Abstract
ATP-sensitive potassium (KATP) channels are cell metabolic sensors that couple cell metabolic status to electric activity, thus regulating many cellular functions. In pancreatic beta cells, KATP channels modulate insulin secretion in response to fluctuations in plasma glucose level, and play an important role in glucose homeostasis. Recent studies show that gain-of-function and loss-of-function mutations in KATP channel subunits cause neonatal diabetes mellitus and congenital hyperinsulinism respectively. These findings lead to significant changes in the diagnosis and treatment for neonatal insulin secretion disorders. This review describes the physiological and pathophysiological functions of KATP channels in glucose homeostasis, their specific roles in neonatal diabetes mellitus and congenital hyperinsulinism, as well as future perspectives of KATP channels in neonatal diseases.
Keywords: ATP-sensitive potassium (KATP) channels, channelopathy, neonatal diabetes, congenital hyperinsulinism, glucose homeostasis, diazoxide, sulfonylureas
Introduction
Adenosine triphosphate (ATP)-sensitive potassium (KATP) channels function as metabolic sensors that are capable of coupling a cell's metabolic status to electrical activity in order to regulate many cellular functions. The KATP channels are expressed extensively in various cell types, including pancreatic beta cells, skeletal muscles, smooth muscles, adipose tissue, cardiomyocytes and neurons, where they regulate cell excitability. In pancreatic beta cells, KATP channels are capable of modulating insulin secretion in response to fluctuations in plasma glucose levels, and thus are an important regulator of glucose homeostasis. KATP channel mutations mediated dysfunctions are associated with a variety of insulin secretion disorders, including neonatal diabetes mellitus and congenital hyperinsulinism. Fortunately, the identification of the role of KATP channels in these diseases has led to the implementation of new and improved clinical diagnoses and treatment practices. This review provides an overview of KATP channels, the role they play in the pathophysiology of neonatal diabetes and congenital hyperinsulinism, and the new therapeutic approaches developed based on our current understanding of these diseases. It also discusses the current issues associated with the use of KATP channel modulators in treating these neonatal diseases.
Structure of KATP channel subunits
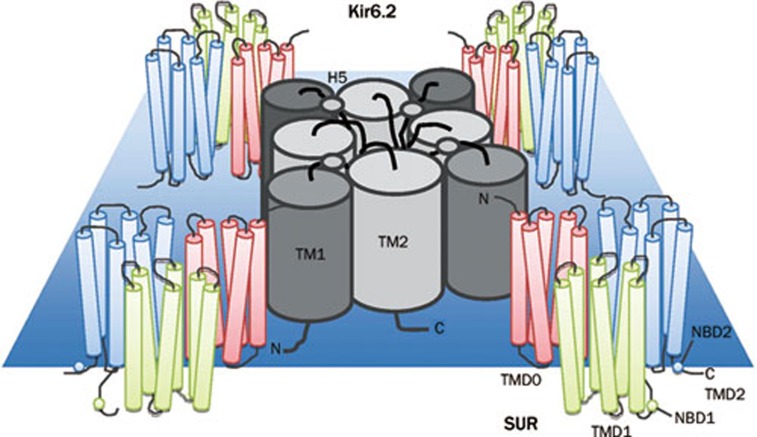
Functional KATP channels are octomeric protein structures composed of four Kir6.x subunits that form the channel pore, surrounded by four sulfonylurea receptors (SURs) that regulate the channel pore activity1 (Figure 1). The Kir6.x subunits (either Kir6.1 or Kir6.2) belong to the superfamily of weak inwardly rectifying, voltage independent potassium (K+) channels2. These channels generate large K+ conductance at potentials negative to the equilibrium potential of potassium (EK), but permit less current flow at more positive potentials. This, in conjunction with their voltage-independence, makes Kir channels one of the major regulators of resting membrane potentials. Furthermore, the unique sensitivity to ATP/ADP levels makes KATP channels the ideal cell metabolic sensors3. That is, KATP channels are able to regulate the membrane excitability in response to the metabolic status of the cell, thus, regulating many cellular functions, such as insulin secretion, excitability and cytoprotection.
Figure 1.
Schematic of the domain structure of a KATP channel. Four Kir6.2 subunits form the channel pore, and are surrounded by four sulfonylurea receptors (SURs) that regulate pore activity. Each Kir6.2 subunit has two domains (TM1 and TM2), linked by an extracellular pore-forming region (H5). Each SUR subunit has 17 transmembrane regions grouped into three domains: TMD0 (TM1-5), TMD1 (TM 6-11), and TMD2 (TM 12-17). Each SUR subunit also has two nucleotide binding domains (NBD).
KATP channels were first discovered in cardiomyocytes4, and their expression was subsequently confirmed in pancreatic beta cells5, 6, skeletal muscles7, 8, vascular smooth muscles9, adipose tissue10, glial cells11, and neurons12, 13, 14. In the majority of these tissues, the channel pore is formed by four Kir6.2 subunits15. Each Kir6.2 subunit has two transmembrane (TM) domains (TM1 and TM2) linked by an extracellular pore-forming region (H5)2, 3. The four TM2 domains in a KATP channel form the channel pore and the H5 region serves as the potassium selectivity filter, which contains the Kir6.2 channel signature sequence GFG (rather than GYG in the K+ channel)2. The amino and carboxyl terminals are both found cytoplasmically, and join together to form the cytoplasmic domain that is responsible for channel gating and ATP binding2, 3, 16. Like other Kir subunits, it lacks the S4 voltage sensor region that is critical for gating in all voltage dependent calcium (Ca2+), sodium (Na+) and potassium (K+) channels. Therefore, KATP channels are constitutively active if other regulatory mechanisms, such as SUR subunits, or ATP are absent.
The SUR regulatory subunits belong to the ATP-binding cassette (ABC) transporter family17. Each SUR subunit has 17 TM regions, grouped into three transmembrane domains (TMDs). TMD1 (TM6-11) and TMD2 (TM12-17) are conserved among members of the ABC family. TMD0 (TM1-5) is unique to SUR, and is essential for trafficking Kir6.2 subunits to the membrane surface18. Similar to all other ABC transporters, SUR subunits contain two nucleotide binding domains (NBDs). Dimerization of these two NBDs creates one single nucleotide binding site and catalytic site. Mg-dependent hydrolysis at this site provides the power stroke necessary to overcome the inhibitory effect of ATP on Kir6.23. Unlike traditional ABC transporters, the SUR regulatory subunits cannot conduct a functional current17.
Two types of SUR regulatory subunits have been identified to date17, 19. They differ primarily in their affinity for sulfonylurea, their tissue distribution and genetic source. SUR1, encoded by the ABCC8 gene located at ch11p15.1, has higher affinity for sulfonylurea and is mainly found in pancreatic beta cells and most neurons. SUR2, encoded by the ABCC9 gene located at ch12p12.1, has lower affinity for sulfonylureas and is expressed mainly in the heart, skeletal muscle and some neurons2, 3. SUR2 has two splice variants, SUR2A and SUR2B. They differ only in the last 42 C-terminal amino acid residues. Interestingly, the C42 of SUR2B shows high homology to the C42 of SUR120. Since most KATP channels identified to date contain the Kir6.2 subunit, the heterogeneity observed between different KATP channels mainly arises from the differential expression of SUR regulatory subunits.
Biophysical properties and regulation of KATP channels
Gating and channel kinetics
Two independent types of gates for KATP channels have been described3, 21, 22. The fast gating is due to the action of the selectivity filter. This ligand-independent gate is affected by mutations near the selectivity filter21. The slow ligand-dependent gate describes changes in the TM2 helices induced by ATP binding22. Mutations in TM2 near the cytoplasmic end have been shown to affect the slow gating in multiple Kir channels3, 23, 24, 25. The kinetics of KATP channels consists of 1 open state and multiple closed states3, 21, 26, 27. The channel only conducts current if all four Kir6.2 subunits are in the open conformation. ATP binding to any one subunit will induce a closed conformation in that subunit, and subsequent channel closure. Therefore the channel has multiple closed states and only one open state.
Trafficking of KATP channels
The membrane expression of KATP channels follows the strict 4:4 SUR:Kir stoichiometry, which has two implications. First, it allows only octameric channels expressed on the membrane, thus serves as quality control mechanisms that prevent expression of partial channels. Second, it indicates a tight coupling between the SUR and Kir subunits during the assembly and trafficking of KATP channels. Indeed, regulation of the membrane expression of the functional channels lies in the presence of a novel ER retention signal, RKR, which is present in both Kir6.2 and SUR subunits28. Appropriate interactions between Kir6.2 and SUR subunits result in shielding of this RKR signal, allowing the complex to exit the ER and be trafficked to the plasma membrane. Furthermore, presence of this RKR signal prevents the membrane expression of Kir tetramers (without SUR), SUR monomers or partial KATP channel complexes28. As such, SUR subunits are the major regulator of the trafficking of Kir6.2 channels.
Numerous factors affect the efficiency of trafficking of KATP channels. Specifically, SUR regulation of Kir6.2 trafficking is dependent on the TMD029 and NBD130 domains of SUR, such that mutations (or deletions) at these regions result in significant decreases of membrane expression of KATP channels. Furthermore, mutations affecting the shielding of the RKR ER retention signal, such as L1544P on SUR1 that causes congenital hyperinsulinism, also decrease trafficking of KATP channels31. Lastly, glucose deprivation stimulates trafficking of Kir6.2 subunits, possibly through an AMP kinase (AMPK)-dependent pathway32.
Regulation by intracellular ATP, Mg-ADP
The primary regulators of KATP channel activity are intracellular nucleotides, ATP and ADP. ATP has two major functions2. First, it exerts a strong inhibitory effect on KATP channels via interaction with the cytoplasmic domain of Kir6.2 subunit33, 34. Each Kir6.2 subunit has one ATP binding site, so the functional channel can accommodate four ATPs35. The ATP binding pocket on each Kir6.2 subunit is formed by residues R50, I182, K185, R201, G3342, 3, 23, 33, 36, 37, 38, 39, 40, 41, 42, 43. Mutations at any one of these locations can reduce ATP mediated inhibitory effect on Kir6.2, resulting in increased KATP channel activity. For example, R201H is one of the most common mutations that cause neonatal diabetes44. Decreased ATP mediated inhibition results in channel over-activity, and the cell remains hyperpolarized regardless of the ATP level. Other mutations that can alter the effect of ATP also change the intrinsic opening kinetics of the channels45. ATP preferentially binds to the closed state of the channel2, 3. Thus mutations that alter the gating characteristics of the channel, such as I296 in neonatal diabetes, reduce the effectiveness of ATP binding and lead to channel overactivity45.
ATP, in the presence of Mg2+, also exerts a weak stimulatory effect on KATP channels via its interaction with SUR regulatory subunits46. It is important to note that the principal role of ATP is inhibition. Binding of ATP to any one of the four Kir6.2 subunits will render the channel closed. Under normal physiological ATP levels, the open probability of KATP channels is less than 0.1% if SUR regulatory subunits are absent2, 3.
Mg-ADP serves as the principle physiological activator of KATP channels, and allows them to operate in an ATP insensitive state. Mg-ADP exerts its stimulatory function via interaction with SUR regulatory subunits2. Each SUR subunit has two nucleotide binding domains (NBDs), and dimerization of the two NBDs generates the catalytic sites for ATP hydrolysis, and thus dimerization is essential for successful transduction of ADP's stimulatory effect47, 48, 49. Mutations that disrupt dimerization reduce ADP mediated activation of KATP channels50. Mutations throughout the SUR1 subunit have been identified as one of the major contributors to the occurrence of congenital hyperinsulinemia hypoglycaemia51. For example, the point mutation G1479R52, 53 in the NBD2 of SUR1 or V187D54 in the TMD0 of SUR1, reduced the channel responsiveness to ADP. The decreased ADP-mediated channel activation leads to membrane depolarization in pancreatic beta cells, and continuous release of insulin into the bloodstream, even when plasma glucose levels are low, thus leading to hyperinsulinemic hypoglycaemia.
Regulation by protein interactions
SUR subunits are essential regulatory subunits of KATP channels. They are necessary for transducing the effect of Mg-ADP, and are the major target site for pharmacological substances. It is unclear how SUR subunits modulate Kir activity; however it has been proposed that the TMD0 domain of SUR anchors SUR to the outer TM1 helix and N-terminus of Kir6.2, thus providing a direct route for information transfer between SUR and the related Kirs55, 56, 57.
KATP channel activity in pancreatic beta cells and cardiomyocytes can by suppressed by the SNARE protein syntaxin 1A via protein-protein interaction58. Two specific mechanisms have been proposed. First, syntaxin 1A interacts with the NBD1 of SUR subunits via its C-terminal H3 domain to decrease the activity of existing plasma membrane KATP channels when ATP levels are lowered58, 59, 60. This effect is subject to ATP regulation, such that ATP dose-dependently inhibits syntaxin 1A binding to SUR1 subunits at physiological concentrations61. Second, it causes downregulation of KATP channel expression, either by accelerating endocytosis of existing surface channels, or by decreasing the biogenesis of KATP channels in the early secretory pathway62. Syntaxin 1A binding to SUR1 subunit is able to counter the stimulatory effects exerted by potassium channel openers (KCOs) such as diazoxide, NNC55-0462, P1075 and cromakalim63, 64. The physiological role of KATP channel regulation by syntaxin 1A is presently unclear. However, through modulating KATP channel activity, syntaxin 1A may play an important role in regulating insulin secretion and in pathologies related to glucose homeostasis.
