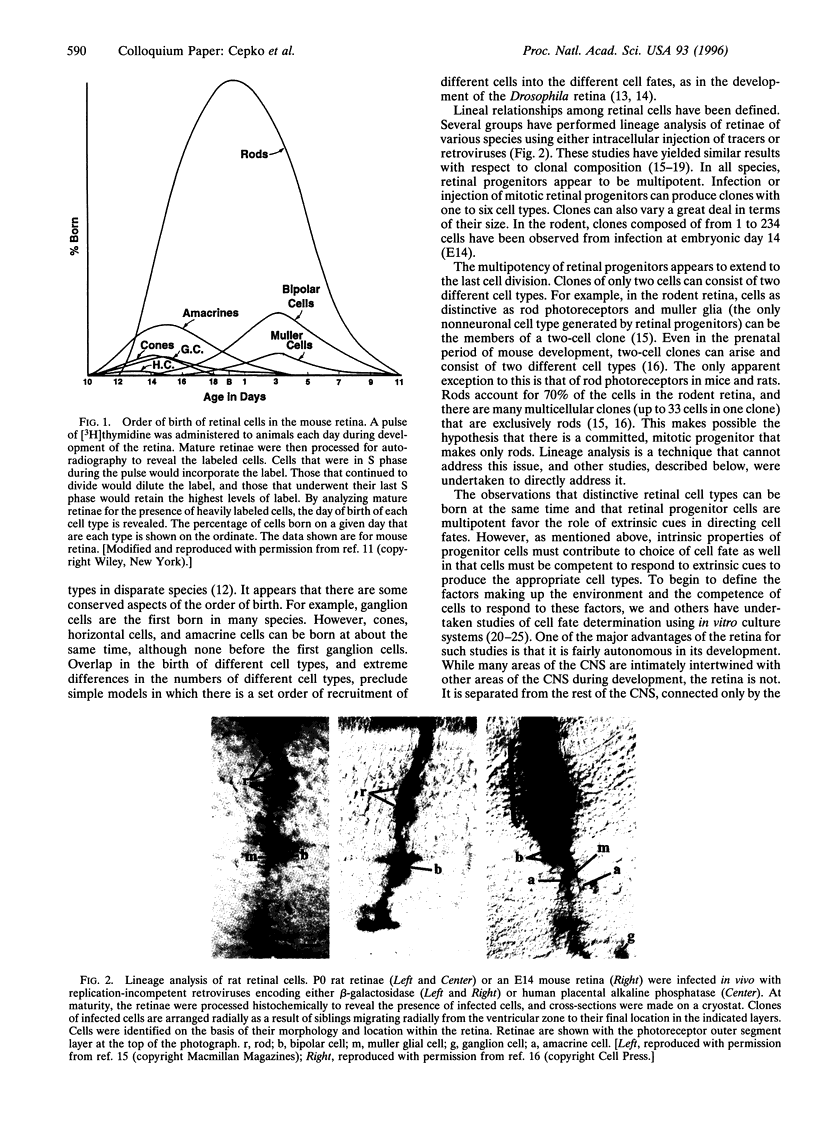
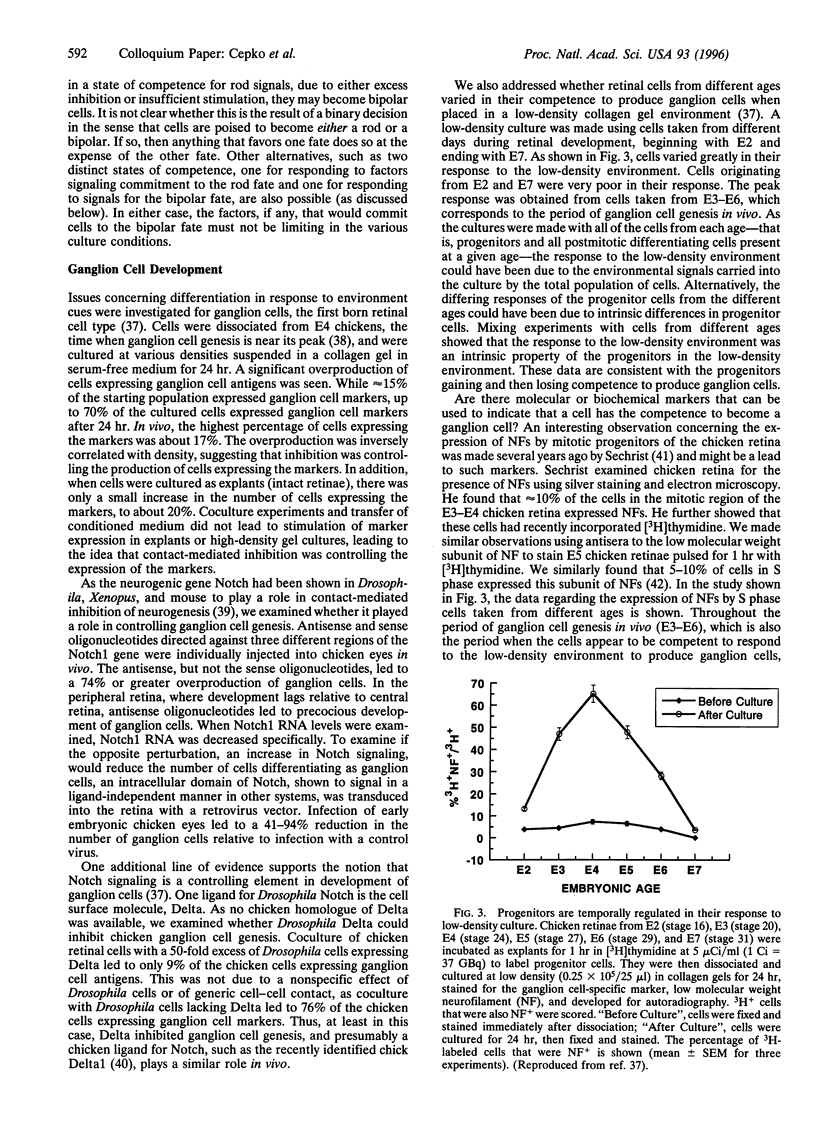
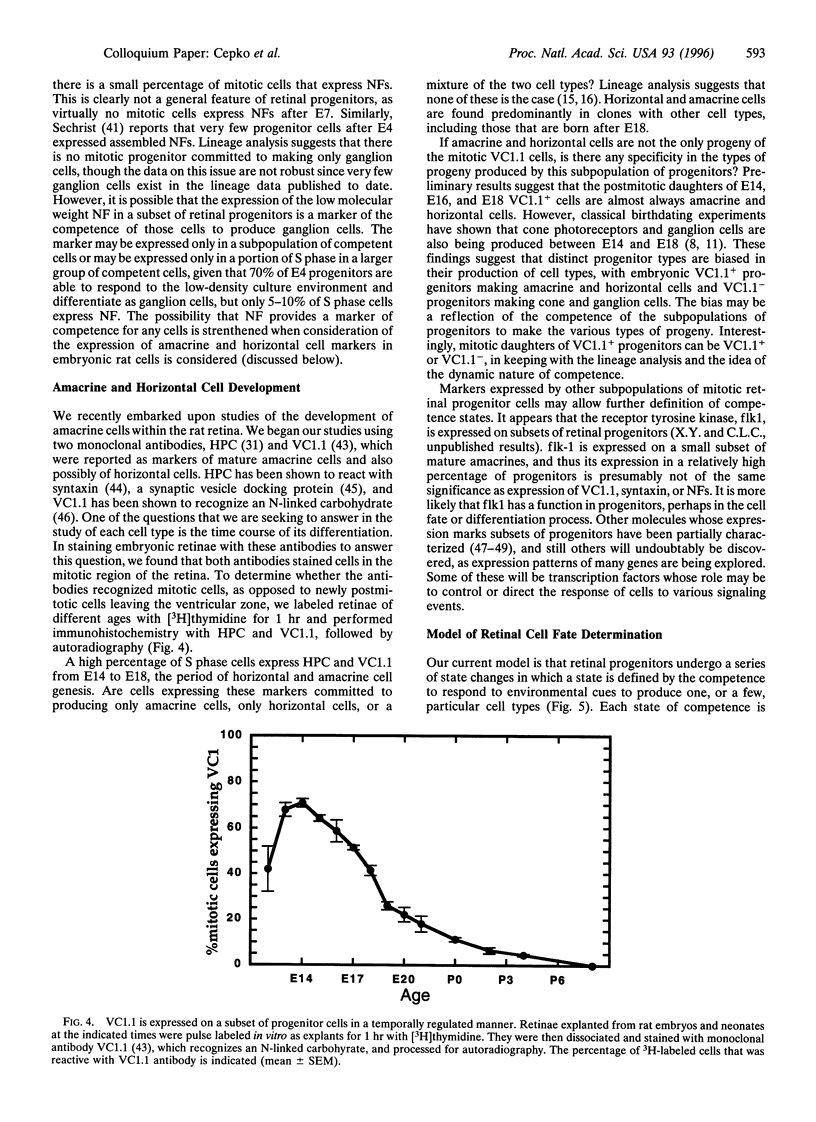
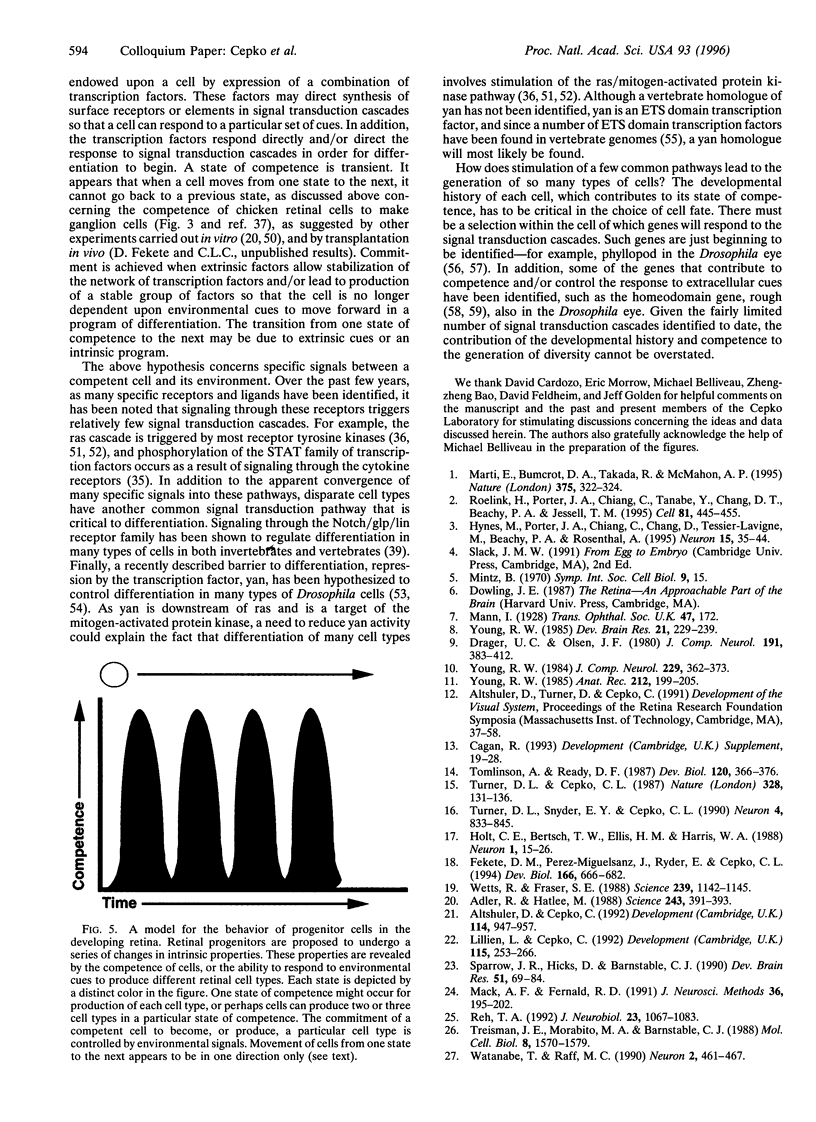
In the vertebrate central nervous system, the retina has been a useful model for studies of cell fate determination. Recent results from studies conducted in vitro and in vivo suggest a model of retinal development in which both the progenitor cells and the environment change over time. The model is based upon the notion that the mitotic cells within the retina change in their response properties, or "competence", during development. These changes presage the ordered appearance of distinct cell types during development and appear to be necessary for the production of the distinct cell types. As the response properties of the cells change, so too do the environmental signals that the cells encounter. Together, intrinsic properties and extrinsic cues direct the choice of cell fate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R., Hatlee M. Plasticity and differentiation of embryonic retinal cells after terminal mitosis. Science. 1989 Jan 20;243(4889):391–393. doi: 10.1126/science.2911751. [DOI] [PubMed] [Google Scholar]
- Altshuler D., Cepko C. A temporally regulated, diffusible activity is required for rod photoreceptor development in vitro. Development. 1992 Apr;114(4):947–957. doi: 10.1242/dev.114.4.947. [DOI] [PubMed] [Google Scholar]
- Altshuler D., Lo Turco J. J., Rush J., Cepko C. Taurine promotes the differentiation of a vertebrate retinal cell type in vitro. Development. 1993 Dec;119(4):1317–1328. doi: 10.1242/dev.119.4.1317. [DOI] [PubMed] [Google Scholar]
- Arimatsu Y., Naegele J. R., Barnstable C. J. Molecular markers of neuronal subpopulations in layers 4, 5, and 6 of cat primary visual cortex. J Neurosci. 1987 Apr;7(4):1250–1263. doi: 10.1523/JNEUROSCI.07-04-01250.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S., Matsuno K., Fortini M. E. Notch signaling. Science. 1995 Apr 14;268(5208):225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- Austin C. P., Feldman D. E., Ida J. A., Jr, Cepko C. L. Vertebrate retinal ganglion cells are selected from competent progenitors by the action of Notch. Development. 