Abstract
Nearly half of breast carcinoma metastases will become clinically evident five or more years after primary tumor ablation. This implies that metastatic cancer cells survived over an extended timeframe without emerging as detectable nodules. The liver is a common metastatic destination, whose parenchymal hepatocytes have been shown to impart a less invasive, dormant phenotype on metastatic cancer cells. We investigated whether hepatic nonparenchymal cells (NPCs) contributed to metastatic breast cancer cell outgrowth and a mesenchymal phenotypic shift indicative of emergence. Co-culture experiments of primary human hepatocytes, NPCs or endothelial cell lines (TMNK-1 orHMEC-1) and breast cancer cell lines (MCF-7 or MDA-MB-231) were conducted. Exposure of carcinoma cells to NPC-conditioned medium isolated soluble factors contributing to outgrowth. To elucidate outgrowth mechanism, epidermal growth factor receptor (EGFR) inhibition co-culture experiments were performed. Flow cytometry analyses and immunofluorescence staining were conducted to quantify breast cancer cell outgrowth and phenotype, respectively. Outgrowth of the MDA-MB-231 cells within primary NPC co-cultures was substantially greater than in hepatocyte-only or hepatocyte+NPC co-cultures. MCF-7 cells co-cultured with human NPCs as well as with the endothelial NPC subtypes grew out significantly more than controls. MCF-7 cells underwent a mesenchymal shift as indicated by spindle morphology, membrane clearance of E-cadherin, and p38 nuclear translocation when in HMEC-1 co-culture. HMEC-1-conditioned medium induced similar results suggesting that secretory factors are responsible for this transition while blocking EGFR blunted theMCF-7 outgrowth. We conclude that NPCs in the metastatic hepatic niche secrete factors that can induce a partial mesenchymal shift in epithelial breast cancer cells thus initiating outgrowth, and that this is in part mediated by EGFR activation. These data suggest that changes in the parenchymal cell and NPC ratios (or activation status) in the liver metastatic microenvironment may contribute to emergence from metastatic dormancy.
Keywords: Mesenchymal to epithelial reverting transition, Epithelial to mesenchymal transition, Nonparenchymal cells, Hepatic microenvironment, Metastatic dormancy, Metastatic emergence, Mesenchymal to epithelial transition
Introduction
Breast cancer metastatic dormancy is a state in which cancer cells avoid clinical detection over many years or decades—these cancer cells likely remain as microscopic foci or single cell deposits [1, 2]. Metastatic dormancy requires a combination of cell survival and escape from apoptosis-signaling pathways, coupled with a phenotype suitable for avoiding the body’s immune response [3, 4]. Thus, metastatic breast cancer dormancy is likely not sustainable by the invasive, mesenchymal phenotype but rather through a partial epithelial reversion in which the cells are in a quiescent state [5–7].
An epithelial hallmark of many non-invasive carcinomas is the membrane expression of E-cadherin that forms homotypic adherins junctions limiting aberrant autocrine EGF signaling and sequestering otherwise non-canonical protein translocation such as nuclear β-catenin [8, 9]. Many human metastatic breast cancer lesions express membranous E-cadherin, whereas their paired primary tumors are E-cadherin negative [10, 11]. This dormancy-associated E-cadherin positive, epithelial phenotype in part protects metastases from chemotherapy [12]. However, a secondary epithelial to mesenchymal transition is thought to underlie latent metastatic outgrowth [6, 13], though the signals that drive this are unknown. To better elucidate the effects of the cellular microenvironment on the outgrowth of breast cancer, we chose to study the cells of a common organ in which breast cancer cells lie dormant, the liver.
Breast cancer cells preferentially home to the liver (with additional organotropism) that harbors cryptic metastases for years to decades [14]. The liver is comprised of parenchymal cells (hepatocytes) that are responsible for the majority of the functions associated with the liver including acute phase protein production and xenobiotic metabolism. Hepatocytes are also the cell type most commonly used in studying the hepatic microenvironment. Nonparenchymal cells (NPCs) provide for the structure and microvasculature in the liver. These cells comprise approximately 40 % of the liver by cell number [15] and are prolific sources of secreted cytokines differentially expressed in normal versus distressed hepatic microenvironments [16, 17].
Because the specific microenvironmental factors involved in the transition from dormancy to metastatic outgrowth are poorly understood, we undertook these investigations to elucidate the potential hepatic microenvironmental cell types that underlie the transition from dormancy to outgrowth in metastatic breast cancer.