Other mechanisms of regulation
Another modulator of KATP channel activity is phosphatidylinositol 4,5-bisphosphate (PIP2). Two possible mechanisms may account for its activating effects2. First, it sustains KATP channel activity by stabilizing the open state, thus decreasing ATP sensitivity and its ability to close the channel. Furthermore, the binding site for PIP2 is also on the cytoplasmic domain of Kir6.2 (R54, R176, 177, R206) and is situated very close to the ATP binding site65, 66, 67. Thus PIP2 binding to the channel subunit may allosterically reduce ATP affinity for the channel. Other modulators of KATP channel activity include protein kinase A (PKA) in smooth muscle cells68, 69 and cytoskeletal actin in the cardiac atrium70. Protein kinase C (PKC) activates the cardiac and pancreatic KATP channels by phosphorylating T180 at the pore-forming subunit Kir6.271. In the hypothalamus, PKC phosphorylation activates the neuronal Kir6.2/SUR1 KATP channels to inhibit hepatic glucose production72. On the other hand, PKC phosphorylation stimulates internalization of KATP channels in cardiomyocytes and CA1 hippocampal neurons, thus functionally decreases KATP channel activity73. Lastly, PKC-mediated activation and upregulation of KATP channels also play an important role in ischemic preconditioning74, 75, 76, 77, 78.
Pharmacology
Sulfonylureas
Sulfonylureas reduce KATP channel activity by binding to SUR subunits. They include acetohexamide, tolbutamide, glipzide, glibenclamide and glimepiride. In pancreatic beta cells, the decreased K+ efflux induced by sulfonylureas leads to membrane depolarization and activation of voltage-gated calcium channels (VGCCs), thus allowing Ca2+ influx and insulin release. As such, sulfonylureas have been used to treat diabetes and related diseases. Sulfonylureas therapy is one of the most established treatments for type 2 diabetes, as it is very effective and cost-efficient in achieving the targeted glycemic goals79. Its major advantage is its rapid effectiveness, and its major side effects include hypoglycaemia and weight gain. With the discovery of the role of Kir6.2 mutations in causing neonatal diabetes mellitus, sulfonylureas have also become the main drug used to treat this disease. Patients achieve better glycemic control with sulfonylureas compared to insulin injections, and many of the side effects observed in type 2 diabetics (eg, hypoglycaemia) are not seen in patients with neonatal diabetes80.
Modulation of sulfonylureas
The effect of sulfonylureas is altered by the presence of cytoplasmic nucleotides such as Mg-ADP. It has been shown that in the cell-attached configuration, sulfonylureas can completely block KATP channels51. In excised membrane patches however, the blockage is only 50%–70%. This difference is attributed to the presence of Mg-ADP, which can strongly activate KATP channels via its interaction with SUR1 and weakly inhibit KATP channel activity through the ATP binding site on Kir6.2. The strong stimulatory effect of Mg-ADP, but not the weak inhibitory effect, is counteracted by sulfonylureas. Therefore, the presence of Mg-ADP appears to enhance sulfonylureas' inhibitory effects51, 81.
Binding sites for tolbutamide and glibenclamide have been described. Both drugs share the high affinity binding site S1237 located in cytoplasmic loop 8 between TM15 and TM16 of SUR82, 83. The other, low affinity binding site for tolbutamide is located on Kir6.284, 85. Another binding site for glibenclamide is located in cytoplasmic loop 3 between TM5 and TM682, 83.
Potassium channel openers
The other class of drugs are K channel openers (KCOs) and these include cromakalim, pinacidil, nicorandil, diazoxide and minoxidil sulphate. Like their name suggests, these drugs bind to SUR regulatory subunits to stimulate KATP channel activity2. In pancreatic beta cells, diazoxide binds to SUR1 subunits to increase KATP channel activity, promoting K+ efflux and cell hyperpolarization. This reduces the amount of insulin released. As such, this drug is currently one of the major drugs used to treat congenital hyperinsulinism86, 87.
Different KATP channels respond differently to these KCOs due to expression of different SUR regulatory subunits. Pancreatic beta cell KATP channels are composed of Kir6.2 and SUR1 subunits, and are readily activated by diazoxide, weakly activated by pinacidil, and unaffected by nicorandil or cromakalim88. In contrast, cardiac KATP channels, which are composed of Kir6.2 and SUR2A subunits, are activated by pinacidil, nicorandil and cromakalim, but not affected by diazoxide89, 90. Interestingly, KATP channels in smooth muscles respond to all of these drugs91. The observed differences in KCO sensitivity is due to differences in SUR subunits. SUR1 shows high sensitivity to diazoxide, and SUR2A shows high sensitivity to pinacidil, nicorandil and cromakalim. The binding site for cromakalim, pinacidil and nicorandil resides within the second TM domain of SUR. The nucleotides L1249 and T1253 in SUR2A, and T1286 and M1290 in SUR2B are necessary and sufficient for KCO binding92, 93, 94. These differences underscore the importance of SUR subunits in determining the function of KATP channels.
Physiological role of KATP channels in glucose homeostasis
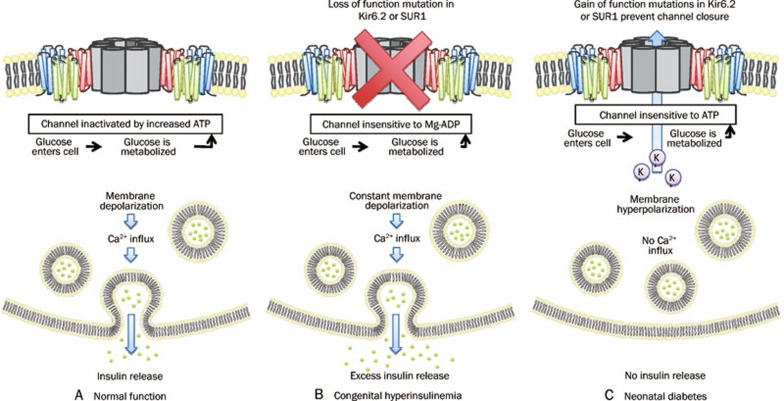
KATP channels across several different tissues contribute to glucose homeostasis. In pancreatic beta cells, KATP channels consisting of Kir6.2 and SUR1 subunits95 promote insulin secretion and thus cause a reduction in blood glucose concentration96 (Figure 2). In their open state, KATP channels permit an efflux of K+ ions to maintain the cell's polarized membrane potential. Following the metabolism of glucose, however, it is believed that the ATP that is produced causes KATP channels to close and thus no longer contribute to the polarization of the cell. The cell is now more depolarized and this triggers the influx of calcium via VGCCs and consequently the release of insulin-containing granules96.
Figure 2.
The role of KATP channels in insulin release from pancreatic β-cells. When glucose enters the cell it is metabolized to yield ATP. (A) During normal function, the KATP channel is inactivated, causing membrane depolarization and insulin release. (B) Loss of function mutations in Kir6.2 or SUR1 result in constant membrane depolarization leading to the excess insulin release characteristic of congenital hyperinsulinemia. (C) Gain of function mutations in Kir6.2 or SUR1 promotes the open state of the channel to cause membrane hyperpolarization and the lack of insulin release characteristic of neonatal diabetes.
KATP channels also play a role in regulating the release of glucagon. Insulin and zinc ions activate KATP channels on pancreatic alpha cells to hyperpolarize them and thus inhibit their ability to release glucagon97. Alternatively, glucose can act to inhibit KATP channels in pancreatic alpha cells98. However, unlike in beta cells where the inhibition of KATP channels is excitatory, the inhibition of KATP channels in alpha cells is inhibitory and therefore inhibits the release of glucagon96, 99. This is because in alpha cells, the resting membrane potential is much closer to the threshold potential for action potential firing than in beta cells. Therefore, the depolarization resulting from KATP channel closure is sufficient to maintain VGSCs in a state where they are unable to reconfigure - thus inducing a depolarization block. In this way, the inactivation of KATP channels is excitatory in beta cells, but inhibitory in alpha cells.
KATP channels may regulate glucose concentration by mediating glucagon release at the level of the hypothalamus. Kir6.2 subunits are required for the increased spontaneous discharge rate of neurons in the ventromedial hypothalamus (VMH) following an increase in the concentration of extracellular glucose96. This increase in VMH activity is likely a reflection of increased firing of glucose-responsive neurons (GRNs) within the VMH96, 100. In addition, neurons within the VMH have been implicated in an autonomic pathway involving the release of adrenaline and terminating with the release of glucagon from pancreatic alpha cells99, 101. VMH KATP channels are composed of Kir6.2 and SUR1 subunits, just like in pancreatic beta cells102. Therefore, the ability for the body to regulate blood glucose levels by releasing both insulin and glucagon likely depends on these specific KATP channels in pancreatic beta cells and VMH cells, respectively. KATP channels in the hypothalamus may modulate the production of glucose103. The infusion of the K+ channel opener diazoxide into the hypothalamus inhibits gluconeogenesis in the liver, and a global knockout of SUR1 prevents the inhibition of gluconeogenesis by insulin103. Insulin acts on KATP channels via the phosphatidylinositol 3-kinase (PI3K)/phosphatidylinositol 3,4,5-triphosphate (PIP3) pathway in the arcuate nucleus of the hypothalamus to inhibit the release of glucose from the liver104. Pro-opiomelanocortin-expressing neurons originating from this nucleus are sensitive to KATP channel activation and partial activation of these neurons results in impaired glucose tolerance105.
Finally, KATP channels may regulate glucose concentration by mediating the uptake of glucose into skeletal muscles. The knockout of Kir6.2 subunits is associated with increased absorption of glucose into skeletal muscle (both basally and in response to insulin)106, as well as an increased sensitivity of blood glucose concentration to insulin102. Similar effects are evident following the knockout of the SUR2 regulatory domain. Therefore, this KATP channel likely acts to inhibit glucose uptake in its open state and promote glucose uptake in its closed state107, 108. Although the mechanism of such action is unknown, evidence suggests that this uptake of glucose is independent of the insulin receptor substrate-1/PI3K signalling pathway that underlies the sensitivity of skeletal muscles to insulin109, 110, 111. It is also believed to be independent of the insulin-independent cAMP-activated protein kinase dependent pathway112, 113. Taken together, KATP channels, acting through different mechanisms and from within various tissues, contribute to the regulation of blood glucose levels, and regulate glucose homeostasis under both physiological and pathological conditions.
Pathophysiological role of KATP channels in glucose homeostasis
Mutations in KATP channel subunits and neonatal diabetes mellitus
Neonatal diabetes mellitus (NDM) is defined as the occurrence of insulin-requiring monogenic diabetes in the first six months of life44, 114, 115. It is a rare disease with incidence in the range of 1/200 000 live births115, 116, 117, 118. It can be transient, which is characterized by spontaneous remission within the first few months of life, or permanent, in which continuous treatment is required from the time of diagnosis. Transient neonatal diabetes mellitus (TNDM) is a result of chromosome abnormalities in the 6q24 locus80. The PLAGL1/ZAC gene, a zinc finger protein that regulates apoptosis and cell cycle arrest and the HYMAI gene are believed to be involved in TNDM, although the mechanism of action is poorly understood80, 119, 120. In contrast, permanent neonatal diabetes mellitus (PNDM) most commonly results from activating mutations in the genes encoding Kir6.2 (KCNJ11) and its regulatory subunit SUR1 (ABCC8)44. Mutations in other genes, including insulin121, 122, 123, 124, 125, 126, insulin promoter factor127, 128, 129, glucokinase130, 131, 132, 133, 134, 135, 136, 137, 138, 139 and FOXP3140, 141 have also been reported to cause PNDM. Understanding the genetic basis of NDM in general has greatly facilitated the correct diagnosis and treatment of this disease. The following sections will focus on the role of KATP channel subunits in PNDM, and the current therapeutic approaches80.
Extensive clinical and molecular studies have firmly established the role of KATP channel subunits, specifically, Kir6.2 and SUR1 in neonatal diabetes. Overexpression of mutant Kir6.2 subunits with reduced ATP sensitivity causes mice to develop severe neonatal diabetes142. More importantly, a polymorphism in Kir6.2 (E23K) is consistently linked with adult diabetes mellitus143, 144, 145, 146, 147, 148. The role of Kir6.2 in PNDM was confirmed in 2004, when Gloyn et al reported that activating dominantly inherited mutations in KCNJ11 were found in 10 out of 29 patients with NDM44. These patients did not secrete insulin in response to glucose or glucagon, but did secrete insulin in response to tolbutamide, which is a KATP channel blocker used to treat type 2 diabetes. Six heterozygous mutations of Kir6.2 were identified, among which the mutations R201H and V59M were most common. When the R201H mutant was co-expressed with SUR1 in Xenopus oocytes, the resulting mutant channels showed 40× decreased sensitivity to ATP inactivation compared to the wild type channels44.
