Abstract
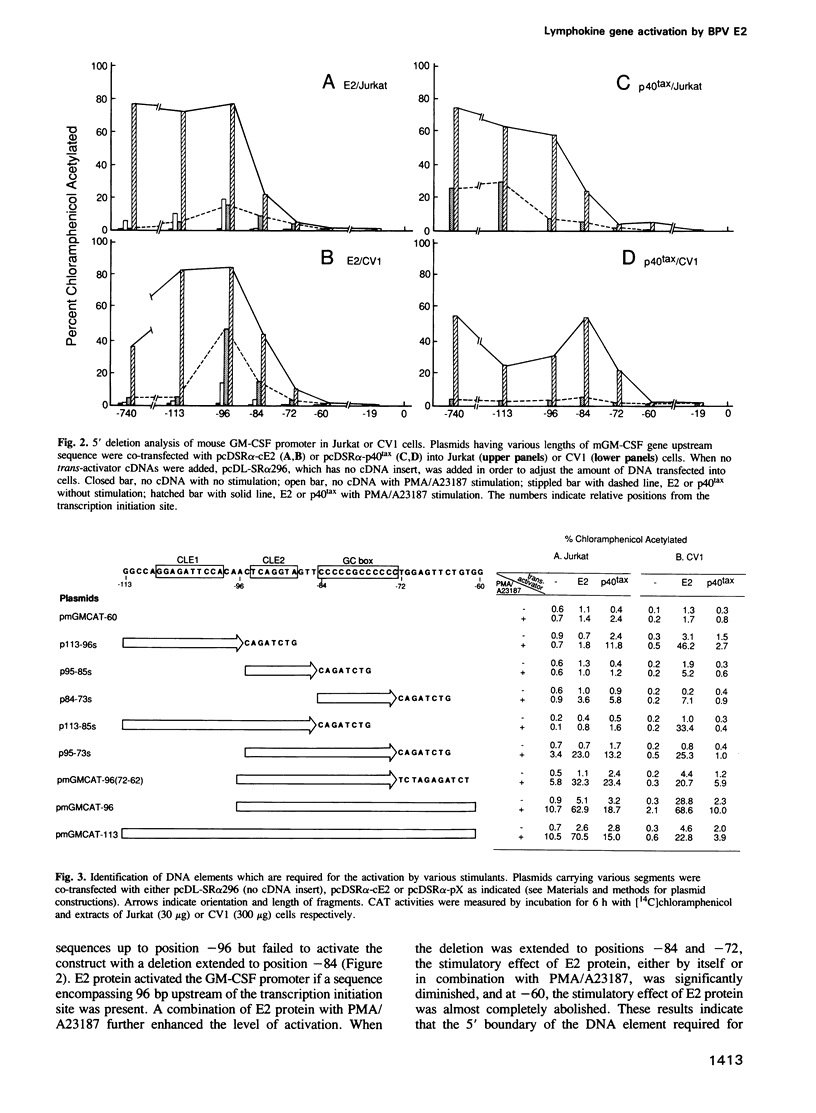
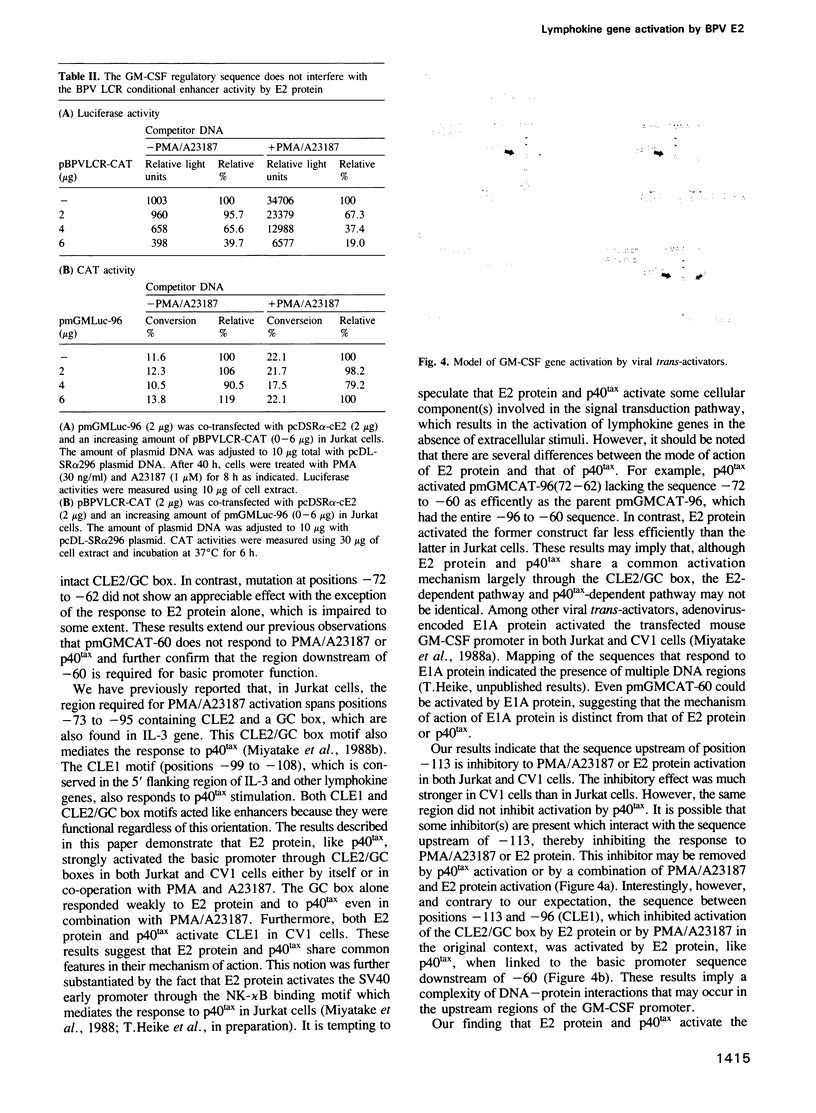
Activation of T cells by antigen, lectin or a combination of phorbol ester (PMA) and calcium ionophore (A23187) leads to the induction of a set of lymphokine genes. Transfection of a human T cell leukemia cell line, Jurkat, or an African green monkey kidney cell line, CV1, with a cDNA encoding E2 protein, a trans-activator of bovine papilloma virus type 1, results in activation of interleukin 2 (IL-2), interleukin 3 (IL-3) and granulocyte/macrophage colony-stimulating factor (GM-CSF) genes in a transient transfection assay. 5' deletion and mutation analyses showed that the sequence between positions -60 and a TATA-like sequence is required for basic promotor function and that the sequence between positions -95 and -73 containing conserved lymphokine element 2 (CLE2) and a GC box (CLE2/GC box) mediates the positive response to E2 protein. The latter has been previously shown to respond to PMA/A23187 stimulation or to p40tax, a trans-activator encoded by human T cell leukemia virus type 1 (HTLV-I). The sequence located between -108 and -99 (CLE1) is inhibitory to E2 protein or PMA/A23187 stimulation. The combination of E2 protein and PMA/A23187 appears to eliminate an inhibitory effect of the upstream region. However, E2 protein, like p40tax, mediates a positive response through CLE1 alone linked to the basic promoter sequence. The level of activation of the long control region (LCR) by E2 protein is unaffected by the number of CLE2/GC box sequences.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Androphy E. J., Lowy D. R., Schiller J. T. Bovine papillomavirus E2 trans-activating gene product binds to specific sites in papillomavirus DNA. Nature. 1987 Jan 1;325(6099):70–73. doi: 10.1038/325070a0. [DOI] [PubMed] [Google Scholar]
- Arai N., Nomura D., Villaret D., DeWaal Malefijt R., Seiki M., Yoshida M., Minoshima S., Fukuyama R., Maekawa M., Kudoh J. Complete nucleotide sequence of the chromosomal gene for human IL-4 and its expression. J Immunol. 1989 Jan 1;142(1):274–282. [PubMed] [Google Scholar]
- Bell T. V., Harley C. B., Stetsko D., Sauder D. N. Expression of mRNA homologous to interleukin 1 in human epidermal cells. J Invest Dermatol. 1987 Apr;88(4):375–379. doi: 10.1111/1523-1747.ep12469041. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Howley P. M., Levinson A. D., Seeburg P. H. The primary structure and genetic organization of the bovine papillomavirus type 1 genome. Nature. 1982 Oct 7;299(5883):529–534. doi: 10.1038/299529a0. [DOI] [PubMed] [Google Scholar]