Results
Primary human NPCs confer a growth advantage to breast cancer cells
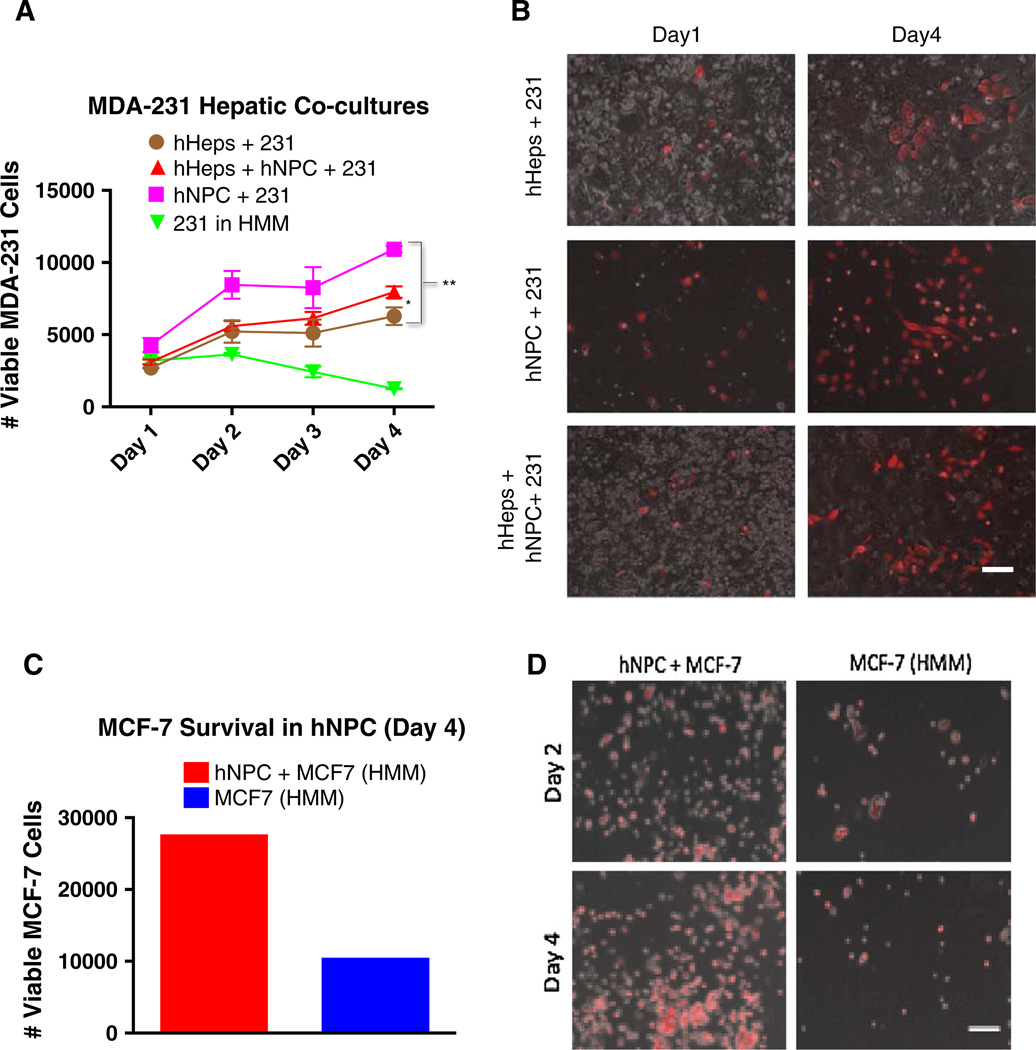
We have previously published that the MDA-MB-231 cell line in hepatocyte co-cultures undergoes a partial mesenchymal-to-epithelial reversion (MErT) in part characterized by the membrane re-expression of E-cadherin and a decreased rate of growth [5]. This partial reversion mimics the more epithelial phenotype seen in clinical metastases [10], and so we chose this model to investigate the effects of NPCs on the outgrowth of MDA-MB-231 cells. Measurement of RFP positive MDA-MB-231 cells by flow cytometry on days 1–4 revealed an increased outgrowth of MDA-MB-231 cells cultured with NPCs that was significantly higher than co-culture with either hepatocytes or hepatocytes and NPCs (Fig. 1a). The MDA-MB-231 outgrowth data were confirmed by microscopy (Fig. 1b).
Fig. 1.
Primary human NPCs confer a growth advantage to breast cancer cells. a Flow cytometry was performed to quantify Annexin V negative, RFP-positive MDA-MB-231 cells in 3 treatment conditions: (1) hepatocyte co-cultures, (2) nonparenchymal co-cultures, and (3) hepatocyte + NPC cultures. Day 0 the hepatocytes or NPC cells were plated on Collagen-coated polystyrene plates and allowed to adhere for 4 h. MDA-231 cells were then inoculated into the cultures. Days 1–4 represent consecutive 24 h harvesting post MDA-231 seeding. b Phase contrast imaging with a fluorescent overlay (Red MDA-MB-231 cells) as representative images on Day 1 versus Day 4. Flow cytometry was performed (c) to quantify Annexin V negative, RFP-positive MCF-7 cells in co-culture with primary human NPCs with representative images of MCF-7-RFP cells in co-culture with primary NPCs (d) compared to their HMM controls demonstrating carcinoma outgrowth in the presence of NPCs. Statistics using ANOVA with Tukey’s test for significance at p values <.01 (between hHeps + 231 and hHeps + hNPC + 231) and p values <.001 (between hHeps + 231 and hNPC + 231). Scale bars 250 µm. All experiments performed at least three independent times
The MCF-7 breast cancer cell line is non-metastatic and expresses high levels of the tumor suppressor protein, E-cadherin. Although MCF-7 cells proliferate in standard culture (RPMI with 10 % serum), from an epithelial phenotype perspective, they serve as a surrogate dormant breast cancer cell type. So, we aimed to test whether NPCs could also induce proliferation among the MCF-7 cells via imaging and flow cytometry. MCF-7 cells co-cultured with primary human NPCs in serum-free hepatocyte maintenance medium (HMM) substantially outgrew the MCF-7 cells mono-cultured in serum-free HMM as the negative control (Fig. 1c, d).