Recent studies have identified more than 40 mutations in Kir6.2 and a similar number in SUR1 that lead to PNDM149. All Kir6.2 mutations are heterozygous (dominantly inherited), but SUR1 mutations are more heterogeneous, with homozygous, heterozygous and compound heterozygous mutations being described80, 149. About 80% of KCNJ11 mutations and 50% of ABCC8 mutations arise de novo150, however, there were two reports of germline mosaicism where two siblings with PNDM were born to unaffected parents151. Interestingly, Kir6.2152, 153, 154, 155, 156, 157, 158, 159 and SUR1160, 161, 162, 163, 164, 165, 166 mutations have also been found in TNDM patients, who do not have mutations at the 6q24 locus.
All mutations reported to date are missense mutations, with the exception of one in-frame KCNJ11 nucleotide deletion167. The functional consequence of Kir6.2 and SUR1 mutations is reduced metabolic sensing capacity of the KATP channel. All mutations on Kir6.2 decrease the channel's sensitivity to ATP2, 134, 168, either directly by interfering with ATP binding37, 44, 159, 169, 170, or indirectly by decreasing the intrinsic open probability of the channel45, 170. Mutations that affect ATP binding directly are clustered near the binding site, and these include the common R201H mutation, and also R50, I182, Y330, and F3332, 3, 44. The second, indirect mechanism by which Kir6.2 mutations increase channel function lies in the fact that ATP sensitivity is decreased when the channel is in the open state. Thus mutations that drive the channel to the open state, such as I196H located at the channel pore, can decrease the ability of ATP to close the channel45. Mutations in SUR1 function mainly increase the Mg-nucleotide mediated activation of the channel or change the intrinsic gating properties of the channel2, 3. Overall, these mutations result in the gain of function of KATP channels so that they are persistently open, leading to beta cell hyperpolarization even in the presence of elevated plasma glucose levels. Hyperpolarization prevents the secretion of insulin, thus resulting in the diabetic phenotype.
Mutations in KATP channel subunits and DEND syndrome
About 20% of patients with PNDM exhibit developmental delay, epilepsy, muscle weakness in addition to neonatal diabetes (DEND syndrome)171. Patients with a milder form, termed intermediate DEND (iDEND), do not have epilepsy. There are fifteen Kir6.2 mutations and two SUR1 mutations that may cause DEND and iDEND149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168. In particular, the V59M mutation in Kir6.2 is the most common cause of iDEND44, 142, 168, 172. The genetic basis of DEND suggests that mutation of this channel is responsible for all of the observed symptoms, and this notion is strengthened by the fact that KATP channel subunits are expressed in the affected tissues, namely the brain, muscle and pancreas.
The diabetic phenotype is the result of KATP channel overactivity in pancreatic beta cells, the same as that observed in PNDM. The muscle dysfunction is neural in origin172. Several lines of evidence illustrate this. Muscle weakness is observed in one patient with a SUR1 mutation, despite the lack of SUR1 expression in skeletal muscles173. Also, muscle weakness and ataxic gait in a patient with a Kir6.2 mutation was improved by treatment with gliclazide, which interacts only with SUR1174. More definitive evidence comes from a recent study by Clark et al, in which hemizygous mice that selectively express the V59M Kir6.2 mutation in muscle or neurons were examined172. Behaviourally, transgenic mice with the neuronal V59M Kir6.2 mutation display the same motor impairments as seen in human DEND. Electrophysiological studies show that V59M-carrying Purkinje neurons (output of cerebellum that regulates motor movement) were hyperpolarized and displayed suppressed electrical firing. This was reversed with the application of tolbutamide, suggesting that neuronal KATP channels containing V59M Kir6.2 were overactive mutants. In contrast, muscle-specific V59M mutation failed to alter muscle membrane properties, and mice with muscular V59M Kir6.2 mutation behave like their wild type counterparts. The differential effects of Kir6.2 V59M mutation on neurons and muscles may be due to the divergence in the identity of SUR regulatory subunits. Neurons, like pancreatic beta-cells, mostly contain KATP channels made up of Kir6.2 and SUR1 subunits, whereas muscle KATP channels are composed of Kir6.2 and SUR2A subunits. In fact, it has been shown that the Kir6.2 V59M mutation specifically enhances the flow of current through KATP channels composed of Kir6.2/SUR1 subunits, but has no effect on Kir6.2/SUR2A KATP channels172. Thus, the V59M mutation selectively targets the pancreas and neurons while sparing the muscle.
The last two symptoms of DEND, namely developmental delay and epilepsy, are neuronal in origin, but their exact causes are unknown. It has been proposed that epilepsy may result from decreased activity of inhibitory interneurons in the hippocampus, as there's a greater density of KATP channels in the inhibitory neurons compared to the excitatory ones11, 173. The cause of developmental delay is unclear, but it may result directly from overactivity of KATP channels, or may be secondary to the symptom of diabetes. Motor and cognitive development requires dynamic changes in neuronal networks and balanced excitatory and inhibitory inputs within the network. Overactivity of KATP channels hinders the occurrence of excitatory synaptic connections, and thus may inhibit neuronal activity and development. On the other hand, developmental delay may be the result of diabetes-induced neuropathy. Therefore, further research is warranted to elucidate this matter.
Therapeutics of neonatal diabetes
Neonatal diabetes was originally thought to be an early onset form of type 1 diabetes and was therefore treated with insulin injections80. However, after the discovery of the mutations of KATP channel subunits, more than 90% of patients were switched to treatments with sulfonylureas (0.05–1.5 mg·kg−1·d−1)175. Most patients exhibited significant improvement in their clinical situations168. Specifically, blood glucose levels were generally reduced, as indicated by HbA1C levels175, 176, 177, 178, 179. There were also less fluctuation in plasma glucose levels, and hypoglycaemic episodes were less common173. In addition, patients with iDEND also see improvement in extrapancreatic symptoms, such as reduced epileptic events, improved cognition and improved muscle tone and balance173, 180, 181, 182, 183. Unfortunately, patients suffering from the severe DEND syndrome are less responsive to sulfonylurea treatment184, 185, 186. Side effects are minimal; a few patients have reported transient diarrhoea and tooth discoloration173, 187, 188.
Sulfonylureas work by interacting with SUR subunits to induce channel closure. This allows for the depolarization of the cell membrane and increased excitability. In pancreatic beta cells, cell depolarization allows for the activation of voltage-gated calcium channels, stimulating an influx of Ca2+ into the cytoplasm and subsequent release of insulin into the bloodstream. This is able to mitigate the diabetic symptoms. Interestingly, there was better insulin response to oral glucose intake than to intravenous glucose in NDM patients treated with sulphonylureas175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189. The presence of food in the gut lumen stimulates the secretion of hormones and signalling molecules such as GLP-1, GIP and ACh, which can amplify the insulin response and it appears that their proper action may require KATP channel closure189. How sulphonylureas affect the secretion of such hormones is unclear at this moment. It has been proposed that secretion of these hormones requires Ca2+ influx, which in patients with PNDM is only possible after sulfonylurea treatment189.
The improvement of neurological symptoms of DEND and iDEND is most likely due to the action of sulfonylureas on neuronal KATP channels. Although there is enhanced cognition, improved muscle tone and abolition of seizures, development does not return to normal with sulfonylurea treatment173. This could be the result of insufficient sulfonylurea potency or the irreversibility of neuronal damage caused by KATP channel overactivity.
A point of concern is that glibenclamides and glyburides, which interact with both SUR1 and SUR2A, are currently used to treat DEND and iDEND. The discovery of the neuronal origin of muscle impairment in DEND and iDEND suggests that more specific drugs, that only target SUR1, could be used in order to avoid unnecessary side effects, especially in the myocardium which also express SUR2A subunits172. Indeed, it has been reported that gliclazide, which only interacts with SUR1, can alleviate the motor symptoms174.
Another minor drawback with sulfonylurea therapy is that its effectiveness decreases as the time between diagnosis and transfer to sulfonylurea therapy increases, although it is relatively successful when used at the early stage of the disease80, 175. Prolonged hypoinsulinemia may result in a loss of beta cells and thus the insulin secreting machinery downstream of KATP channels may not be functional173. In mouse models of neonatal diabetes, prolonged lack of insulin leads to a progressive decrease in beta cell mass and a loss of normal islet architecture is observed190. In such cases, even though sulfonylurea may enhance KATP channel closure, it cannot relieve the hyperglycemia. Thus, it is beneficial to start sulfonylurea treatment as early as possible if neonatal diabetes is diagnosed. However, sulfonylurea treatment is only effective for those with mutations in KATP channel subunits, which only account for half of all NDM patients. For patients with mutations in other genes such as insulin and glucokinase, it may not be beneficial. Therefore, it may be advantageous for all diabetic patients to undergo genetic testing in the first six months of life in order to obtain a definitive diagnosis , and permit the earliest possible commencement of treatment80.
KATP channels and congenital hyperinsulinism
Congenital hyperinsulinism (CHI) is characterized by continuous and unregulated insulin secretion despite low plasma glucose levels51, 191, 192. The incidence of CHI is 1/30 000 to 1/50 000 live births per year, however in some isolated areas where inbreeding is common, the disease incidence may reach 1/2500193. This disease cannot be detected in utero, and babies with CHI have no gross characteristic differences from normal babies51. The first clinical signs are vague, and include cyanosis, respiratory distress, sweating, hypothermia, poor feeding and hunger. It is important that the correct diagnosis is made promptly, as delayed treatment will result in permanent brain damage and mental retardation due to insufficient energy supply for brain metabolism51.
There are two distinct forms of CHI, categorized based on their histological differences. Focal CHI is characterized by the presence of a small endocrine lesion in the pancreas. In the lesion area, the islets are normally structured, but hyperplastic; outside of the legion, the islets are normal194, 195, 196. Complete resection of the lesion can cure the patient. On the other hand, in patients with diffuse CHI, the islets of Langerhans show large beta cells with abnormally large nuclei, which is indicative of hyperactivity. Treatment for diffuse CHI patients usually involves partial pancreatectomy196, which often leads to pancreatic insufficiency and iatrogenic diabetes mellitus51.
Mutations in KATP channel subunits are the most common cause of CHI51. The SUR1 gene ABCC8 and Kir6.2 gene KCNJ11 are located at ch11p15. Mutations at this genetic locus are linked to both diffuse and focal CHI197. Diffuse CHI predominantly arises from autosomal recessive mutations of KATP channel subunits, although dominant ones have been reported51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193. Focal CHI results from loss of heterozygosity at this locus198, 199, 200, 201, 202, 203, 204. In the normal part of the pancreas, the cells inherit a mutated SUR1 gene from the father and a normal SUR1 gene from the mother. These cells have a normal phenotype, due to expression of the normal maternal SUR1 gene. In the focal lesion, the cells have lost the normal maternal chromosome during fetal life and contain two copies of the mutated SUR1 genes inherited from the father. They have also lost the maternally imprinted tumor suppressor genes P57kip2, although they still express the paternally derived insulin-like growth factor II gene. This combination enables the growth of the focal lesion. About 40%–65% of patients with CHI have focal CHI51.
Mutations of KATP channels responsible for CHI are mostly found in SUR subunits, and a few in Kir6.2 subunits51, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196, 197, 198, 199, 200, 201, 202, 203, 204, 205. Indeed, over 150 mutations have been identified in the SUR1 subunit and these account for 50% of all CHI cases149. These loss-of-function mutations have been grouped into two classes168. In class I, there is a total loss of KATP channels in the plasma membrane, resulting in no KATP current51. This type of mutation accounts for 10% of all diffuse CHI patients and 55% of focal CHI patients. The most common cause is defects in trafficking168. For example, the mutation R1437Q(23)X in exon35 of ABCC8 causes truncation of the C-terminus of SUR1, which contains the signal sequence necessary for exiting the ER51, 206. Thus, the channel protein is retained in the ER and cannot be expressed in the membrane. In class II mutations, KATP channels are present in the membrane (although less than normal) but show reduced sensitivity to Mg-nucleotide activation or reduced intrinsic channel open probability51, 168. These mutations account for more than 60% of diffuse CHI cases and 45% of focal CHI cases. For example, point mutations such as G1479R in NBD2 of SUR152 or V187D in the TMD0 of SUR1207 lead to reduced responsiveness to ADP activation in the expressed channels. Overall, these mutations result in a loss-of-function of KATP channels in the pancreatic beta cells, leading to constitutive exocytosis of insulin-containing secretory vesicles. In addition to KATP channel related mutations, CHI also arises from autosomal dominant mutations in genes involved in glucose metabolism, including mutations in glutamate dehydrogenase (GDH)208, 209, 210, 211, 212, 213, 214, 215, 216, glucokinase217, 218 and short-chain L-3-hydroxyacyl-CoA dehydrogenase (SCHAD)106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196, 197, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215, 216, 217, 218, 219.