1995 Nov;121(11):3637–3650. doi: 10.1242/dev.121.11.3637. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Hofstein R., Akagawa K. A marker of early amacrine cell development in rat retina. Brain Res. 1985 Jun;352(2):286–290. doi: 10.1016/0165-3806(85)90116-6. [DOI] [PubMed] [Google Scholar]
- Basler K., Yen D., Tomlinson A., Hafen E. Reprogramming cell fate in the developing Drosophila retina: transformation of R7 cells by ectopic expression of rough. Genes Dev. 1990 May;4(5):728–739. doi: 10.1101/gad.4.5.728. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Calakos N., Scheller R. H. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992 Jul 10;257(5067):255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Cagan R. Cell fate specification in the developing Drosophila retina. Dev Suppl. 1993:19–28. [PubMed] [Google Scholar]
- Chang H. C., Solomon N. M., Wassarman D. A., Karim F. D., Therrien M., Rubin G. M., Wolff T. phyllopod functions in the fate determination of a subset of photoreceptors in Drosophila. Cell. 1995 Feb 10;80(3):463–472. doi: 10.1016/0092-8674(95)90497-2. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Dickson B. J., Domínguez M., van der Straten A., Hafen E. Control of Drosophila photoreceptor cell fates by phyllopod, a novel nuclear protein acting downstream of the Raf kinase. Cell. 1995 Feb 10;80(3):453–462. doi: 10.1016/0092-8674(95)90496-4. [DOI] [PubMed] [Google Scholar]
- Dräger U. C., Olsen J. F. Origins of crossed and uncrossed retinal projections in pigmented and albino mice. J Comp Neurol. 1980 Jun;191(3):383–412. doi: 10.1002/cne.901910306. [DOI] [PubMed] [Google Scholar]
- Fekete D. M., Perez-Miguelsanz J., Ryder E. F., Cepko C. L. Clonal analysis in the chicken retina reveals tangential dispersion of clonally related cells. Dev Biol. 1994 Dec;166(2):666–682. doi: 10.1006/dbio.1994.1346. [DOI] [PubMed] [Google Scholar]
- Guillemot F., Cepko C. L. Retinal fate and ganglion cell differentiation are potentiated by acidic FGF in an in vitro assay of early retinal development. Development. 1992 Mar;114(3):743–754. doi: 10.1242/dev.114.3.743. [DOI] [PubMed] [Google Scholar]