Endothelial cell lines confer a grow-out advantage to breast cancer cells
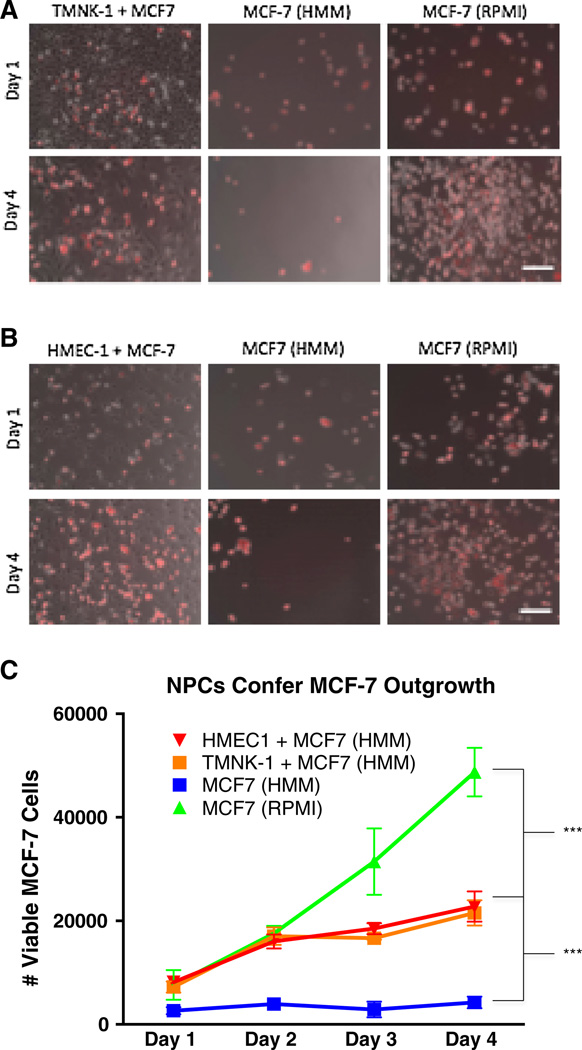
Liver NPCs are comprised of stellate cells, kupffer cells, and liver sinusoidal endothelial cells [18]. We aimed to determine which NPC type could be responsible for the outgrowth phenotype observed in MDA-MB-231 and MCF-7 cells. As micrometastases form in the hepatic sub-endothelial space [19], we investigated whether endothelial cells promote expansion and alter the phenotype of the epithelial breast cancer cells. For this, we used the noninvasive breast cancer line MCF-7, and two different types of endothelial cells: an immortalized human liver sinusoidal cell line (TMNK-1) [20] to represent the hepatic microvasculature, and a human microvascular cell line (HMEC-1) [21]. Co-cultures of the MCF-7 cells and NPC cell lines were performed by first seeding the NPC cells in their respective growth medium for 3–4 h to allow attachment. Next, the growth medium was aspirated, and the culture plates rinsed twice in phosphate buffered saline (PBS) before seeding the MCF-7 cells. The MCF-7 cells exogenously expressing RFP were seeded under three culture conditions: (1) co-cultured with TMNK-1 cells in serum-free HMM, (2) mono-cultured MCF-7 in serum-free HMM, and (3) mono-cultured MCF-7 in their normal growth medium (RPMI with 10 % serum). Low viability of MCF-7 cells in HMM medium was observed throughout the 4-day experimental timeframe (negative control), while the MCF-7 cells experienced exponential growth in RPMI with 10 % serum (positive control). Notably, the MCF-7 cells substantially outgrew in co-culture with the TMNK-1 cells in HMM as observed by microscopy and flow cytometry, suggesting that the TMNK-1 cells provide a survival and grow-out advantage (Fig. 2a, c).
Fig. 2.
NPC lines co-cultured with MCF-7-RFP cells confer carcinoma outgrowth. a Phase contrast imaging with a fluorescence overlay (Red MCF-7 cells) are representative images on Day 1 and Day 4 across 3 conditions: TMNK-1 co-culture in HMM, MCF-7-RFP in HMM, and MCF-7-RFP in RPMI. The MCF-7 cells experience outgrowth in the presence of TMNK-1 cells. b Same conditions and results as in a but with HMEC-1 cells. The negative control MCF-7 cells alone in HMM (serum free) fail to outgrow, while exponentially outgrowing in the RPMI (10 % serum) positive controls as expected. Flow cytometry for Annexin V negative, RFP positive cells (MCF-7-RFP) used to quantify cell counts in a separate biological replicate and performed in triplicate wells (c). Scale bars 250 µm. Day 1 is 24 h after MCF-7-RFP seeding. Statistics using ANOVA with Tukey’s test for significance at p values <.001. All experiments performed at least three independent times
After co-culturing MCF-7 cells with the liver-specific endothelial cell line (TMNK-1), we investigated whether other endothelial cell lines would induce the same MCF-7 outgrowth phenotype. We, therefore, co-cultured MCF-7 cells with the HMEC-1 cell line. The HMEC-1 co-cultured MCF-7 cells experienced outgrowth similar to the TMNK-1 co-cultures by microscopy and flow cytometry (Fig. 2b, c).