- Chen I. S., Slamon D. J., Rosenblatt J. D., Shah N. P., Quan S. G., Wachsman W. The x gene is essential for HTLV replication. Science. 1985 Jul 5;229(4708):54–58. doi: 10.1126/science.2990037. [DOI] [PubMed] [Google Scholar]
- Coleman D. L., Kupper T. S., Flood P. M., Fultz C. C., Horowitz M. C. Characterization of a keratinocyte-derived T cell growth factor distinct from interleukin 2 and B cell stimulatory factor 1. J Immunol. 1987 May 15;138(10):3314–3318. [PubMed] [Google Scholar]
- Cross S. L., Feinberg M. B., Wolf J. B., Holbrook N. J., Wong-Staal F., Leonard W. J. Regulation of the human interleukin-2 receptor alpha chain promoter: activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell. 1987 Apr 10;49(1):47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- Depper J. M., Leonard W. J., Krönke M., Waldmann T. A., Greene W. C. Augmented T cell growth factor receptor expression in HTLV-1-infected human leukemic T cells. J Immunol. 1984 Oct;133(4):1691–1695. [PubMed] [Google Scholar]
- Gasson J. C., Weisbart R. H., Kaufman S. E., Clark S. C., Hewick R. M., Wong G. G., Golde D. W. Purified human granulocyte-macrophage colony-stimulating factor: direct action on neutrophils. Science. 1984 Dec 14;226(4680):1339–1342. doi: 10.1126/science.6390681. [DOI] [PubMed] [Google Scholar]
- Giri I., Yaniv M. Structural and mutational analysis of E2 trans-activating proteins of papillomaviruses reveals three distinct functional domains. EMBO J. 1988 Sep;7(9):2823–2829. doi: 10.1002/j.1460-2075.1988.tb03138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen T. H., Turek L. P., Mercurio F. M., Cripe T. P., Olson B. J., Anderson R. D., Seidl D., Karin M., Schiller J. Sequence-specific and general transcriptional activation by the bovine papillomavirus-1 E2 trans-activator require an N-terminal amphipathic helix-containing E2 domain. EMBO J. 1988 Dec 20;7(13):4245–4253. doi: 10.1002/j.1460-2075.1988.tb03322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley-Nelson P., Androphy E. J., Lowy D. R., Schiller J. T. The specific DNA recognition sequence of the bovine papillomavirus E2 protein is an E2-dependent enhancer. EMBO J. 1988 Feb;7(2):525–531. doi: 10.1002/j.1460-2075.1988.tb02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Seiki M., Taniguchi T., Tsuru S., Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 1986 Nov;5(11):2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Kupper T. S., Ballard D. W., Chua A. O., McGuire J. S., Flood P. M., Horowitz M. C., Langdon R., Lightfoot L., Gubler U. Human keratinocytes contain mRNA indistinguishable from monocyte interleukin 1 alpha and beta mRNA. Keratinocyte epidermal cell-derived thymocyte-activating factor is identical to interleukin 1. J Exp Med. 1986 Dec 1;164(6):2095–2100. doi: 10.1084/jem.164.6.