- Guillemot F., Joyner A. L. Dynamic expression of the murine Achaete-Scute homologue Mash-1 in the developing nervous system. Mech Dev. 1993 Aug;42(3):171–185. doi: 10.1016/0925-4773(93)90006-j. [DOI] [PubMed] [Google Scholar]
- Henrique D., Adam J., Myat A., Chitnis A., Lewis J., Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995 Jun 29;375(6534):787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- Hernández-Sánchez C., Frade J. M., de la Rosa E. J. Heterogeneity among neuroepithelial cells in the chick retina revealed by immunostaining with monoclonal antibody PM1. Eur J Neurosci. 1994 Jan 1;6(1):105–114. doi: 10.1111/j.1460-9568.1994.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Holt C. E., Bertsch T. W., Ellis H. M., Harris W. A. Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron. 1988 Mar;1(1):15–26. doi: 10.1016/0896-6273(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Hynes M., Porter J. A., Chiang C., Chang D., Tessier-Lavigne M., Beachy P. A., Rosenthal A. Induction of midbrain dopaminergic neurons by Sonic hedgehog. Neuron. 1995 Jul;15(1):35–44. doi: 10.1016/0896-6273(95)90062-4. [DOI] [PubMed] [Google Scholar]
- Inoue A., Obata K., Akagawa K. Cloning and sequence analysis of cDNA for a neuronal cell membrane antigen, HPC-1. J Biol Chem. 1992 May 25;267(15):10613–10619. [PubMed] [Google Scholar]
- Ip N. Y., McClain J., Barrezueta N. X., Aldrich T. H., Pan L., Li Y., Wiegand S. J., Friedman B., Davis S., Yancopoulos G. D. The alpha component of the CNTF receptor is required for signaling and defines potential CNTF targets in the adult and during development. Neuron. 1993 Jan;10(1):89–102. doi: 10.1016/0896-6273(93)90245-m. [DOI] [PubMed] [Google Scholar]
- Jasoni C. L., Walker M. B., Morris M. D., Reh T. A. A chicken achaete-scute homolog (CASH-1) is expressed in a temporally and spatially discrete manner in the developing nervous system. Development. 1994 Apr;120(4):769–783. doi: 10.1242/dev.120.4.769. [DOI] [PubMed] [Google Scholar]