NPCs confer a partial mesenchymal phenotypic shift
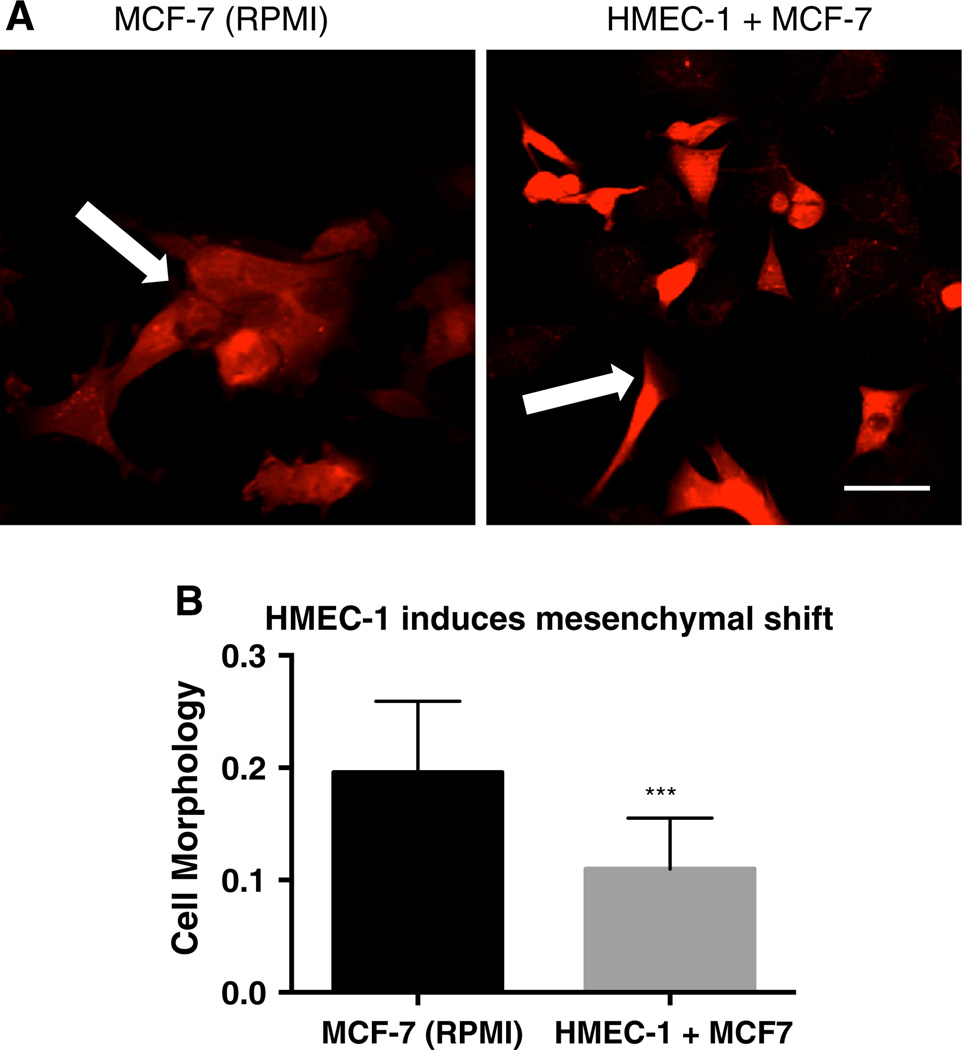
To investigate the effect of NPCs on breast cancer cell phenotype, we co-cultured the epithelial MCF-7 cells with HMEC-1 cells. MCF-7 cells in standard growth medium are characterized by a cobblestone appearance and tight cell–cell contacts (Fig. 3a, left). In MCF-7 and HMEC-1 co-cultures, a sub-population of the MCF-7 cells became spindle-shaped and did not readily form cell–cell contacts indicative of a more mesenchymal phenotype (Fig. 3a, right, b).
Fig. 3.
HMEC-1 co-culture confers a mesenchymal phenotype. Fluorescent imaging depicts MCF-7-RFP cells in growth medium (a, left column) versus in HMEC-1 co-culture in HMM (a, right column) after 4 days of co-culture. b MCF-7 mesenchymal phenotypic shift in a quantified by the ratio of the midpoint diameter divided by cell perimeter. Scale bar 50 µm. All experiments performed at least three independent times. p value <.001 using a two-tailed Student’s t test
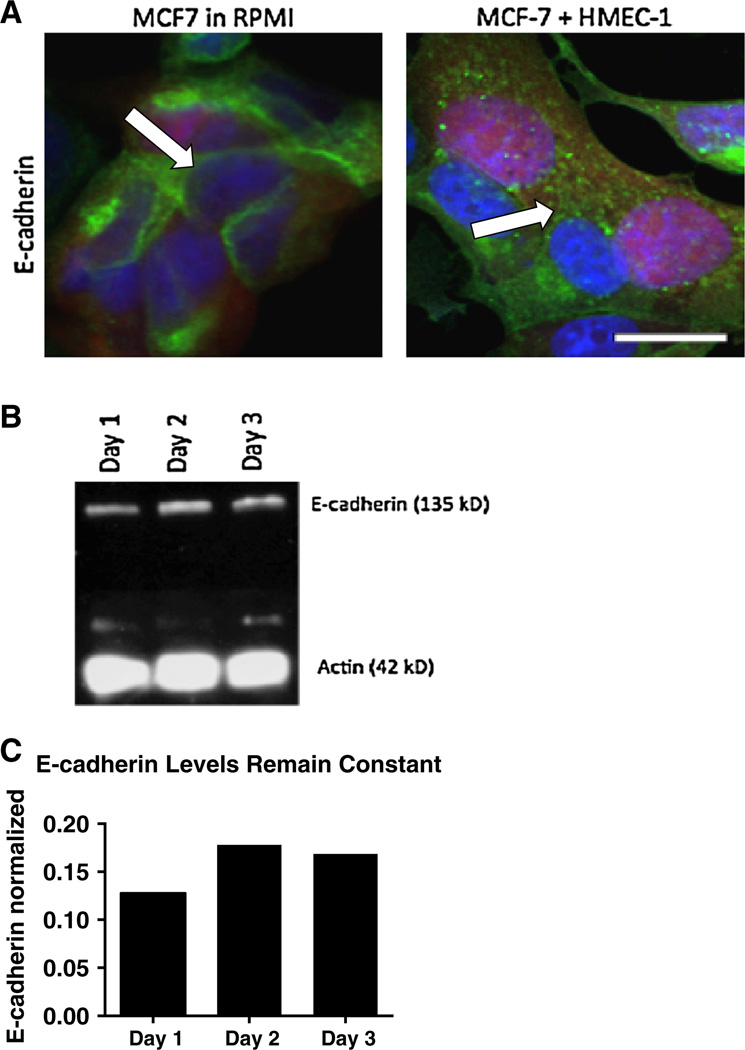
The epithelial cell phenotype of MCF-7 cells was confirmed by E-cadherin membrane staining that forms cell–cell adherins junctions in the RPMI controls (Fig. 4a, left). In the HMEC-1 co-cultures, a sub-population of MCF-7 cells fail to maintain membrane E-cadherin expression with E-cadherin noted in cytosolic vesicles (Fig. 4a, right). Immunoblotting revealed that total cell E-cadherin protein levels remain constant (Fig. 4b, c) throughout the HMEC-1 co-cultures even as the E-cadherin localization changes.