Therapeutics of congenital hyperinsulinism
Understanding the molecular mechanisms that underlie CHI provides the basis for establishing effective treatment protocols. Of the utmost importance is the correct diagnosis of the type of CHI. Following the identification of KATP channels in the pathology of CHI, the K+ channel opener, diazoxide, has become a diagnostic tool and a treatment method86, 87. When CHI is suspected, the first step in diagnosis is to determine the diazoxide responsiveness. The diazoxide-responsive CHI patients can be managed with diazoxide treatment with regular monitoring. Non-responsive patients are given a genetic test of the ABCC8 and KCNJ11 genes to determine whether it is a case of focal or diffuse CHI. For the diffuse CHI patients, treatment involves a high calorie diet, somatostatin (Octreotide) therapy and a near-total pancreatectomy. Regular follow-up is required to monitor growth, development, as well as the occurrence of diabetes mellitus later in life. For focal CHI patients, a complete resection of the focal lesion is commonly used and can cure patients86, 87.
The mainstream drug used in CHI treatment, diazoxide (10-20 mg·kg−1·d−1), is a KATP channel opener and binds to SUR1 subunits to promote KATP channel opening51. This prevents the depolarization of pancreatic beta cells and insulin secretion. Diazoxide is easily administered orally and provides significant relief for CHI caused by mutations in GDH, glucokinase and SCHAD. However, it is not effective for type I KATP-related CHI, in which a lack of KATP channels has been found. Therefore, diazoxide has no targets to exert its effects. More potent diazoxide analogues such as HEI713, BPDZ73, BPDZ44, and BPDZ154 have been synthesized51. Although effective in stimulating KATP channel opening in vitro, their clinical potential has not yet been determined.
Diazoxide also poses serious side effects. It causes sodium/water retention that can lead to complications such as congestive heart defects, poor cardiac reserve, hyperuricemia and hypotension in patients with heart problems. Moreover, diazoxide can decrease immune function and long term use has been linked with hyperosmolar nonketotic coma51.
Other drugs used for CHI treatment include L-type Ca2+ channel antagonists (nifedipine, verapamil), glucagon, somatostatin and corticosteroids51. VGCC antagonists prevent Ca2+ influx and may decrease insulin secretion. They have been shown to be therapeutically beneficial in some220, 221, 222, 223, but not all CHI patients224. Glucagon is used because it stimulates glycogenolysis and gluconeogenesis, but it is disfavoured because it also acts as an insulin secretagogue, thus promoting insulin hypersecretion. Long-term use of somatostatin is widely accepted, as it potently inhibits insulin release via activation of hyperpolarizing K+ channels and it independently inhibits VGCCs. Short term use of steroids helps to maintain adequate blood glucose levels51.
Perspectives/future directions
Neonatal diabetes mellitus
The identification of the role of KATP channels in neonatal diabetes has revolutionized the treatment for this disease. With the groundbreaking report in 2004 by Gloyn et al that identified mutations in Kir6.2 subunits as one of the major causes of neonatal diabetes44, more than 90% of patients have been switched to treatment with sulfonylureas, which induce KATP channel closure. Sulfonylureas provide better glucose control than previous treatment methods, and to date, the side effects reported (eg, tooth discoloration) have been minimal. Hypoglycemic episodes, which is the major side effect associated with sulfonylurea treatment in type 2 diabetes, are not observed in patients with neonatal diabetes. However, long-term monitoring is needed to determine whether the other side effects previously reported with sulfonylurea use, such as liver dysfunction, skin allergic reactions, pancytopenia, hyponatremia or cardiovascular abnormalities will appear in patients with NDM. One possible way to minimize potential side effect is to develop derivatives of sulfonylureas specific to pancreatic cells, thus minimizing the unwanted effect on other tissues.
DEND and iDEND
For patients with DEND or iDEND, sulfonylurea treatment not only provides better glucose control, but also alleviates some of the extrapancreatic symptoms. As such, sulfonylurea therapy reduces occurrences of epileptic events, and improves muscle and cognitive functions173. However, sulfonylureas do not improve developmental delay. The main difficulty is that the cause of developmental delay is unclear. It could arise from prolonged over-activity of neuronal KATP channels, or could be secondary to sustained high glucose levels prior to treatment. It is unclear how well sulfonylureas cross the blood brain barrier, thus the potency and efficacy of these drugs on neurons is unknown. It has been suggested that higher potency drugs may better improve the neural symptoms.
Another interesting point is that there are no cardiac symptoms observed in DEND or iDEND, even though KATP channels are widely expressed in cardiomyocytes. Pancreatic beta cells and neurons express Kir6.2 and SUR1 subunits while skeletal muscle and cardiomyocytes express SUR2A and Kir6.2 subunits. The study by Clark et al (2010) demonstrating the neural origin of motor symptoms suggest that skeletal muscle KATP channels, which are composed of SUR2A and Kir6.2 subunits, are not affected, thus, it is not surprising that DEND or iDEND patients have no cardiac symptoms172.
This differential response between KATP channels to the same genetic mutation has several implications. For one, it indicates that more specific sulfonylureas — ones that selectively target SUR1 — should be preferred over those that bind to both SUR2A and SUR1 when treating DEND/iDEND. Next, it underlines the importance of neuronal KATP channels in DEND and iDEND. This area is relatively unexplored at this moment. Understanding the effect of these mutations on neuronal networks would provide a better idea about the molecular basis of the observed symptoms, and allow development of better therapeutic approaches. Lastly, it highlights the importance of SUR subunit regulation in KATP channel activity. Mutations implicated in DEND and iDEND are mostly found in the Kir6.2 subunit, and these subunits are expressed in most tissues. The expression of SUR subunits, however, is tissue-specific and thus SUR subunits have a better capability of regulating mutant channels.
Congenital hyperinsulinism
Identification of the role of KATP channels has improved the diagnostic and treatment process for many patients suffering with congenital hyperinsulinism (CHI). Since congenital CHI usually results from the loss of KATP function, KATP channel openers such as diazoxide are used to counteract the deficit in KATP function. In cases where KATP channels are not trafficked to membrane, diazoxide treatment may be ineffective. Nonetheless, diazoxide is useful in two ways. One, it serves as a diagnostic tool for determining the specific type of CHI. Patients in which diazoxide treatment is effective likely do not have mutations in KATP channel subunits and thus diazoxide can be administered as the key drug for these patients. Since diazoxide is not very effective for CHI patients with KATP channel mutations, alternative approaches for treating these patients may be to examine novel regulators of KATP channels. One such molecule is syntaxin 1A, which inhibits KATP channels61. Therefore, decreases in syntaxin 1A levels can increase KATP channel activity. In contrast to diazoxide which only increases the activity of existing KATP channels, syntaxin 1A has been shown to regulate the membrane expression of wild-type KATP channels. Thus, it would be worthwhile to examine whether syntaxin 1A can provide additional regulation and therefore be effective for treating congenital hyperinsulinism.
Conclusion
Understanding the genetic basis of neonatal diabetes and congenital hyperinsulinism has significantly improved the diagnosis and treatment of patients with these diseases. The current use of KATP channel modulators by these patients has greatly alleviated their symptoms and improved their quality of life. However, assessment of the long-term effects of these treatment methods is warranted and better optimization of the treatment protocol is needed in order to deliver the best possible care to patients.
Acknowledgments
Zhong-ping FENG holds a New Investigator Award from the Heart and Stroke Foundation of Canada.
References
- Shyng S, Nichols CG. Octameric stoichiometry of the KATP channel complex. J Gen Physiol. 1997;110:655–64. doi: 10.1085/jgp.110.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–6. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–8. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–8. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic beta-cells. Nature. 1984;311:271–3. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Spruce AE, Standen NB, Stanfield PR. Studies of the unitary properties of adenosine-5′-triphosphate-regulated potassium channels of frog skeletal muscle. J Physiol. 1987;382:213–36. doi: 10.1113/jphysiol.1987.sp016364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce AE, Standen NB, Stanfield PR. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature. 1985;316:736–8. doi: 10.1038/316736a0. [DOI] [PubMed] [Google Scholar]
- Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177–80. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Gabrielsson BG, Karlsson AC, Lonn M, Olofsson LE, Johansson JM, Torgerson JS, et al. Molecular characterization of a local sulfonylurea system in human adipose tissue. Mol Cell Biochem. 2004;258:65–71. doi: 10.1023/b:mcbi.0000012837.11847.c8. [DOI] [PubMed] [Google Scholar]
- Zawar C, Plant TD, Schirra C, Konnerth A, Neumcke B. Cell-type specific expression of ATP-sensitive potassium channels in the rat hippocampus. J Physiol. 1999;514:327–41. doi: 10.1111/j.1469-7793.1999.315ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford ML, Sturgess NC, Trout NJ, Gardner NJ, Hales CN. Adenosine-5′-triphosphate-sensitive ion channels in neonatal rat cultured central neurones. Pflugers Arch. 1988;412:297–304. doi: 10.1007/BF00582512. [DOI] [PubMed] [Google Scholar]
- Ashford ML, Boden PR, Treherne JM. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch. 1990;415:479–83. doi: 10.1007/BF00373626. [DOI] [PubMed] [Google Scholar]
- Ashford ML, Boden PR, Treherne JM. Tolbutamide excites rat glucoreceptive ventromedial hypothalamic neurones by indirect inhibition of ATP-K+ channels. Br J Pharmacol. 1990;101:531–40. doi: 10.1111/j.1476-5381.1990.tb14116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattersley AT, Ashcroft FM. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005;54:2503–13. doi: 10.2337/diabetes.54.9.2503. [DOI] [PubMed] [Google Scholar]
- Nishida M, Cadene M, Chait BT, MacKinnon R. Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. EMBO J. 2007;26:4005–15. doi: 10.1038/sj.emboj.7601828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, Boyd AE III, Gonzalez G, et al. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–6. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- Babenko AP, Bryan J. SUR-dependent modulation of KATP channels by an N-terminal KIR6.2 peptide. Defining intersubunit gating interactions. J Biol Chem. 2002;277:43997–4004. doi: 10.1074/jbc.M208085200. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, et al. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–7. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, et al. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem. 1996;271:24321–4. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- Proks P, Capener CE, Jones P, Ashcroft FM. Mutations within the P-loop of Kir6.2 modulate the intraburst kinetics of the ATP-sensitive potassium channel. J Gen Physiol. 2001;118:341–53. doi: 10.1085/jgp.118.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. The open pore conformation of potassium channels. Nature. 2002;417:523–6. doi: 10.1038/417523a. [DOI] [PubMed] [Google Scholar]
