Abstract
The segmentation gene, giant, is located in 3A1 within a cloned chromosome region surrounding the zeste locus. Rearrangement breakpoints associated with giant mutations were localized on the genomic clone map, and nearby transcription units were identified. One transcription unit is active during early embryogenesis and its transcripts are spatially localized from blastoderm into extended germband stages, consistent with expected expression patterns predicted by the 'gap' phenotype of giant mutants. Germ line transformation experiments using a 10-kb DNA fragment containing this transcription unit gave complete rescue of the abdominal giant defect but only partial correction of the head defect. The effect of mutations in three other gap loci, Kr, kni and hb, were also analyzed.
Full text
PDF


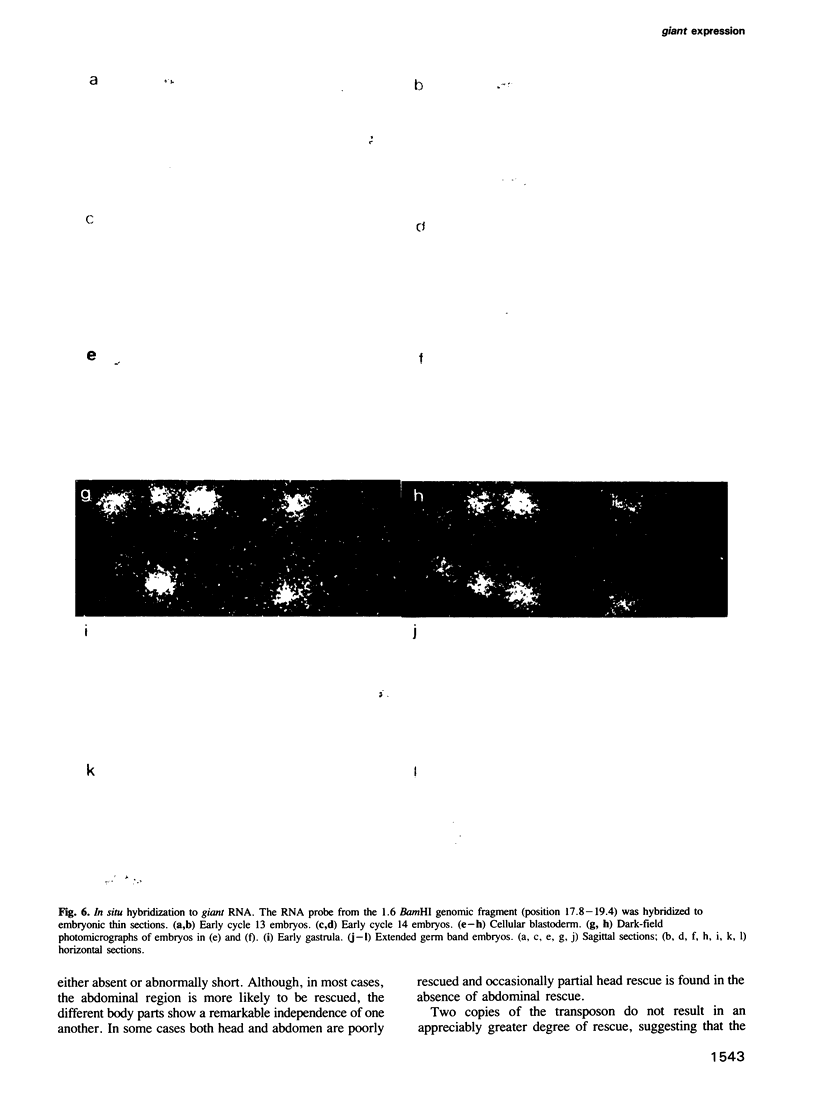
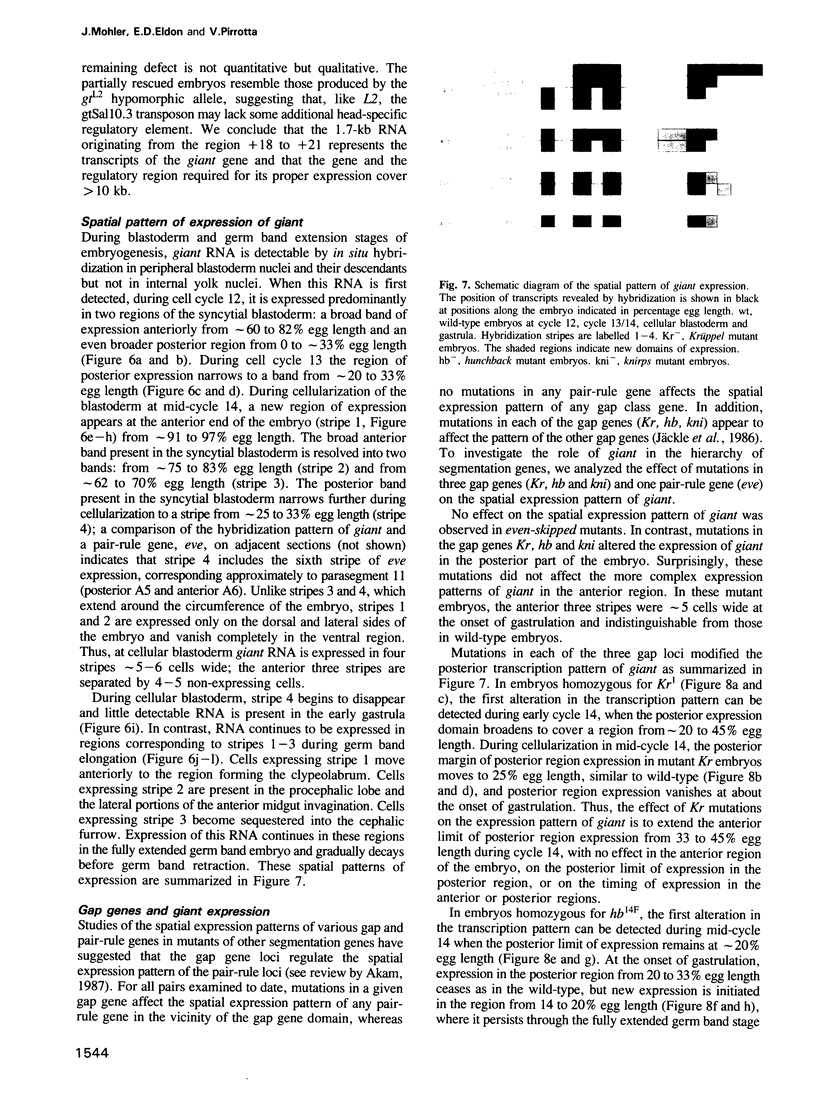
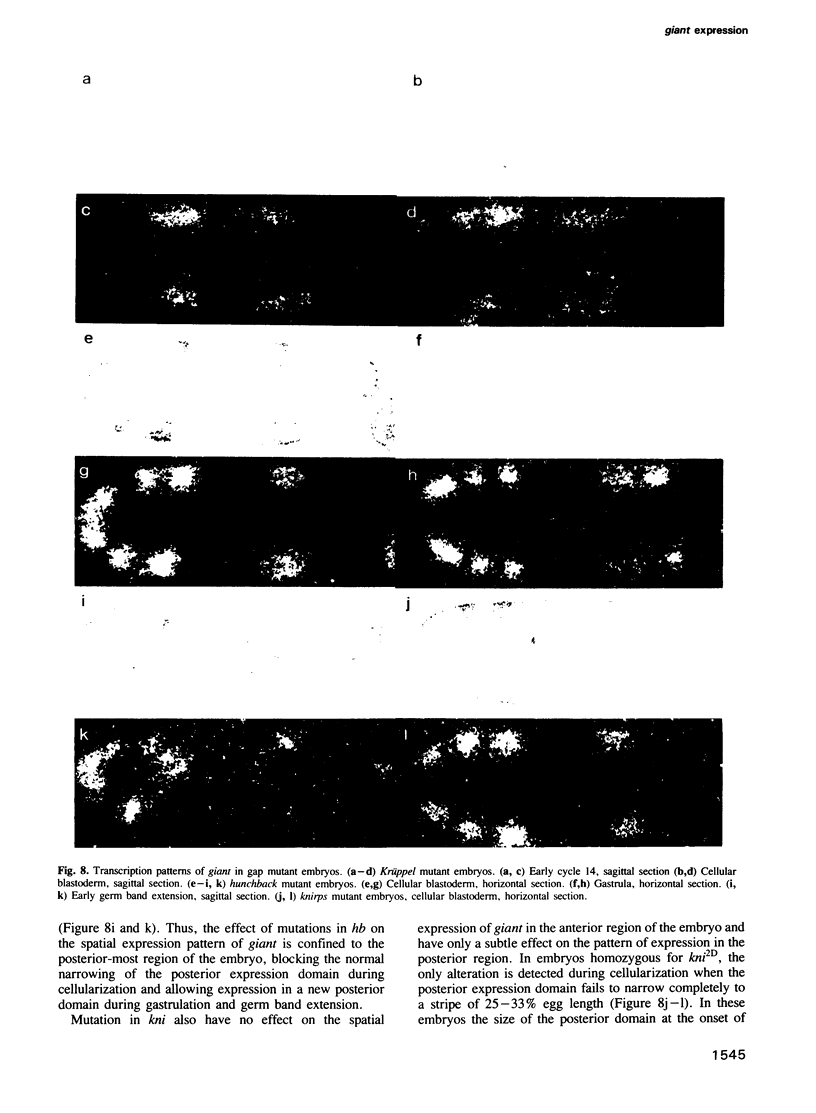



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987 Sep;101(1):1–22. [PubMed] [Google Scholar]
- Baker N. E. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J. 1987 Jun;6(6):1765–1773. doi: 10.1002/j.1460-2075.1987.tb02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner S., Bopp D., Burri M., Noll M. Structure of two genes at the gooseberry locus related to the paired gene and their spatial expression during Drosophila embryogenesis. Genes Dev. 1987 Dec;1(10):1247–1267. doi: 10.1101/gad.1.10.1247. [DOI] [PubMed] [Google Scholar]
- Carroll S. B., Scott M. P. Zygotically active genes that affect the spatial expression of the fushi tarazu segmentation gene during early Drosophila embryogenesis. Cell. 1986 Apr 11;45(1):113–126. doi: 10.1016/0092-8674(86)90543-x. [DOI] [PubMed] [Google Scholar]
- Frasch M., Levine M. Complementary patterns of even-skipped and fushi tarazu expression involve their differential regulation by a common set of segmentation genes in Drosophila. Genes Dev. 1987 Nov;1(9):981–995. doi: 10.1101/gad.1.9.981. [DOI] [PubMed] [Google Scholar]