Fig. 4.
HMEC-1 co-culture confers E-cadherin membrane de-localization to MCF-7 cells. a Confocal imaging for E-cadherin (green) shows membrane-bound staining in RPMI controls while co-cultured MCF-7 cells (red) internalize E-cadherin within the cytoplasm on day 4 of co-culture. b Immunoblot for E-cadherin in MCF-7 cells cocultured with HMEC-1 cells on days 1–3 with E-cadherin immunoblot quantification normalized to actin (c). Scale bar 50 µm. All experiments performed at least three independent times
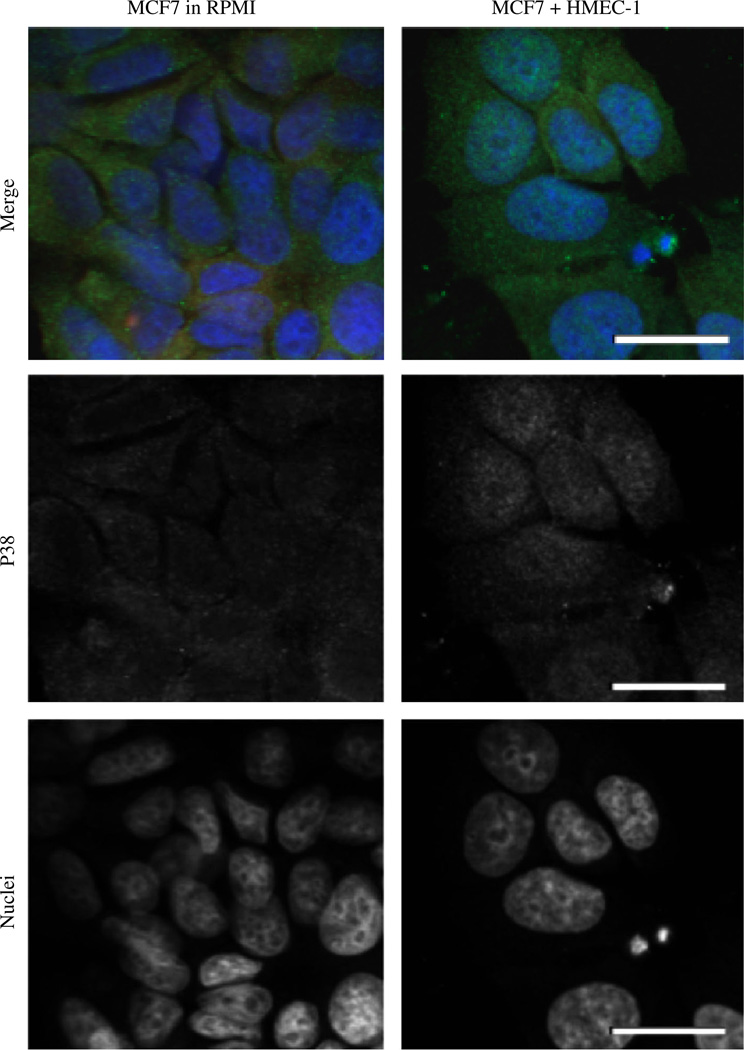
It has been previously been demonstrated that the mitogen-activated protein kinase, p38, is involved with pathways supporting cell cycle arrest in the G0/G1 checkpoints, as it forms complexes in the cytoplasm [22]. Further studies have suggested that changes in p38 to extracellular signal-regulated kinase ratios contribute to metastatic dormancy or emergence [23, 24]. The nuclear translocation of p38 from the cytosol has been implicated in stress response pathways including the upregulation of tumor necrosis factor alpha (TNF-α) [25]—with TNF-α being an important promoter of carcinoma invasion and metastasis [26]. So we investigated the effect of MCF-7 p38 differential localization in the HMEC-1 co-cultures. MCF-7 cells co-cultured with HMEC-1 cells exhibited p38 concentrated in the cell nuclei whereas it was found to be cytosolic in the control cultures (Fig. 5).
Fig. 5.