- Enkvetchakul D, Nichols CG. Gating mechanism of KATP channels: function fits form. J Gen Physiol. 2003;122:471–80. doi: 10.1085/jgp.200308878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackin H, Nanazashvili M, Palmer LG, Krambis M, Walters DE. Structural locus of the pH gate in the Kir1.1 inward rectifier channel. Biophys J. 2005;88:2597–606. doi: 10.1529/biophysj.104.051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drain P, Geng X, Li L. Concerted gating mechanism underlying KATP channel inhibition by ATP. Biophys J. 2004;86:2101–12. doi: 10.1016/S0006-3495(04)74269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkvetchakul D, Loussouarn G, Makhina E, Shyng SL, Nichols CG. The kinetic and physical basis of KATP channel gating: toward a unified molecular understanding. Biophys J. 2000;78:2334–48. doi: 10.1016/S0006-3495(00)76779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drain P, Li L, Wang J. KATP channel inhibition by ATP requires distinct functional domains of the cytoplasmic C terminus of the pore-forming subunit. Proc Natl Acad Sci U S A. 1998;95:13953–8. doi: 10.1073/pnas.95.23.13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron. 1999;22:537–48. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- Hosy E, Dupuis JP, Vivaudou M. Impact of disease-causing SUR1 mutations on the KATP channel subunit interface probed with a rhodamine protection assay. J Biol Chem. 2010;285:3084–91. doi: 10.1074/jbc.M109.043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masia R, Caputa G, Nichols CG. Regulation of KATP channel expression and activity by the SUR1 nucleotide binding fold 1. Channels (Austin) 2007;1:315–23. doi: 10.4161/chan.5083. [DOI] [PubMed] [Google Scholar]
- Taschenberger G, Mougey A, Shen S, Lester LB, LaFranchi S, Shyng SL. Identification of a familial hyperinsulinism-causing mutation in the sulfonylurea receptor 1 that prevents normal trafficking and function of KATP channels. J Biol Chem. 2002;277:17139–46. doi: 10.1074/jbc.M200363200. [DOI] [PubMed] [Google Scholar]
- Lim A, Park SH, Sohn JW, Jeon JH, Park JH, Song DK, et al. Glucose deprivation regulates KATP channel trafficking via AMP-activated protein kinase in pancreatic beta-cells. Diabetes. 2009;58:2813–9. doi: 10.2337/db09-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antcliff JF, Haider S, Proks P, Sansom MS, Ashcroft FM. Functional analysis of a structural model of the ATP-binding site of the KATP channel Kir6.2 subunit. EMBO J. 2005;24:229–39. doi: 10.1038/sj.emboj.7600487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong K, Tang LQ, MacGregor GG, Leng Q, Hebert SC. Novel nucleotide-binding sites in ATP-sensitive potassium channels formed at gating interfaces. EMBO J. 2005;24:1318–29. doi: 10.1038/sj.emboj.7600626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markworth E, Schwanstecher C, Schwanstecher M. ATP4-mediates closure of pancreatic beta-cell ATP-sensitive potassium channels by interaction with 1 of 4 identical sites. Diabetes. 2000;49:1413–8. doi: 10.2337/diabetes.49.9.1413. [DOI] [PubMed] [Google Scholar]
- Cukras CA, Jeliazkova I, Nichols CG. The role of NH2-terminal positive charges in the activity of inward rectifier KATP channels. J Gen Physiol. 2002;120:437–46. doi: 10.1085/jgp.20028621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John SA, Weiss JN, Xie LH, Ribalet B. Molecular mechanism for ATP-dependent closure of the K+ channel Kir6.2. J Physiol. 2003;552:23–34. doi: 10.1113/jphysiol.2003.048843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang J, Drain P. The I182 region of K(ir)6.2 is closely associated with ligand binding in KATP channel inhibition by ATP. Biophys J. 2000;79:841–52. doi: 10.1016/S0006-3495(00)76340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proks P, Gribble FM, Adhikari R, Tucker SJ, Ashcroft FM. Involvement of the N-terminus of Kir6.2 in the inhibition of the KATP channel by ATP. J Physiol. 1999;514:19–25. doi: 10.1111/j.1469-7793.1999.019af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann F, Ryder TJ, Tucker SJ, Ashcroft FM. The role of lysine 185 in the Kir6.2 subunit of the ATP-sensitive channel in channel inhibition by ATP. J Physiol. 1999;520:661–9. doi: 10.1111/j.1469-7793.1999.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, Lippiat JD, Ashcroft FM, Rutter GA. ATP-dependent interaction of the cytosolic domains of the inwardly rectifying K+ channel Kir6.2 revealed by fluorescence resonance energy transfer. Proc Natl Acad Sci U S A. 2004;101:76–81. doi: 10.1073/pnas.0306347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker SJ, Gribble FM, Proks P, Trapp S, Ryder TJ, Haug T, et al. Molecular determinants of KATP channel inhibition by ATP. EMBO J. 1998;17:3290–6. doi: 10.1093/emboj/17.12.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Haider S, Jones P, Sansom MS, Ashcroft FM. Identification of residues contributing to the ATP binding site of Kir6.2. EMBO J. 2003;22:2903–12. doi: 10.1093/emboj/cdg282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838–49. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- Proks P, Girard C, Haider S, Gloyn AL, Hattersley AT, Sansom MS, et al. A gating mutation at the internal mouth of the Kir6.2 pore is associated with DEND syndrome. EMBO Rep. 2005;6:470–5. doi: 10.1038/sj.embor.7400393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM, Kakei M. ATP-sensitive K+ channels in rat pancreatic beta-cells: modulation by ATP and Mg2+ ions. J Physiol. 1989;416:349–67. doi: 10.1113/jphysiol.1989.sp017765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JD, Proks P, Lippiat JD, Sansom MS, Ashcroft FM. Identification of a functionally important negatively charged residue within the second catalytic site of the SUR1 nucleotide-binding domains. Diabetes. 2004;53:S123–7. doi: 10.2337/diabetes.53.suppl_3.s123. [DOI] [PubMed] [Google Scholar]
- Masia R, Nichols CG. Functional clustering of mutations in the dimer interface of the nucleotide binding folds of the sulfonylurea receptor. J Biol Chem. 2008;283:30322–9. doi: 10.1074/jbc.M804318200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergani P, Lockless SW, Nairn AC, Gadsby DC. CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains. Nature. 2005;433:876–80. doi: 10.1038/nature03313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Kurachi Y. The nucleotide-binding domains of sulfonylurea receptor 2A and 2B play different functional roles in nicorandil-induced activation of ATP-sensitive K+ channels. Mol Pharmacol. 2004;65:1198–207. doi: 10.1124/mol.65.5.1198. [DOI] [PubMed] [Google Scholar]
- Dunne MJ, Cosgrove KE, Shepherd RM, Aynsley-Green A, Lindley KJ. Hyperinsulinism in infancy: from basic science to clinical disease. Physiol Rev. 2004;84:239–75. doi: 10.1152/physrev.00022.2003. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Shyng SL, Nestorowicz A, Glaser B, Clement JP, Gonzalez G, et al. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science. 1996;272:1785–7. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- Sharma N, Crane A, Gonzalez G, Bryan J, Aguilar-Bryan L. Familial hyperinsulinism and pancreatic beta-cell ATP-sensitive potassium channels. Kidney Int. 2000;57:803–8. doi: 10.1046/j.1523-1755.2000.00918.x. [DOI] [PubMed] [Google Scholar]
- Otonkoski T, Ammala C, Huopio H, Cote GJ, Chapman J, Cosgrove K, et al. A point mutation inactivating the sulfonylurea receptor causes the severe form of persistent hyperinsulinemic hypoglycemia of infancy in Finland. Diabetes. 1999;48:408–15. doi: 10.2337/diabetes.48.2.408. [DOI] [PubMed] [Google Scholar]
- Babenko AP, Bryan J. Sur domains that associate with and gate KATP pores define a novel gatekeeper. J Biol Chem. 2003;278:41577–80. doi: 10.1074/jbc.C300363200. [DOI] [PubMed] [Google Scholar]
- Fang K, Csanady L, Chan KW. The N-terminal transmembrane domain (TMD0) and a cytosolic linker (L0) of sulphonylurea receptor define the unique intrinsic gating of KATP channels. J Physiol. 2006;576:379–89. doi: 10.1113/jphysiol.2006.112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosy E, Derand R, Revilloud J, Vivaudou M. Remodelling of the SUR-Kir6.2 interface of the KATP channel upon ATP binding revealed by the conformational blocker rhodamine 123. J Physiol. 2007;582:27–39. doi: 10.1113/jphysiol.2007.134288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasyk EA, Kang Y, Huang X, Cui N, Sheu L, Gaisano HY. Syntaxin-1A binds the nucleotide-binding folds of sulphonylurea receptor 1 to regulate the KATP channel. J Biol Chem. 2004;279:4234–40. doi: 10.1074/jbc.M309667200. [DOI] [PubMed] [Google Scholar]
- Cui N, Kang Y, He Y, Leung YM, Xie H, Pasyk EA, et al. H3 domain of syntaxin 1A inhibits KATP channels by its actions on the sulfonylurea receptor 1 nucleotide-binding folds-1 and -2. J Biol Chem. 2004;279:53259–65. doi: 10.1074/jbc.M410171200. [DOI] [PubMed] [Google Scholar]
- Kang Y, Leung YM, Manning-Fox JE, Xia F, Xie H, Sheu L, et al. Syntaxin-1A inhibits cardiac KATP channels by its actions on nucleotide binding folds 1 and 2 of sulfonylurea receptor 2A. J Biol Chem. 2004;279:47125–31. doi: 10.1074/jbc.M404954200. [DOI] [PubMed] [Google Scholar]
- Kang Y, Zhang Y, Liang T, Leung YM, Ng B, Xie H, et al. ATP modulates interaction of Syntaxin-1A with sulfonylurea receptor 1 to regulate pancreatic {beta}-cell KATP channels. J Biol Chem. 2011;286:5876–83. doi: 10.1074/jbc.M109.089607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PC, Bruederle CE, Gaisano HY, Shyng SL. Syntaxin 1A regulates surface expression of {beta}-cell ATP-sensitive potassium channels. Am J Physiol Cell Physiol. 2011;300:C506–16. doi: 10.1152/ajpcell.00429.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng B, Kang Y, Xie H, Sun H, Gaisano HY. Syntaxin-1A inhibition of P-1075, cromakalim, and diazoxide actions on mouse cardiac ATP-sensitive potassium channel. Cardiovasc Res. 2008;80:365–74. doi: 10.1093/cvr/cvn210. [DOI] [PubMed] [Google Scholar]
- Ng B, Kang Y, Elias CL, He Y, Xie H, Hansen JB, et al. The actions of a novel potent islet beta-cell specific ATP-sensitive K+ channel opener can be modulated by syntaxin-1A acting on sulfonylurea receptor 1. Diabetes. 2007;56:2124–34. doi: 10.2337/db07-0030. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, et al. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–4. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Shyng SL, Cukras CA, Harwood J, Nichols CG. Structural determinants of PIP(2) regulation of inward rectifier KATP channels. J Gen Physiol. 2000;116:599–608. doi: 10.1085/jgp.116.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng SL, Barbieri A, Gumusboga A, Cukras C, Pike L, Davis JN, et al. Modulation of nucleotide sensitivity of ATP-sensitive potassium channels by phosphatidylinositol-4-phosphate 5-kinase. Proc Natl Acad Sci U S A. 2000;97:937–41. doi: 10.1073/pnas.97.2.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn KV, Giblin JP, Tinker A. Multisite phosphorylation mechanism for protein kinase A activation of the smooth muscle ATP-sensitive K+ channel. Circ Res. 2004;94:1359–66. doi: 10.1161/01.RES.0000128513.34817.c4. [DOI] [PubMed] [Google Scholar]
- Shi Y, Wu Z, Cui N, Shi W, Yang Y, Zhang X, et al. PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1205–14. doi: 10.1152/ajpregu.00337.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wagoner DR. Mechanosensitive gating of atrial ATP-sensitive potassium channels. Circ Res. 1993;72:973–83. doi: 10.1161/01.res.72.5.973. [DOI] [PubMed] [Google Scholar]
- Light PE, Bladen C, Winkfein RJ, Walsh MP, French RJ. Molecular basis of protein kinase C-induced activation of ATP-sensitive potassium channels. Proc Natl Acad Sci U S A. 2000;97:9058–63. doi: 10.1073/pnas.160068997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, Wang PY, Chari M, Lam CK, Caspi L, Ono H, et al. Hypothalamic protein kinase C regulates glucose production. Diabetes. 2008;57:2061–5. doi: 10.2337/db08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Huang CS, Jan YN, Jan LY. ATP-sensitive potassium channel traffic regulation by adenosine and protein kinase C. Neuron. 2003;38:417–32. doi: 10.1016/s0896-6273(03)00256-3. [DOI] [PubMed] [Google Scholar]
- Speechly-Dick ME, Grover GJ, Yellon DM. Does ischemic preconditioning in the human involve protein kinase C and the ATP-dependent K+ channel? Studies of contractile function after simulated ischemia in an atrial in vitro model. Circ Res. 1995;77:1030–5. doi: 10.1161/01.res.77.5.1030. [DOI] [PubMed] [Google Scholar]