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P. F., Spalholz B. A., Howley P. M. A transcriptional repressor encoded by BPV-1 shares a common carboxy-terminal domain with the E2 transactivator. Cell. 1987 Jul 3;50(1):69–78. doi: 10.1016/0092-8674(87)90663-5. [DOI] [PubMed] [Google Scholar]
- Luger T. A., Wirth U., Köck A. Epidermal cells synthesize a cytokine with interleukin 3-like properties. J Immunol. 1985 Feb;134(2):915–919. [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Characterization of the bovine papilloma virus plasmid maintenance sequences. Cell. 1984 Feb;36(2):391–401. doi: 10.1016/0092-8674(84)90232-0. [DOI] [PubMed] [Google Scholar]
- Maruyama M., Shibuya H., Harada H., Hatakeyama M., Seiki M., Fujita T., Inoue J., Yoshida M., Taniguchi T. Evidence for aberrant activation of the interleukin-2 autocrine loop by HTLV-1-encoded p40x and T3/Ti complex triggering. Cell. 1987 Jan 30;48(2):343–350. doi: 10.1016/0092-8674(87)90437-5. [DOI] [PubMed] [Google Scholar]
- McBride A. A., Schlegel R., Howley P. M. The carboxy-terminal domain shared by the bovine papillomavirus E2 transactivator and repressor proteins contains a specific DNA binding activity. EMBO J. 1988 Feb;7(2):533–539. doi: 10.1002/j.1460-2075.1988.tb02842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake S., Seiki M., Malefijt R. D., Heike T., Fujisawa J., Takebe Y., Nishida J., Shlomai J., Yokota T., Yoshida M. Activation of T cell-derived lymphokine genes in T cells and fibroblasts: effects of human T cell leukemia virus type I p40x protein and bovine papilloma virus encoded E2 protein. Nucleic Acids Res. 1988 Jul 25;16(14A):6547–6566. doi: 10.1093/nar/16.14.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Rabson M. S., Yee C., Yang Y. C., Howley P. M. Bovine papillomavirus type 1 3' early region transformation and plasmid maintenance functions. J Virol. 1986 Nov;60(2):626–634. doi: 10.1128/jvi.60.2.626-634.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver N., Rabson M. S., Yang Y. C., Byrne J. C., Howley P. M. Localization and analysis of bovine papillomavirus type 1 transforming functions. J Virol. 1984 Nov;52(2):377–388. doi: 10.1128/jvi.52.2.377-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Hirano T., Ishibashi K., Nakano N., Taga T., Sugamura K., Yamamura Y., Kishimoto T. Immortalization of BGDF (BCGF II)- and BCDF-producing T cells by human T cell leukemia virus (HTLV) and characterization of human BGDF (BCGF II). J Immunol. 1985 Mar;134(3):1728–1733. [PubMed] [Google Scholar]