HMEC-1 cells induce p38 nuclear translocation in MCF-7 cells. Confocal imaging for phosphorylated p38 (Green) shows a nuclear translocation in HMEC-1 co-cultures versus the RPMI control. Scale bar 50 µm. All experiments performed at least three independent times
NPC-induced MCF-7 outgrowth is partially mediated by soluble factor secretion through epidermal growth factor receptor (EGFR) activation
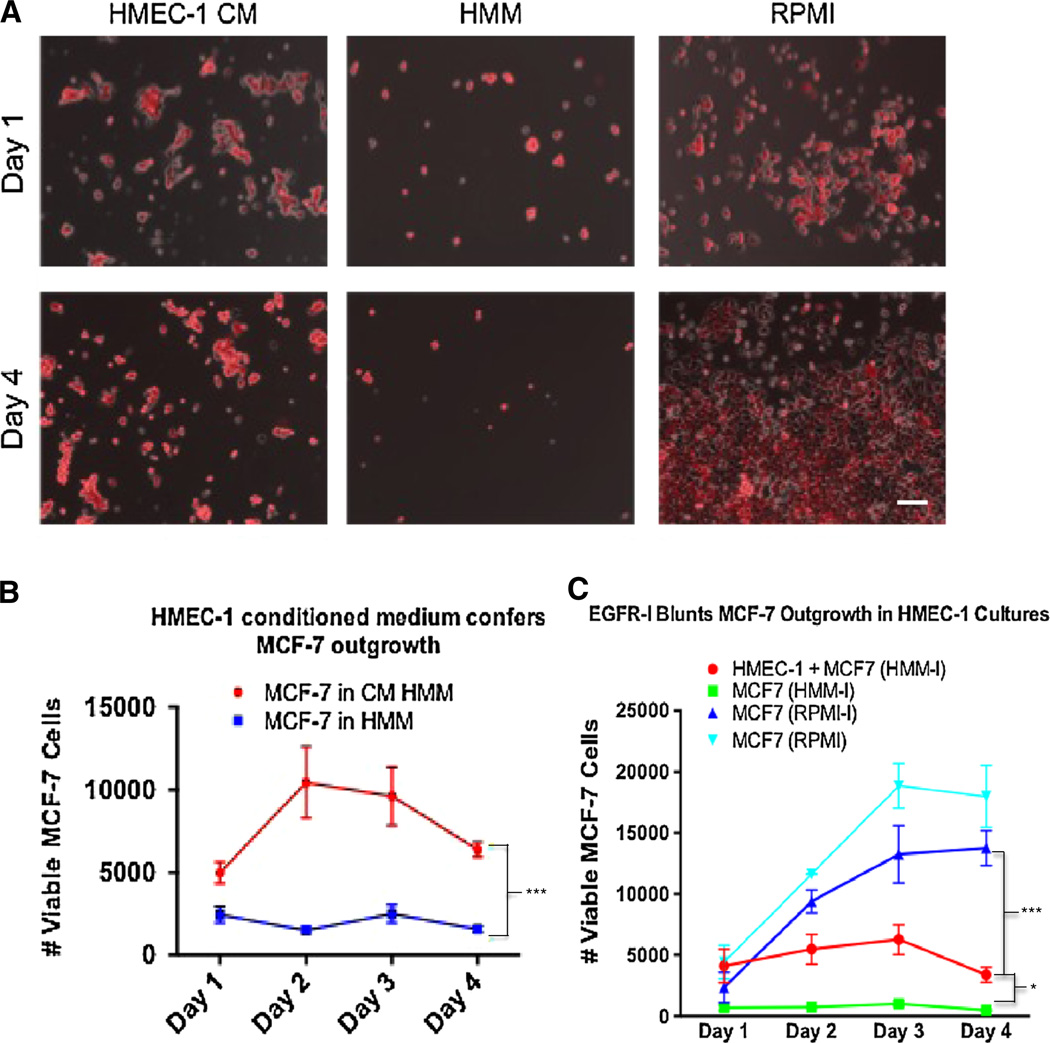
We investigated whether paracrine soluble factor signaling from the HMEC-1 cells to the MCF-7 cells was at least in part responsible for the breast cancer cell outgrowth phenotype. MCF-7 cells were cultured in HMEC-1-conditioned medium cultures (in HMM), which prompted an outgrowth phenotype as noted in the HMEC-1 co-cultures (Fig. 2b, c) but to a lesser extent (Fig. 6a, b).
Fig. 6.
HMEC-1-conditioned medium supports MCF-7 outgrowth in part mediated by EGFR activation. a Phase contrast images of MCF-7-RFP cells with fluorescent overlay across three culture conditions: (1) HMEC-1 conditioned medium, (2) HMM, and (3) RPMI growth medium. b MCF-7 cells in conditioned medium exhibited a grow-out advantage over MCF-7 cells cultured in the HMM controls. c EGFR inhibition blunts MCF-7 outgrowth in HMEC-1 cultures. 500 nM of EGFR inhibitor was introduced into the three treatment groups while MCF-7 positive growth controls in RPMI were free from inhibitor. Statistics using ANOVA with Tukey’s test for significance at p values <.01. Scale bar 250 µm. All experiments performed at least three independent times
In order to uncover a plausible mechanism for the NPC-induced MCF-7 outgrowth and partial EMT switch, and given that MCF-7 cells express EGFR (though not aberrantly) and that hepatic NPCs are known to secrete EGFR ligands [27], we investigated whether the mechanism of HMEC-1 signaling to MCF-7 cells involved the EGFR pathway. The HMEC-1 and MCF-7 co-cultures were treated with an EGFR receptor inhibitor, and we evaluated outgrowth by flow cytometry to determine if the outgrowth phenotype would be attenuated. Inhibition of EGFR significantly blunted MCF-7 cell outgrowth while the positive control of EGFR inhibition in MCF-7 RPMI cultures promoted outgrowth (Fig. 6c).
Discussion
Despite advances in successfully treating breast cancer within the primary site, breast cancer has the insidious propensity to recur many years or decades after primary tumor diagnosis and intervention [3, 14]. This recurrence is typically refractory to current treatments including radiation, chemotherapy, and surgical resection [4, 28]. The long latency period between primary tumor diagnosis and clinical metastatic presentation is termed metastatic dormancy. One mechanistic trigger of metastatic dormancy may be additional genetic aberrations within the metastatic cells conferring a growth arrest, or even a balanced equation between carcinoma proliferation and apoptosis. However, another explanation that has been largely overlooked in comparison is the role of the stromal cells within the metastatic niche conferring dormancy or emergence (as well as drug resistance). It has been previously demonstrated that the epithelial versus mesenchymal status of breast carcinoma cells impacts their proliferation rate, survival signaling, and resistance to chemotherapeutics [12]. However, only experimental methods such as exogenous EMT inducers (e.g., Snail, TWIST) or introduction of EGFR receptor ligands into culture have demonstrated a mesenchymal shift indicative of an outgrowth phenotype. To date, very few investigations of endogenous factors inducing a metastatic breast cancer outgrowth phenotype have been conducted.