- Kelley M. W., Turner J. K., Reh T. A. Retinoic acid promotes differentiation of photoreceptors in vitro. Development. 1994 Aug;120(8):2091–2102. doi: 10.1242/dev.120.8.2091. [DOI] [PubMed] [Google Scholar]
- Kimmel B. E., Heberlein U., Rubin G. M. The homeo domain protein rough is expressed in a subset of cells in the developing Drosophila eye where it can specify photoreceptor cell subtype. Genes Dev. 1990 May;4(5):712–727. doi: 10.1101/gad.4.5.712. [DOI] [PubMed] [Google Scholar]
- Lillien L., Cepko C. Control of proliferation in the retina: temporal changes in responsiveness to FGF and TGF alpha. Development. 1992 May;115(1):253–266. doi: 10.1242/dev.115.1.253. [DOI] [PubMed] [Google Scholar]
- Lin L. F., Mismer D., Lile J. D., Armes L. G., Butler E. T., 3rd, Vannice J. L., Collins F. Purification, cloning, and expression of ciliary neurotrophic factor (CNTF). Science. 1989 Nov 24;246(4933):1023–1025. doi: 10.1126/science.2587985. [DOI] [PubMed] [Google Scholar]
- Mack A. F., Fernald R. D. Thin slices of teleost retina continue to grow in culture. J Neurosci Methods. 1991 Feb;36(2-3):195–202. doi: 10.1016/0165-0270(91)90045-2. [DOI] [PubMed] [Google Scholar]
- Martí E., Bumcrot D. A., Takada R., McMahon A. P. Requirement of 19K form of Sonic hedgehog for induction of distinct ventral cell types in CNS explants. Nature. 1995 May 25;375(6529):322–325. doi: 10.1038/375322a0. [DOI] [PubMed] [Google Scholar]
- Naegele J. R., Barnstable C. J. A carbohydrate epitope defined by monoclonal antibody VC1.1 is found on N-CAM and other cell adhesion molecules. Brain Res. 1991 Sep 13;559(1):118–129. doi: 10.1016/0006-8993(91)90294-6. [DOI] [PubMed] [Google Scholar]
- O'Neill E. M., Rebay I., Tjian R., Rubin G. M. The activities of two Ets-related transcription factors required for Drosophila eye development are modulated by the Ras/MAPK pathway. Cell. 1994 Jul 15;78(1):137–147. doi: 10.1016/0092-8674(94)90580-0. [DOI] [PubMed] [Google Scholar]
- Pawson T., Bernstein A. Receptor tyrosine kinases: genetic evidence for their role in Drosophila and mouse development. Trends Genet. 1990 Nov;6(11):350–356. doi: 10.1016/0168-9525(90)90276-c. [DOI] [PubMed] [Google Scholar]