- Spalholz B. A., Lambert P. F., Yee C. L., Howley P. M. Bovine papillomavirus transcriptional regulation: localization of the E2-responsive elements of the long control region. J Virol. 1987 Jul;61(7):2128–2137. doi: 10.1128/jvi.61.7.2128-2137.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalholz B. A., Yang Y. C., Howley P. M. Transactivation of a bovine papilloma virus transcriptional regulatory element by the E2 gene product. Cell. 1985 Aug;42(1):183–191. doi: 10.1016/s0092-8674(85)80114-8. [DOI] [PubMed] [Google Scholar]
- Stanley E., Metcalf D., Sobieszczuk P., Gough N. M., Dunn A. R. The structure and expression of the murine gene encoding granulocyte-macrophage colony stimulating factor: evidence for utilisation of alternative promoters. EMBO J. 1985 Oct;4(10):2569–2573. doi: 10.1002/j.1460-2075.1985.tb03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck W., Rösl F., Zentgraf H. Origin of replication in episomal bovine papilloma virus type 1 DNA isolated from transformed cells. EMBO J. 1984 Sep;3(9):2173–2178. doi: 10.1002/j.1460-2075.1984.tb02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Wiskocil R. L., Stobo J. D. The role of T3 surface molecules in the activation of human T cells: a two-stimulus requirement for IL 2 production reflects events occurring at a pre-translational level. J Immunol. 1984 Jul;133(1):123–128. [PubMed] [Google Scholar]
- Yang Y. C., Okayama H., Howley P. M. Bovine papillomavirus contains multiple transforming genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1030–1034. doi: 10.1073/pnas.82.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Arai N., de Vries J., Spits H., Banchereau J., Zlotnik A., Rennick D., Howard M., Takebe Y., Miyatake S. Molecular biology of interleukin 4 and interleukin 5 genes and biology of their products that stimulate B cells, T cells and hemopoietic cells. Immunol Rev. 1988 Feb;102:137–187. doi: 10.1111/j.1600-065x.1988.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Seiki M. Recent advances in the molecular biology of HTLV-1: trans-activation of viral and cellular genes. Annu Rev Immunol. 1987;5:541–559. doi: 10.1146/annurev.iy.05.040187.002545. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]