Further, developing a physiologically relevant dormant metastatic experimental system has been a significant challenge [7, 29, 30] though progress is being made. However, as an alternative to studying metastatic dormancy directly, we aimed to study physiologic components in the liver microenvironment that could be responsible for emergence from metastatic dormancy. The principle being that if we can uncover metastatic breast cancer proliferation inducers (a phenotypic switch back to a mesenchymal phenotype), then we might be able to backward integrate into what may prompt the precursor dormancy period. We therefore directed our attention to the hepatic stromal cells with particular emphasis on the endothelial cells. We investigated the endothelial cell effect because breast cancer hepatic metastases must first extravasate and form under the endothelial space [19].
Though these investigations, we first identified that the highly invasive and mesenchymal MDA-MB-231 cell outgrowth is attenuated by primary human hepatocyte co-culture, but that this outgrowth is exacerbated by the inclusion of human NPCs. This provided evidence that the hepatic NPCs (physiologically present in the liver) provided a favorable outgrowth environment for the breast cancer cells in this culture system. However, considering that the MDA-MB-231 cells are highly metastatic and mesenchymal, they don’t serve as surrogate dormant, epithelial-like metastases. We, therefore, co-cultured the epithelial, non-metastatic human immortalized breast cancer cell line (MCF-7) with human NPCs and confirmed the same carcinoma outgrowth phenotype. In order to further identify which of the NPC types were responsible for the outgrowth, we co-cultured the MCF-7 cells with two human immortalized endothelial cell lines and confirmed the MCF-7 outgrowth phenotype—suggesting that the outgrowth can be driven by NPCs with the endothelial cells being at least partly responsible.
Because we hypothesized that the emergence from metastatic dormancy required a secondary reversion to a mesenchymal phenotype, we investigated and discovered that the endothelial cells drove the E-cadherin positive MCF-7 breast cancer cells to a partial mesenchymal phenotype, while they simultaneously conferred this grow-out advantage. Moreover, theMCF-7 E-cadherin was internalized into the cytosol in endothelial co-cultures while total MCF-7 E-cadherin remained constant, suggesting that E-cadherin may be readily available for membrane re-localization to potentially establish a secondary MErT for survival if outgrowth were interrupted. We further investigated whether MCF-7 p38 was modulated by the endothelial cell co-cultures and discovered a p38 nuclear translocation suggesting a nuclear factor role for metastatic outgrowth.
These investigations have certain limitations that merit further investigation. Although we used the MCF-7 cell line as a surrogate dormant carcinoma—we did so by epithelial phenotype only, as these cells proliferate in standard culture. The MCF-7 p38 nuclear localization in endothelial co-cultures may be conflated by a potential stress response induced by the otherwise non-permissive HMM, though the negative control is not possible because the MCF-7 cells alone in HMM perish. It is yet to be determined which soluble factors are responsible for activating EGFR, and we did not investigate whether GPCRs might compensate for EGFR blocking. However, these are natural investigative progressions that we aim to address in future work.
In conclusion, these data suggest that perturbations of the parenchymal hepatocytes and NPCs in the liver metastatic microenvironment may differentially contribute to metastatic dormancy, stability, or emergence. Therefore, dormancy and emergence from dormancy may hinge upon the inactivated or activated status of the hepatic NPCs, thus making the metastatic niche a potential therapeutic target to combat metastatic disease that may one day illuminate new drugs, and therapies that could be effective against larger patient sub-populations independent of the carcinoma’s genetic gradations. Further still, considerations for long-term hormonal adjuvant therapy (e.g., tamoxifen) as effective combatants for reducing late breast cancer recurrence have received significant scrutiny while holding promise [31]. However, the mechanism of action from these endocrine adjuvants may be through in part the mitigation of the activated status of the NPCs in the metastatic niche versus direct action against the carcinoma cells. So, further investigations to optimize endocrine inhibiting adjuvants among resident, metastatic stromal cells may further improve patient outcomes of endocrine-resistant breast cancer.
Materials and methods
Cells and cell culture
RFP expressing MDA-MB-231 and MCF-7 cell lines were transfected as previously described [5]. To maintain selection for RFP positive breast cancer cells, MCF-7 cells were cultured with 900 µg/ml G418, and MDA-MB-231 were cultured with 5 µg/ml puromycin in RPMI-1640 (Life Technologies, Carslbad, CA) supplemented with 10 % FBS until used in the experiments. HMEC-1 cells were a kind gift from Dr. Richard Bodnar of the Pittsburgh Veterans Affairs (Pittsburgh, PA). HMEC-1 cells were cultured in MCDB 131 medium (Life Technologies, Carlsbad, CA) with 10 % FBS, 10 ng/ml EGF, 1 µg/ml hydrocortisone, and 10 mM l-glutamine until used for experiments. TMNK-1 cells were a kind gift from Dr. Alex Soto-Gutierrez of the University of Pittsburgh. TMNK-1 cells were cultured in DMEM (Life Technologies, Carlsbad, CA) with 4.5 g/ml glucose, 1 % penicillin–streptomycin, and 10 % FBS until used for experiments. HMEC-1 and TMNK-1 co-cultures with MCF-7 or MDA-MB-231 cells were performed in serum free HMM (Lonza, Anaheim, CA) supplemented with SingleQuots®(Lonza, Anaheim, CA).
Co-culture
TMNK-1 or HMEC-1 cells (NPC cell lines) were trypsinized from T75 flasks at 80 % confluence and resuspended in their respective growth medium with 10 % FBS to inactivate the trypsin. NPC were spun into pellets, their growth medium aspirated, and resuspended in HMM. 20,000 NPC cells/cm2 were seeded into 12-well polystyrene plates and allowed to attach for 3–4 h before breast cancer cell co-culture. Breast cancer cell lines were prepared the same way as the NPC cell lines but seeded at 1,000 cells/cm2 after the NPC attachment period.