- Hu K, Duan D, Li GR, Nattel S. Protein kinase C activates ATP-sensitive K+ current in human and rabbit ventricular myocytes. Circ Res. 1996;78:492–8. doi: 10.1161/01.res.78.3.492. [DOI] [PubMed] [Google Scholar]
- Light PE, Sabir AA, Allen BG, Walsh MP, French RJ. Protein kinase C-induced changes in the stoichiometry of ATP binding activate cardiac ATP-sensitive K+ channels. A possible mechanistic link to ischemic preconditioning. Circ Res. 1996;79:399–406. doi: 10.1161/01.res.79.3.399. [DOI] [PubMed] [Google Scholar]
- Zhuang JG, Zhang Y, Zhou ZN. Hypoxic preconditioning upregulates KATP channels through activation of protein kinase C in rat ventricular myocytes. Acta Pharmacol Sin. 2000;21:845–9. [PubMed] [Google Scholar]
- Hu K, Li GR, Nattel S. Adenosine-induced activation of ATP-sensitive K+ channels in excised membrane patches is mediated by PKC. Am J Physiol. 1999;276:H488–95. doi: 10.1152/ajpheart.1999.276.2.H488. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeley SA, Tucker SE, Naylor RN, Bell GI, Philipson LH. Neonatal diabetes mellitus: a model for personalized medicine. Trends Endocrinol Metab. 2010;21:464–72. doi: 10.1016/j.tem.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble FM, Reimann F. Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003;46:875–91. doi: 10.1007/s00125-003-1143-3. [DOI] [PubMed] [Google Scholar]
- Ashfield R, Gribble FM, Ashcroft SJ, Ashcroft FM. Identification of the high-affinity tolbutamide site on the SUR1 subunit of the KATP channel. Diabetes. 1999;48:1341–7. doi: 10.2337/diabetes.48.6.1341. [DOI] [PubMed] [Google Scholar]
- Mikhailov MV, Mikhailova EA, Ashcroft SJ. Molecular structure of the glibenclamide binding site of the beta-cell KATP channel. FEBS Lett. 2001;499:154–60. doi: 10.1016/s0014-5793(01)02538-8. [DOI] [PubMed] [Google Scholar]
- Gribble FM, Tucker SJ, Ashcroft FM. The interaction of nucleotides with the tolbutamide block of cloned ATP-sensitive K+ channel currents expressed in Xenopus oocytes: a reinterpretation. J Physiol. 1997;504:35–45. doi: 10.1111/j.1469-7793.1997.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros L, Virsolvy A, Salazar G, Bataille D, Blache P. Characterization of low-affinity binding sites for glibenclamide on the Kir6.2 subunit of the beta-cell KATP channel. Biochem Biophys Res Commun. 1999;257:766–70. doi: 10.1006/bbrc.1999.0529. [DOI] [PubMed] [Google Scholar]
- Arnoux JB, de LP, Ribeiro MJ, Hussain K, Blankenstein O, Mohnike K, et al. Congenital hyperinsulinism. Early Hum Dev. 2010;86:287–94. doi: 10.1016/j.earlhumdev.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Kapoor RR, Flanagan SE, James C, Shield J, Ellard S, Hussain K. Hyperinsulinaemic hypoglycaemia. Arch Dis Child. 2009;94:450–7. doi: 10.1136/adc.2008.148171. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM, Gribble FM. New windows on the mechanism of action of KATP channel openers. Trends Pharmacol Sci. 2000;21:439–45. doi: 10.1016/s0165-6147(00)01563-7. [DOI] [PubMed] [Google Scholar]
- Isomoto S, Kurachi Y. Function, regulation, pharmacology, and molecular structure of ATP-sensitive K+ channels in the cardiovascular system. J Cardiovasc Electrophysiol. 1997;8:1431–46. doi: 10.1111/j.1540-8167.1997.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Terzic A, Jahangir A, Kurachi Y. Cardiac ATP-sensitive K+ channels: regulation by intracellular nucleotides and K+ channel-opening drugs. Am J Physiol. 1995;269:C525–45. doi: 10.1152/ajpcell.1995.269.3.C525. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- Moreau C, Jacquet H, Prost AL, D'hahan N, Vivaudou M. The molecular basis of the specificity of action of KATP channel openers. EMBO J. 2000;19:6644–51. doi: 10.1093/emboj/19.24.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau C, Gally F, Jacquet-Bouix H, Vivaudou M. The size of a single residue of the sulfonylurea receptor dictates the effectiveness of KATP channel openers. Mol Pharmacol. 2005;67:1026–33. doi: 10.1124/mol.104.008698. [DOI] [PubMed] [Google Scholar]
- Moreau C, Prost AL, Derand R, Vivaudou M. SUR, ABC proteins targeted by KATP channel openers. J Mol Cell Cardiol. 2005;38:951–63. doi: 10.1016/j.yjmcc.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, et al. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci U S A. 1998;95:10402–6. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami K, Miki T, Kadowaki T, Seino S. Roles of ATP-sensitive K+ channels as metabolic sensors: studies of Kir6.x null mice. Diabetes. 2004;53:S176–80. doi: 10.2337/diabetes.53.suppl_3.s176. [DOI] [PubMed] [Google Scholar]
- Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes. 2005;54:1808–15. doi: 10.2337/diabetes.54.6.1808. [DOI] [PubMed] [Google Scholar]
- MacDonald PE, De Marinis YZ, Ramracheya R, Salehi A, Ma X, Johnson PR, et al. A KATP channel-dependent pathway within alpha cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol. 2007;5:e143. doi: 10.1371/journal.pbio.0050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28:84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969;222:282–4. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- Taborsky GJ Jr, Ahren B, Havel PJ. Autonomic mediation of glucagon secretion during hypoglycemia: implications for impaired alpha-cell responses in type 1 diabetes. Diabetes. 1998;47:995–1005. doi: 10.2337/diabetes.47.7.995. [DOI] [PubMed] [Google Scholar]
- Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, et al. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci. 2001;4:507–12. doi: 10.1038/87455. [DOI] [PubMed] [Google Scholar]
- Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, et al. Hypothalamic KATP channels control hepatic glucose production. Nature. 2005;434:1026–31. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–49. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–32. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- Hardy OT, Hohmeier HE, Becker TC, Manduchi E, Doliba NM, Gupta RK, et al. Functional genomics of the beta-cell: short-chain 3-hydroxyacyl-coenzyme A dehydrogenase regulates insulin secretion independent of K+ currents. Mol Endocrinol. 2007;21:765–73. doi: 10.1210/me.2006-0411. [DOI] [PubMed] [Google Scholar]
- Chutkow WA, Samuel V, Hansen PA, Pu J, Valdivia CR, Makielski JC, et al. Disruption of Sur2-containing KATP channels enhances insulin-stimulated glucose uptake in skeletal muscle. Proc Natl Acad Sci U S A. 2001;98:11760–4. doi: 10.1073/pnas.201390398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Minami K, Zhang L, Morita M, Gonoi T, Shiuchi T, et al. ATP-sensitive potassium channels participate in glucose uptake in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab. 2002;283:E1178–84. doi: 10.1152/ajpendo.00313.2002. [DOI] [PubMed] [Google Scholar]
- Araki E, Lipes MA, Patti ME, Bruning JC, Haag B3, Johnson RS, et al. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–90. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- Minami K, Morita M, Saraya A, Yano H, Terauchi Y, Miki T, et al. ATP-sensitive K+ channel-mediated glucose uptake is independent of IRS-1/phosphatidylinositol 3-kinase signaling. Am J Physiol Endocrinol Metab. 2003;285:E1289–96. doi: 10.1152/ajpendo.00278.2003. [DOI] [PubMed] [Google Scholar]
- Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372:182–6. doi: 10.1038/372182a0. [DOI] [PubMed] [Google Scholar]
- Mu J, Brozinick JT Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–94. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- Musi N, Fujii N, Hirshman MF, Ekberg I, Froberg S, Ljungqvist O, et al. AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes. 2001;50:921–7. doi: 10.2337/diabetes.50.5.921. [DOI] [PubMed] [Google Scholar]
- Shield JP, Gardner RJ, Wadsworth EJ, Whiteford ML, James RS, Robinson DO, et al. Aetiopathology and genetic basis of neonatal diabetes. Arch Dis Child Fetal Neonatal Ed. 1997;76:F39–42. doi: 10.1136/fn.76.1.f39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling MA. ATP-sensitive potassium channels--neonatal diabetes mellitus and beyond. N Engl J Med. 2006;355:507–10. doi: 10.1056/NEJMe068142. [DOI] [PubMed] [Google Scholar]
- Stanik J, Gasperikova D, Paskova M, Barak L, Javorkova J, Jancova E, et al. Prevalence of permanent neonatal diabetes in Slovakia and successful replacement of insulin with sulfonylurea therapy in KCNJ11 and ABCC8 mutation carriers. J Clin Endocrinol Metab. 2007;92:1276–82. doi: 10.1210/jc.2006-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann B, Schober E, Waldhoer T, Koehle J, Flanagan SE, Mackay DJ, et al. Incidence of neonatal diabetes in Austria-calculation based on the Austrian Diabetes Register. Pediatr Diabetes. 2010;11:18–23. doi: 10.1111/j.1399-5448.2009.00530.x. [DOI] [PubMed] [Google Scholar]
- Slingerland AS, Shields BM, Flanagan SE, Bruining GJ, Noordam K, Gach A, et al. Referral rates for diagnostic testing support an incidence of permanent neonatal diabetes in three European countries of at least 1 in 260 000 live births. Diabetologia. 2009;52:1683–5. doi: 10.1007/s00125-009-1416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple IK, James RS, Crolla JA, Sitch FL, Jacobs PA, Howell WM, et al. An imprinted gene(s) for diabetes. Nat Genet. 1995;9:110–2. doi: 10.1038/ng0295-110. [DOI] [PubMed] [Google Scholar]
- Mackay DJ, Coupe AM, Shield JP, Storr JN, Temple IK, Robinson DO. Relaxation of imprinted expression of ZAC and HYMAI in a patient with transient neonatal diabetes mellitus. Hum Genet. 2002;110:139–44. doi: 10.1007/s00439-001-0671-5. [DOI] [PubMed] [Google Scholar]
- Garin I, Edghill EL, Akerman I, Rubio-Cabezas O, Rica I, Locke JM, et al. Recessive mutations in the INS gene result in neonatal diabetes through reduced insulin biosynthesis. Proc Natl Acad Sci U S A. 2010;107:3105–10. doi: 10.1073/pnas.0910533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahamed A, Unnikrishnan AG, Pendsey SS, Nampoothiri S, Bhavani N, Praveen VP, et al. Permanent neonatal diabetes mellitus due to a C96Y heterozygous mutation in the insulin gene. A case report. JOP. 2008;9:715–8. [PubMed] [Google Scholar]
- Molven A, Ringdal M, Nordbo AM, Raeder H, Stoy J, Lipkind GM, et al. Mutations in the insulin gene can cause MODY and autoantibody-negative type 1 diabetes. Diabetes. 2008;57:1131–5. doi: 10.2337/db07-1467. [DOI] [PubMed] [Google Scholar]
- Polak M, Dechaume A, Cave H, Nimri R, Crosnier H, Sulmont V, et al. Heterozygous missense mutations in the insulin gene are linked to permanent diabetes appearing in the neonatal period or in early infancy: a report from the French ND (Neonatal Diabetes) Study Group. Diabetes. 2008;57:1115–9. doi: 10.2337/db07-1358. [DOI] [PubMed] [Google Scholar]
- Edghill EL, Flanagan SE, Patch AM, Boustred C, Parrish A, Shields B, et al. Insulin mutation screening in 1 044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes. 2008;57:1034–42. doi: 10.2337/db07-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoy J, Edghill EL, Flanagan SE, Ye H, Paz VP, Pluzhnikov A, et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci U S A. 2007;104:15040–4. doi: 10.1073/pnas.0707291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997;15:106–10. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- Schwitzgebel VM, Mamin A, Brun T, Ritz-Laser B, Zaiko M, Maret A, et al. Agenesis of human pancreas due to decreased half-life of insulin promoter factor 1. J Clin Endocrinol Metab. 2003;88:4398–406. doi: 10.1210/jc.2003-030046. [DOI] [PubMed] [Google Scholar]
- Thomas IH, Saini NK, Adhikari A, Lee JM, Kasa-Vubu JZ, Vazquez DM, et al. Neonatal diabetes mellitus with pancreatic agenesis in an infant with homozygous IPF-1 Pro63fsX60 mutation. Pediatr Diabetes. 2009;10:492–6. doi: 10.1111/j.1399-5448.2009.00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiec M, Antosik K, Fendler W, Deja G, Jarosz-Chobot P, Mysliwiec M, et al. Novel glucokinase mutations in patients with monogenic diabetes — clinical outline of gck-md and potential for founder effect in slavic population Clin Genet 2011 Feb 23. doi: 10.1111/j.1399-0004.2011.01656.x [DOI] [PubMed]