- Prada Carmen, Puga José, Pérez-Méndez Luisa, López Rosario, Ramírez Galo. Spatial and Temporal Patterns of Neurogenesis in the Chick Retina. Eur J Neurosci. 1991 Jun;3(6):559–569. doi: 10.1111/j.1460-9568.1991.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Rebay I., Rubin G. M. Yan functions as a general inhibitor of differentiation and is negatively regulated by activation of the Ras1/MAPK pathway. Cell. 1995 Jun 16;81(6):857–866. doi: 10.1016/0092-8674(95)90006-3. [DOI] [PubMed] [Google Scholar]
- Reh T. A. Cellular interactions determine neuronal phenotypes in rodent retinal cultures. J Neurobiol. 1992 Oct;23(8):1067–1083. doi: 10.1002/neu.480230811. [DOI] [PubMed] [Google Scholar]
- Reh T. A., Kljavin I. J. Age of differentiation determines rat retinal germinal cell phenotype: induction of differentiation by dissociation. J Neurosci. 1989 Dec;9(12):4179–4189. doi: 10.1523/JNEUROSCI.09-12-04179.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelink H., Porter J. A., Chiang C., Tanabe Y., Chang D. T., Beachy P. A., Jessell T. M. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995 May 5;81(3):445–455. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Ullrich A. Growth factor signaling by receptor tyrosine kinases. Neuron. 1992 Sep;9(3):383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- Sechrist J. W. Neurocytogenesis. I. Neurofibrils, neurofilaments, and the terminal mitotic cycle. Am J Anat. 1969 Jan;124(1):117–133. doi: 10.1002/aja.1001240108. [DOI] [PubMed] [Google Scholar]
- Sparrow J. R., Hicks D., Barnstable C. J. Cell commitment and differentiation in explants of embryonic rat neural retina. Comparison with the developmental potential of dissociated retina. Brain Res Dev Brain Res. 1990 Jan 1;51(1):69–84. doi: 10.1016/0165-3806(90)90259-2. [DOI] [PubMed] [Google Scholar]
- Tomlinson A., Ready D. F. Neuronal differentiation in Drosophila ommatidium. Dev Biol. 1987 Apr;120(2):366–376. doi: 10.1016/0012-1606(87)90239-9. [DOI] [PubMed] [Google Scholar]