- Gergen J. P., Wieschaus E. F. The localized requirements for a gene affecting segmentation in Drosophila: analysis of larvae mosaic for runt. Dev Biol. 1985 Jun;109(2):321–335. doi: 10.1016/0012-1606(85)90459-2. [DOI] [PubMed] [Google Scholar]
- Gergen J. P., Wieschaus E. Dosage requirements for runt in the segmentation of Drosophila embryos. Cell. 1986 Apr 25;45(2):289–299. doi: 10.1016/0092-8674(86)90393-4. [DOI] [PubMed] [Google Scholar]
- Harding K., Rushlow C., Doyle H. J., Hoey T., Levine M. Cross-regulatory interactions among pair-rule genes in Drosophila. Science. 1986 Aug 29;233(4767):953–959. doi: 10.1126/science.3755551. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Pinchin S. M. Pattern abnormalities induced by ectopic expression of the Drosophila gene hairy are associated with repression of ftz transcription. Cell. 1987 Nov 6;51(3):405–415. doi: 10.1016/0092-8674(87)90636-2. [DOI] [PubMed] [Google Scholar]
- Jürgens G. Head and tail development of the Drosophila embryo involves spalt, a novel homeotic gene. EMBO J. 1988 Jan;7(1):189–196. doi: 10.1002/j.1460-2075.1988.tb02799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipple D. C., Seifert E., Rosenberg U. B., Preiss A., Jäckle H. Spatial and temporal patterns of Krüppel gene expression in early Drosophila embryos. Nature. 1985 Sep 5;317(6032):40–44. doi: 10.1038/317040a0. [DOI] [PubMed] [Google Scholar]
- Kornberg T., Sidén I., O'Farrell P., Simon M. The engrailed locus of Drosophila: in situ localization of transcripts reveals compartment-specific expression. Cell. 1985 Jan;40(1):45–53. doi: 10.1016/0092-8674(85)90307-1. [DOI] [PubMed] [Google Scholar]
- Mariani C., Pirrotta V., Manet E. Isolation and characterization of the zeste locus of Drosophila. EMBO J. 1985 Aug;4(8):2045–2052. doi: 10.1002/j.1460-2075.1985.tb03890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narachi M. A., Boyd J. B. The giant (gt) mutants of Drosophila melanogaster alter DNA metabolism. Mol Gen Genet. 