- Hussain K. Mutations in pancreatic ss-cell Glucokinase as a cause of hyperinsulinaemic hypoglycaemia and neonatal diabetes mellitus. Rev Endocr Metab Disord. 2010;11:179–83. doi: 10.1007/s11154-010-9147-z. [DOI] [PubMed] [Google Scholar]
- Barbetti F, Cobo-Vuilleumier N, Dionisi-Vici C, Toni S, Ciampalini P, Massa O, et al. Opposite clinical phenotypes of glucokinase disease: Description of a novel activating mutation and contiguous inactivating mutations in human glucokinase (GCK) gene. Mol Endocrinol. 2009;23:1983–9. doi: 10.1210/me.2009-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osbak KK, Colclough K, Saint-Martin C, Beer NL, Bellanne-Chantelot C, Ellard S, et al. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum Mutat. 2009;30:1512–26. doi: 10.1002/humu.21110. [DOI] [PubMed] [Google Scholar]
- Turkkahraman D, Bircan I, Tribble ND, Akcurin S, Ellard S, Gloyn AL. Permanent neonatal diabetes mellitus caused by a novel homozygous (T168A) glucokinase (GCK) mutation: initial response to oral sulphonylurea therapy. J Pediatr. 2008;153:122–6. doi: 10.1016/j.jpeds.2007.12.037. [DOI] [PubMed] [Google Scholar]
- Njolstad PR, Sagen JV, Bjorkhaug L, Odili S, Shehadeh N, Bakry D, et al. Permanent neonatal diabetes caused by glucokinase deficiency: inborn error of the glucose-insulin signaling pathway. Diabetes. 2003;52:2854–60. doi: 10.2337/diabetes.52.11.2854. [DOI] [PubMed] [Google Scholar]
- Gloyn AL. Glucokinase (GCK) mutations in hyper- and hypoglycemia: maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemia of infancy. Hum Mutat. 2003;22:353–62. doi: 10.1002/humu.10277. [DOI] [PubMed] [Google Scholar]
- Vaxillaire M, Samson C, Cave H, Metz C, Froguel P, Polak M. Glucokinase gene mutations are not a common cause of permanent neonatal diabetes in France. Diabetologia. 2002;45:454–5. doi: 10.1007/s00125-001-0741-1. [DOI] [PubMed] [Google Scholar]
- Njolstad PR, Sovik O, Cuesta-Munoz A, Bjorkhaug L, Massa O, Barbetti F, et al. Neonatal diabetes mellitus due to complete glucokinase deficiency. N Engl J Med. 2001;344:1588–92. doi: 10.1056/NEJM200105243442104. [DOI] [PubMed] [Google Scholar]
- Rubio-Cabezas O, Diaz GF, Aragones A, Argente J, Campos-Barros A. Permanent neonatal diabetes caused by a homozygous nonsense mutation in the glucokinase gene. Pediatr Diabetes. 2008;9:245–9. doi: 10.1111/j.1399-5448.2007.00361.x. [DOI] [PubMed] [Google Scholar]
- Rubio-Cabezas O, Minton JA, Caswell R, Shield JP, Deiss D, Sumnik Z, et al. Clinical heterogeneity in patients with FOXP3 mutations presenting with permanent neonatal diabetes. Diabetes Care. 2009;32:111–6. doi: 10.2337/dc08-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- Girard CA, Wunderlich FT, Shimomura K, Collins S, Kaizik S, Proks P, et al. Expression of an activating mutation in the gene encoding the KATP channel subunit Kir6.2 in mouse pancreatic beta cells recapitulates neonatal diabetes. J Clin Invest. 2009;119:80–90. doi: 10.1172/JCI35772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming KS, Soliman D, Matemisz LC, Niazi O, Lang Y, Gloyn AL, et al. Coexpression of the type 2 diabetes susceptibility gene variants KCNJ11 E23K and ABCC8 S1369A alter the ATP and sulfonylurea sensitivities of the ATP-sensitive K+ channel. Diabetes. 2009;58:2419–24. doi: 10.2337/db09-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villareal DT, Koster JC, Robertson H, Akrouh A, Miyake K, Bell GI, et al. Kir6.2 variant E23K increases ATP-sensitive K+ channel activity and is associated with impaired insulin release and enhanced insulin sensitivity in adults with normal glucose tolerance. Diabetes. 2009;58:1869–78. doi: 10.2337/db09-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistiakov DA, Potapov VA, Khodirev DC, Shamkhalova MS, Shestakova MV, Nosikov VV. Genetic variations in the pancreatic ATP-sensitive potassium channel, beta-cell dysfunction, and susceptibility to type 2 diabetes. Acta Diabetol. 2009;46:43–9. doi: 10.1007/s00592-008-0056-5. [DOI] [PubMed] [Google Scholar]
- Riedel MJ, Steckley DC, Light PE. Current status of the E23K Kir6.2 polymorphism: implications for type-2 diabetes. Hum Genet. 2005;116:133–45. doi: 10.1007/s00439-004-1216-5. [DOI] [PubMed] [Google Scholar]
- Riedel MJ, Boora P, Steckley D, de Vries G, Light PE. Kir6.2 polymorphisms sensitize beta-cell ATP-sensitive potassium channels to activation by acyl CoAs: a possible cellular mechanism for increased susceptibility to type 2 diabetes. Diabetes. 2003;52:2630–5. doi: 10.2337/diabetes.52.10.2630. [DOI] [PubMed] [Google Scholar]
- Gloyn AL, Weedon MN, Owen KR, Turner MJ, Knight BA, Hitman G, et al. Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes. 2003;52:568–72. doi: 10.2337/diabetes.52.2.568. [DOI] [PubMed] [Google Scholar]
- Flanagan SE, Clauin S, Bellanne-Chantelot C, de Lonlay P, Harries LW, Gloyn AL, et al. Update of mutations in the genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat. 2009;30:170–80. doi: 10.1002/humu.20838. [DOI] [PubMed] [Google Scholar]
- Remedi MS, Koster JC. KATP channelopathies in the pancreas. Pflugers Arch. 2010;460:307–20. doi: 10.1007/s00424-009-0756-x. [DOI] [PubMed] [Google Scholar]
- Gloyn AL, Cummings EA, Edghill EL, Harries LW, Scott R, Costa T, et al. Permanent neonatal diabetes due to paternal germline mosaicism for an activating mutation of the KCNJ11 gene encoding the Kir6.2 subunit of the beta-cell potassium adenosine triphosphate channel. J Clin Endocrinol Metab. 2004;89:3932–5. doi: 10.1210/jc.2004-0568. [DOI] [PubMed] [Google Scholar]
- Ioannou YS, Ellard S, Hattersley A, Skordis N. KCNJ11 activating mutations cause both transient and permanent neonatal diabetes mellitus in Cypriot patients. Pediatr Diabetes. 2011;12:133–7. doi: 10.1111/j.1399-5448.2010.00743.x. [DOI] [PubMed] [Google Scholar]
- Kochar IP, Kulkarni KP. Transient neonatal diabetes due to Kcnj11 mutation. Indian Pediatr. 2010;47:359–60. [PubMed] [Google Scholar]
- Khadilkar VV, Khadilkar AV, Kapoor RR, Hussain K, Hattersley AT, Ellard S. KCNJ11 activating mutation in an Indian family with remitting and relapsing diabetes. Indian J Pediatr. 2010;77:551–4. doi: 10.1007/s12098-010-0062-9. [DOI] [PubMed] [Google Scholar]
- Stanik J, Lethby M, Flanagan SE, Gasperikova D, Milosovicova B, Lever M, et al. Coincidence of a novel KCNJ11 missense variant R365H with a paternally inherited 6q24 duplication in a patient with transient neonatal diabetes. Diabetes Care. 2008;31:1736–7. doi: 10.2337/dc08-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amato E, Tammaro P, Craig TJ, Tosi A, Giorgetti R, Lorini R, et al. Variable phenotypic spectrum of diabetes mellitus in a family carrying a novel KCNJ11 gene mutation. Diabet Med. 2008;25:651–6. doi: 10.1111/j.1464-5491.2008.02443.x. [DOI] [PubMed] [Google Scholar]
- Flanagan SE, Patch AM, Mackay DJ, Edghill EL, Gloyn AL, Robinson D, et al. Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes. 2007;56:1930–7. doi: 10.2337/db07-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji T, Nagashima K, Kurokawa K, Kawai M, Oishi M, Akazawa Y, et al. The C42R mutation in the Kir6.2 (KCNJ11) gene as a cause of transient neonatal diabetes, childhood diabetes, or later-onset, apparently type 2 diabetes mellitus. J Clin Endocrinol Metab. 2005;90:3174–8. doi: 10.1210/jc.2005-0096. [DOI] [PubMed] [Google Scholar]
- Gloyn AL, Reimann F, Girard C, Edghill EL, Proks P, Pearson ER, et al. Relapsing diabetes can result from moderately activating mutations in KCNJ11. Hum Mol Genet. 2005;14:925–34. doi: 10.1093/hmg/ddi086. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Garin I, Castano L, Argente J, Munoz-Calvo MT, Perez de NG, et al. Neonatal diabetes caused by mutations in sulfonylurea receptor 1: interplay between expression and Mg-nucleotide gating defects of ATP-sensitive potassium channels. J Clin Endocrinol Metab. 2010;95:E473–8. doi: 10.1210/jc.2010-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet H, Proks P, Lafond M, Aittoniemi J, Sansom MS, Flanagan SE, et al. A mutation (R826W) in nucleotide-binding domain 1 of ABCC8 reduces ATPase activity and causes transient neonatal diabetes. EMBO Rep. 2008;9:648–54. doi: 10.1038/embor.2008.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaxillaire M, Dechaume A, Busiah K, Cave H, Pereira S, Scharfmann R, et al. New ABCC8 mutations in relapsing neonatal diabetes and clinical features. Diabetes. 2007;56:1737–41. doi: 10.2337/db06-1540. [DOI] [PubMed] [Google Scholar]
- Babenko AP, Polak M, Cave H, Busiah K, Czernichow P, Scharfmann R, et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med. 2006;355:456–66. doi: 10.1056/NEJMoa055068. [DOI] [PubMed] [Google Scholar]
- Batra CM, Gupta N, Atwal G, Gupta V. Transient neonatal diabetes due to activating mutation in the ABCC8 gene encoding SUR1. Indian J Pediatr. 2009;76:1169–72. doi: 10.1007/s12098-009-0222-y. [DOI] [PubMed] [Google Scholar]
- Flanagan SE, Patch AM, Mackay DJ, Edghill EL, Gloyn AL, Robinson D, et al. Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes. 2007;56:1930–7. doi: 10.2337/db07-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patch AM, Flanagan SE, Boustred C, Hattersley AT, Ellard S. Mutations in the ABCC8 gene encoding the SUR1 subunit of the KATP channel cause transient neonatal diabetes, permanent neonatal diabetes or permanent diabetes diagnosed outside the neonatal period. Diabetes Obes Metab. 2007;9:28–39. doi: 10.1111/j.1463-1326.2007.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig TJ, Shimomura K, Holl RW, Flanagan SE, Ellard S, Ashcroft FM. An in-frame deletion in Kir6.2 (KCNJ11) causing neonatal diabetes reveals a site of interaction between Kir6.2 and SUR1. J Clin Endocrinol Metab. 2009;94:2551–7. doi: 10.1210/jc.2009-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTaggart JS, Clark RH, Ashcroft FM. The role of the KATP channel in glucose homeostasis in health and disease: more than meets the islet. J Physiol. 2010;588:3201–9. doi: 10.1113/jphysiol.2010.191767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest. 2005;115:2047–58. doi: 10.1172/JCI25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proks P, Antcliff JF, Lippiat J, Gloyn AL, Hattersley AT, Ashcroft FM. Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features. Proc Natl Acad Sci U S A. 2004;101:17539–44. doi: 10.1073/pnas.0404756101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon DD, Stanley CA.Permanent neonatal diabetes mellitus. 1993. In: Pagon RA, Bird TD, Dolan CR, Stephens K, editors. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2008 Feb 08 [updated 2008 Mar 04]. [PubMed]
- Clark RH, McTaggart JS, Webster R, Mannikko R, Iberl M, Sim XL, et al. Muscle dysfunction caused by a KATP channel mutation in neonatal diabetes is neuronal in origin. Science. 2010;329:458–61. doi: 10.1126/science.1186146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM. New uses for old drugs: neonatal diabetes and sulphonylureas. Cell Metab. 2010;11:179–81. doi: 10.1016/j.cmet.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Koster JC, Cadario F, Peruzzi C, Colombo C, Nichols CG, Barbetti F. The G53D mutation in Kir6.2 (KCNJ11) is associated with neonatal diabetes and motor dysfunction in adulthood that is improved with sulfonylurea therapy. J Clin Endocrinol Metab. 2008;93:1054–61. doi: 10.1210/jc.2007-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson ER, Flechtner I, Njolstad PR, Malecki MT, Flanagan SE, Larkin B, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355:467–77. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- Zung A, Glaser B, Nimri R, Zadik Z. Glibenclamide treatment in permanent neonatal diabetes mellitus due to an activating mutation in Kir6.2. J Clin Endocrinol Metab. 2004;89:5504–7. doi: 10.1210/jc.2004-1241. [DOI] [PubMed] [Google Scholar]
- Wambach JA, Marshall BA, Koster JC, White NH, Nichols CG. Successful sulfonylurea treatment of an insulin-naive neonate with diabetes mellitus due to a KCNJ11 mutation. Pediatr Diabetes. 2010;11:286–8. doi: 10.1111/j.1399-5448.2009.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum-Hasan J, Polychronakos C, Brill H. Familial permanent neonatal diabetes with KCNJ11 mutation and the response to glyburide therapy--a three-year follow-up. J Pediatr Endocrinol Metab. 2008;21:895–903. doi: 10.1515/jpem.2008.21.9.895. [DOI] [PubMed] [Google Scholar]