- Treisman J. E., Morabito M. A., Barnstable C. J. Opsin expression in the rat retina is developmentally regulated by transcriptional activation. Mol Cell Biol. 1988 Apr;8(4):1570–1579. doi: 10.1128/mcb.8.4.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. L., Cepko C. L. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987 Jul 9;328(6126):131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Turner D. L., Snyder E. Y., Cepko C. L. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron. 1990 Jun;4(6):833–845. doi: 10.1016/0896-6273(90)90136-4. [DOI] [PubMed] [Google Scholar]
- Waid D. K., McLoon S. C. Immediate differentiation of ganglion cells following mitosis in the developing retina. Neuron. 1995 Jan;14(1):117–124. doi: 10.1016/0896-6273(95)90245-7. [DOI] [PubMed] [Google Scholar]
- Wassarman D. A., Therrien M., Rubin G. M. The Ras signaling pathway in Drosophila. Curr Opin Genet Dev. 1995 Feb;5(1):44–50. doi: 10.1016/s0959-437x(95)90052-7. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Hahn S. L., Giovane A. The Ets family of transcription factors. Eur J Biochem. 1993 Jan 15;211(1-2):7–18. doi: 10.1007/978-3-642-78757-7_2. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Raff M. C. Rod photoreceptor development in vitro: intrinsic properties of proliferating neuroepithelial cells change as development proceeds in the rat retina. Neuron. 1990 Mar;4(3):461–467. doi: 10.1016/0896-6273(90)90058-n. [DOI] [PubMed] [Google Scholar]
- Wetts R., Fraser S. E. Multipotent precursors can give rise to all major cell types of the frog retina. Science. 1988 Mar 4;239(4844):1142–1145. doi: 10.1126/science.2449732. [DOI] [PubMed] [Google Scholar]
- Yamamori T., Fukada K., Aebersold R., Korsching S., Fann M. J., Patterson P. H. The cholinergic neuronal differentiation factor from heart cells is identical to leukemia inhibitory factor. Science. 1989 Dec 15;246(4936):1412–1416. doi: 10.1126/science.2512641. [DOI] [PubMed] [Google Scholar]
- Young R. W. Cell death during differentiation of the retina in the mouse. J Comp Neurol. 1984 Nov 1;229(3):362–373. doi: 10.1002/cne.902290307. [DOI] [PubMed] [Google Scholar]
- Young R. W. Cell differentiation in the retina of the mouse. Anat Rec. 1985 Jun;212(2):199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]