1985;199(3):500–506. doi: 10.1007/BF00330765. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980 Oct 30;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Petschek J. P., Perrimon N., Mahowald A. P. Region-specific defects in l(1)giant embryos of Drosophila melanogaster. Dev Biol. 1987 Jan;119(1):175–189. doi: 10.1016/0012-1606(87)90219-3. [DOI] [PubMed] [Google Scholar]
- Pirrotta V., Manet E., Hardon E., Bickel S. E., Benson M. Structure and sequence of the Drosophila zeste gene. EMBO J. 1987 Mar;6(3):791–799. doi: 10.1002/j.1460-2075.1987.tb04821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royden C. S., Pirrotta V., Jan L. Y. The tko locus, site of a behavioral mutation in D. melanogaster, codes for a protein homologous to prokaryotic ribosomal protein S12. Cell. 1987 Oct 23;51(2):165–173. doi: 10.1016/0092-8674(87)90144-9. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Vectors for P element-mediated gene transfer in Drosophila. Nucleic Acids Res. 1983 Sep 24;11(18):6341–6351. doi: 10.1093/nar/11.18.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. B., Imberski R. B., Kelly T. J. Analysis of metamorphosis in Drosophila melanogaster: characterization of giant, an ecdysteroid-deficient mutant. Dev Biol. 1984 May;103(1):85–95. doi: 10.1016/0012-1606(84)90010-1. [DOI] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. A transposable P vector that confers selectable G418 resistance to Drosophila larvae. EMBO J. 1985 Jan;4(1):167–171. doi: 10.1002/j.1460-2075.1985.tb02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. P transposons controlled by the heat shock promoter. Mol Cell Biol. 1986 May;6(5):1640–1649. doi: 10.1128/mcb.6.5.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G., Akam M. Altered distributions of Ultrabithorax transcripts in extra sex combs mutant embryos of Drosophila. EMBO J. 1985 Dec 1;4(12):3259–3264. doi: 10.1002/j.1460-2075.1985.tb04075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]