- Klupa T, Skupien J, Mirkiewicz-Sieradzka B, Gach A, Noczynska A, Szalecki M, et al. Diabetic retinopathy in permanent neonatal diabetes due to Kir6.2 gene mutations: the results of a minimum 2-year follow-up after the transfer from insulin to sulphonylurea. Diabet Med. 2009;26:663–4. doi: 10.1111/j.1464-5491.2009.02711.x. [DOI] [PubMed] [Google Scholar]
- Mohamadi A, Clark LM, Lipkin PH, Mahone EM, Wodka EL, Plotnick LP. Medical and developmental impact of transition from subcutaneous insulin to oral glyburide in a 15-yr-old boy with neonatal diabetes mellitus and intermediate DEND syndrome: extending the age of KCNJ11 mutation testing in neonatal DM. Pediatr Diabetes. 2010;11:203–7. doi: 10.1111/j.1399-5448.2009.00548.x. [DOI] [PubMed] [Google Scholar]
- Stoy J, Greeley SA, Paz VP, Ye H, Pastore AN, Skowron KB, et al. Diagnosis and treatment of neonatal diabetes: a United States experience. Pediatr Diabetes. 2008;9:450–9. doi: 10.1111/j.1399-5448.2008.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slingerland AS, Nuboer R, Hadders-Algra M, Hattersley AT, Bruining GJ. Improved motor development and good long-term glycaemic control with sulfonylurea treatment in a patient with the syndrome of intermediate developmental delay, early-onset generalised epilepsy and neonatal diabetes associated with the V59M mutation in the KCNJ11 gene. Diabetologia. 2006;49:2559–63. doi: 10.1007/s00125-006-0407-0. [DOI] [PubMed] [Google Scholar]
- Slingerland AS, Hurkx W, Noordam K, Flanagan SE, Jukema JW, Meiners LC, et al. Sulphonylurea therapy improves cognition in a patient with the V59M KCNJ11 mutation. Diabet Med. 2008;25:277–81. doi: 10.1111/j.1464-5491.2007.02373.x. [DOI] [PubMed] [Google Scholar]
- Masia R, Koster JC, Tumini S, Chiarelli F, Colombo C, Nichols CG, et al. An ATP-binding mutation (G334D) in KCNJ11 is associated with a sulfonylurea-insensitive form of developmental delay, epilepsy, and neonatal diabetes. Diabetes. 2007;56:328–36. doi: 10.2337/db06-1275. [DOI] [PubMed] [Google Scholar]
- Sumnik Z, Kolouskova S, Wales JK, Komarek V, Cinek O. Sulphonylurea treatment does not improve psychomotor development in children with KCNJ11 mutations causing permanent neonatal diabetes mellitus accompanied by developmental delay and epilepsy (DEND syndrome) Diabet Med. 2007;24:1176–8. doi: 10.1111/j.1464-5491.2007.02228.x. [DOI] [PubMed] [Google Scholar]
- Della MT, Battistim C, Radonsky V, Savoldelli RD, Damiani D, Kok F, et al. Glibenclamide unresponsiveness in a Brazilian child with permanent neonatal diabetes mellitus and DEND syndrome due to a C166Y mutation in KCNJ11 (Kir6.2) gene. Arq Bras Endocrinol Metabol. 2008;52:1350–5. doi: 10.1590/s0004-27302008000800024. [DOI] [PubMed] [Google Scholar]
- Kumaraguru J, Flanagan SE, Greeley SA, Nuboer R, Stoy J, Philipson LH, et al. Tooth discoloration in patients with neonatal diabetes after transfer onto glibenclamide: a previously unreported side effect. Diabetes Care. 2009;32:1428–30. doi: 10.2337/dc09-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codner E, Flanagan SE, Ugarte F, Garcia H, Vidal T, Ellard S, et al. Sulfonylurea treatment in young children with neonatal diabetes: dealing with hyperglycemia, hypoglycemia, and sick days. Diabetes Care. 2007;30:e28–9. doi: 10.2337/dc06-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R, Proks P. ATP-sensitive potassium channels in health and disease. Adv Exp Med Biol. 2010;654:165–92. doi: 10.1007/978-90-481-3271-3_8. [DOI] [PubMed] [Google Scholar]
- Remedi MS, Kurata HT, Scott A, Wunderlich FT, Rother E, Kleinridders A, et al. Secondary consequences of beta cell inexcitability: identification and prevention in a murine model of KATP-induced neonatal diabetes mellitus. Cell Metab. 2009;9:140–51. doi: 10.1016/j.cmet.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain K. Congenital hyperinsulinism. Semin Fetal Neonatal Med. 2005;10:369–76. doi: 10.1016/j.siny.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Hussain K, Cosgrove KE. From congenital hyperinsulinism to diabetes mellitus: the role of pancreatic beta-cell KATP channels. Pediatr Diabetes. 2005;6:103–13. doi: 10.1111/j.1399-543X.2005.00109.x. [DOI] [PubMed] [Google Scholar]
- Glaser B, Thornton P, Otonkoski T, Junien C. Genetics of neonatal hyperinsulinism. Arch Dis Child Fetal Neonatal Ed. 2000;82:F79–86. doi: 10.1136/fn.82.2.F79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempoux C, Guiot Y, Dubois D, Nollevaux MC, Saudubray JM, Nihoul-Fekete C, et al. Pancreatic beta-cell proliferation in persistent hyperinsulinemic hypoglycemia of infancy: an immunohistochemical study of 18 cases. Mod Pathol. 1998;11:444–9. [PubMed] [Google Scholar]
- Sempoux C, Guiot Y, Rahier J. The focal form of persistent hyperinsulinemic hypoglycemia of infancy. Diabetes. 2001;50:S182–3. doi: 10.2337/diabetes.50.2007.s182. [DOI] [PubMed] [Google Scholar]
- Sempoux C, Guiot Y, Jaubert F, Rahier J. Focal and diffuse forms of congenital hyperinsulinism: the keys for differential diagnosis. Endocr Pathol. 2004;15:241–6. doi: 10.1385/ep:15:3:241. [DOI] [PubMed] [Google Scholar]
- Glaser B, Landau H, Permutt MA. Neonatal hyperinsulinism. Trends Endocrinol Metab. 1999;10:55–61. doi: 10.1016/s1043-2760(98)00102-7. [DOI] [PubMed] [Google Scholar]
- Fournet JC, Mayaud C, de Lonlay P, Verkarre V, Rahier J, Brunelle F, et al. Loss of imprinted genes and paternal SUR1 mutations lead to focal form of congenital hyperinsulinism. Horm Res. 2000;53:2–6. doi: 10.1159/000053197. [DOI] [PubMed] [Google Scholar]
- Fournet JC, Mayaud C, de Lonlay P, Gross-Morand MS, Verkarre V, Castanet M, et al. Unbalanced expression of 11p15 imprinted genes in focal forms of congenital hyperinsulinism: association with a reduction to homozygosity of a mutation in ABCC8 or KCNJ11. Am J Pathol. 2001;158:2177–84. doi: 10.1016/S0002-9440(10)64689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournet JC, Junien C. Genetics of congenital hyperinsulinism. Endocr Pathol. 2004;15:233–40. doi: 10.1385/ep:15:3:233. [DOI] [PubMed] [Google Scholar]
- de Lonlay P, Fournet JC, Touati G, Groos MS, Martin D, Sevin C, et al. Heterogeneity of persistent hyperinsulinaemic hypoglycaemia. A series of 175 cases. Eur J Pediatr. 2002;161:37–48. doi: 10.1007/s004310100847. [DOI] [PubMed] [Google Scholar]
- de Lonlay P, Fournet JC, Rahier J, Gross-Morand MS, Poggi-Travert F, Foussier V, et al. Somatic deletion of the imprinted 11p15 region in sporadic persistent hyperinsulinemic hypoglycemia of infancy is specific of focal adenomatous hyperplasia and endorses partial pancreatectomy. J Clin Invest. 1997;100:802–7. doi: 10.1172/JCI119594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser B, Ryan F, Donath M, Landau H, Stanley CA, Baker L, et al. Hyperinsulinism caused by paternal-specific inheritance of a recessive mutation in the sulfonylurea-receptor gene. Diabetes. 1999;48:1652–7. doi: 10.2337/diabetes.48.8.1652. [DOI] [PubMed] [Google Scholar]
- Verkarre V, Fournet JC, de Lonlay P, Gross-Morand MS, Devillers M, Rahier J, et al. Paternal mutation of the sulfonylurea receptor (SUR1) gene and maternal loss of 11p15 imprinted genes lead to persistent hyperinsulinism in focal adenomatous hyperplasia. J Clin Invest. 1998;102:1286–91. doi: 10.1172/JCI4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huopio H, Shyng SL, Otonkoski T, Nichols CG. KATP channels and insulin secretion disorders. Am J Physiol Endocrinol Metab. 2002;283:E207–16. doi: 10.1152/ajpendo.00047.2002. [DOI] [PubMed] [Google Scholar]
- James C, Kapoor RR, Ismail D, Hussain K. The genetic basis of congenital hyperinsulinism. J Med Genet. 2009;46:289–99. doi: 10.1136/jmg.2008.064337. [DOI] [PubMed] [Google Scholar]
- Chan KW, Zhang H, Logothetis DE. N-terminal transmembrane domain of the SUR controls trafficking and gating of Kir6 channel subunits. EMBO J. 2003;22:3833–43. doi: 10.1093/emboj/cdg376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero LC, Pozo RJ, Munoz Calvo MT, Martos MG, Donoso MA, Rubio CO, et al. Hyperinsulinism-hyperammonemia syndrome due to a de novo mutation in exon 7 (G979A) of the glutamate dehydrogenase gene with excellent response to diazoxide. An Pediatr (Barc) 2004;61:433–7. doi: 10.1016/s1695-4033(04)78419-2. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Koda N, Kadowaki H, Ogawa Y, Kimura S, Kadowaki T, et al. A Japanese case of congenital hyperinsulinism with hyperammonemia due to a mutation in glutamate dehydrogenase (GLUD1) gene. Intern Med. 2001;40:32–7. doi: 10.2169/internalmedicine.40.32. [DOI] [PubMed] [Google Scholar]
- Marquard J, Palladino AA, Stanley CA, Mayatepek E, Meissner T. Rare forms of congenital hyperinsulinism. Semin Pediatr Surg. 2011;20:38–44. doi: 10.1053/j.sempedsurg.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Palladino AA, Stanley CA. The hyperinsulinism/hyperammonemia syndrome. Rev Endocr Metab Disord. 2010;11:171–8. doi: 10.1007/s11154-010-9146-0. [DOI] [PubMed] [Google Scholar]
- Chik KK, Chan CW, Lam CW, Ng KL. Hyperinsulinism and hyperammonaemia syndrome due to a novel missense mutation in the allosteric domain of the glutamate dehydrogenase 1 gene. J Paediatr Child Health. 2008;44:517–9. doi: 10.1111/j.1440-1754.2008.01361.x. [DOI] [PubMed] [Google Scholar]
- de Lonlay P, Giurgea I, Sempoux C, Touati G, Jaubert F, Rahier J, et al. Dominantly inherited hyperinsulinaemic hypoglycaemia. J Inherit Metab Dis. 2005;28:267–76. doi: 10.1007/s10545-005-7057-0. [DOI] [PubMed] [Google Scholar]
- Stanley CA. Hyperinsulinism/hyperammonemia syndrome: insights into the regulatory role of glutamate dehydrogenase in ammonia metabolism. Mol Genet Metab. 2004;81:S45–51. doi: 10.1016/j.ymgme.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Fujioka H, Okano Y, Inada H, Asada M, Kawamura T, Hase Y, et al. Molecular characterisation of glutamate dehydrogenase gene defects in Japanese patients with congenital hyperinsulinism/hyperammonaemia. Eur J Hum Genet. 2001;9:931–7. doi: 10.1038/sj.ejhg.5200749. [DOI] [PubMed] [Google Scholar]
- Kelly A, Stanley CA. Disorders of glutamate metabolism. Ment Retard Dev Disabil Res Rev. 2001;7:287–95. doi: 10.1002/mrdd.1040. [DOI] [PubMed] [Google Scholar]
- Meissner T, Marquard J, Cobo-Vuilleumier N, Maringa M, Rodriguez-Bada P, Garcia-Gimeno MA, et al. Diagnostic difficulties in glucokinase hyperinsulinism. Horm Metab Res. 2009;41:320–6. doi: 10.1055/s-0028-1102922. [DOI] [PubMed] [Google Scholar]
- Christesen HB, Tribble ND, Molven A, Siddiqui J, Sandal T, Brusgaard K, et al. Activating glucokinase (GCK) mutations as a cause of medically responsive congenital hyperinsulinism: prevalence in children and characterisation of a novel GCK mutation. Eur J Endocrinol. 2008;159:27–34. doi: 10.1530/EJE-08-0203. [DOI] [PubMed] [Google Scholar]
- Molven A, Matre GE, Duran M, Wanders RJ, Rishaug U, Njolstad PR, et al. Familial hyperinsulinemic hypoglycemia caused by a defect in the SCHAD enzyme of mitochondrial fatty acid oxidation. Diabetes. 2004;53:221–7. doi: 10.2337/diabetes.53.1.221. [DOI] [PubMed] [Google Scholar]
- Bas F, Darendeliler F, Demirkol D, Bundak R, Saka N, Gunoz H. Successful therapy with calcium channel blocker (nifedipine) in persistent neonatal hyperinsulinemic hypoglycemia of infancy. J Pediatr Endocrinol Metab. 1999;12:873–8. doi: 10.1515/jpem.1999.12.6.873. [DOI] [PubMed] [Google Scholar]
- Eichmann D, Hufnagel M, Quick P, Santer R. Treatment of hyperinsulinaemic hypoglycaemia with nifedipine. Eur J Pediatr. 1999;158:204–6. doi: 10.1007/s004310051049. [DOI] [PubMed] [Google Scholar]
- Lindley KJ, Dunne MJ, Kane C, Shepherd RM, Squires PE, James RF, et al. Ionic control of beta cell function in nesidioblastosis. A possible therapeutic role for calcium channel blockade. Arch Dis Child. 1996;74:373–8. doi: 10.1136/adc.74.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbag P, Pathak A, Vaidya M, Shahid SK. Persistent hyperinsulinemic hypoglycemia of infancy-successful therapy with nifedipine. Indian J Pediatr. 2002;69:271–2. doi: 10.1007/BF02734240. [DOI] [PubMed] [Google Scholar]
- Straub SG, Cosgrove KE, Ammala C, Shepherd RM, O'Brien RE, Barnes PD, et al. Hyperinsulinism of infancy: the regulated release of insulin by KATP channel-independent pathways. Diabetes. 2001;50:329–39. doi: 10.2337/diabetes.50.2.329. [DOI] [PubMed] [Google Scholar]