Imaging
Phase contrast images were captured by an Olympus inverted scope and digitally captured using Spot AdvancedTM software (Diagnostics Instruments, Macomb, Michigan). Confocal images were captured on an Olympus Fluoview 1000 scope (Olympus, Center Valley, PA) and captured using Fluoview ViewerTM. Immunofluorescence was performed by 24-h primary antibody incubation against E-cadherin Cat# 610182 (BD Biosciences) and phospho p38 Cat# 4631S (Cell Signaling, Danvers, MA) each at 1:200 dilution. Alexa Fluor®488 rabbit anti-mouse (Life Technologies) secondary antibody was incubated for 45 min at room temperature at 1:500 dilution. Hoechst was incubated for 10 min at 1:500 dilution at room temperature.
Cell morphology quantification
Images from MCF-7+HMEC-1 co-cultures in HMM versus MCF-7 in RPMI growth medium were imported into ImageJ Version 1.44i (U. S. National Institutes of Health, Bethesda, Maryland). Two fields per experimental condition were analyzed. Cell perimeters and midpoint widths were manually traced and measured in pixel units using the ImageJ functions. The ratio of width versus perimeter was computed for each cell and the mean values, standard deviations, and Student’s t test (2 tailed) were calculated using Prism 6 (GraphPad Software, Inc., La Jolla, CA).
Immunoblotting
A 7.5 % SDS–PAGE gel resolved cell lysates and were subsequently transferred to a PVDF membrane. Membranes were blocked with 1 % serum albumin for 1 h and incubated overnight with E-cadherin primary antibody Cat# 3195 (Cell Signaling, Danvers, MA) or Actin (Abcam, Cambridge, MA). Chemiluminescence was detected on film following incubation with peroxidase-conjugated secondary antibodies.
Flow cytometry
Cell cultures in each well were incubated in trypsin for 30 min until dissociated. 2 ml of PBS with 2 % FBS were added to each well, transferred to flow tubes, and pelleted. The media was aspirated, and cell pellets incubated with the reconstituted components of the Alexa Fluor®488 Annexin V/Dead Cell Apoptosis Kit (Life Technologies, Carlsbad, CA) for 15 min. The reaction was terminated by adding 100 µl of the Annexin binding buffer to each tube. 10 µl of CountBrightTM Absolute Counting Beads (Life Technologies, Carlsbad, CA) were added to each tube to compute the number of RFP positive, Annexin V negative breast cancer cells. Cell suspensions were run on the BD LSRFortessaTM flow cytometer and BD FACSDiva Software (BD Biosciences, Franklin Lakes, NJ).
EGFR inhibition
PD 153035 hydrochloride Cat# 1037 (Tocris Bioscience, Minneapolis, MN) was incubated in EGFR inhibition treatment cultures at a 500 nM concentration throughout the experiment. HMEC-1 cells were plated and seeded with MCF-7 cells as described above except that the MCF-7 cells were resuspended in the EGFR inhibitor media where applied.
Statistical analysis
Graphical data are provided as mean ± standard deviation from three independent technical replicates. Except where otherwise noted, p-value significance was evaluated using ANOVA and Tukey method and set at a minimum 0.05. Images were representative of at least three independent fields per well.
Acknowledgments
These studies were supported by Grants from the NIH NCATS program (TR000496) and by a Merit Award from the VA. The authors thank members of Wells laboratory and Linda Griffith and her laboratory members at MIT.
Abbreviations
- MErT
Mesenchymal to epithelial reverting transition
- EMT
Epithelial to mesenchymal transition
- MET
Mesenchymal to epithelial transition
- NPCs
Nonparenchymal cells
- EGFR
Epidermal growth factor receptor
- HMM
Hepatocyte maintenance medium
Footnotes
Conflict of interest The authors have no conflicts to declare.
Contributor Information
Donald P. Taylor, Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, USA Department of Pathology, University of Pittsburgh and Pittsburgh VA Health System, Pittsburgh, PA, USA.
Amanda Clark, Department of Pathology, University of Pittsburgh and Pittsburgh VA Health System, Pittsburgh, PA, USA.
Sarah Wheeler, Department of Pathology, University of Pittsburgh and Pittsburgh VA Health System, Pittsburgh, PA, USA.
Alan Wells, Email: wellsa@upmc.edu, Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, USA; Department of Pathology, University of Pittsburgh and Pittsburgh VA Health System, Pittsburgh, PA, USA; University of Pittsburgh School of Medicine, 3550 Terrace Street, S713 Scaife Hall, Pittsburgh, PA 15261, USA.
References
- 1.Demicheli R. Tumour dormancy: findings and hypotheses from clinical research on breast cancer. Semin Cancer Biol. 2001;11(4):297–306. doi: 10.1006/scbi.2001.0385. [DOI] [PubMed] [Google Scholar]
- 2.Taylor DP, Wells JZ, Savol A, Chennubhotla C, Wells A. Modeling boundary conditions for balanced proliferation in metastatic latency. Clin Cancer Res. 2013;19(5):1063–1070. doi: 10.1158/1078-0432.CCR-12-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguirre-Ghiso JA. The problem of cancer dormancy: understanding the basic mechanisms and identifying therapeutic opportunities. Cell Cycle. 2006;5(16):1740–1743. doi: 10.4161/cc.5.16.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Townson JL, Chambers AF. Dormancy of solitary metastatic cells. Cell Cycle. 2006;5(16):1744–1750. doi: 10.4161/cc.5.16.2864. [DOI] [PubMed] [Google Scholar]
- 5.Chao YL, Shepard CR, Wells A. Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Mol Cancer. 2010;9:179. doi: 10.1186/1476-4598-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunasinghe NP, Wells A, Thompson EW, Hugo HJ. Mesenchymal-epithelial transition (MET) as a mechanism for metastatic colonisation in breast cancer. Cancer Metastasis Rev. 2012 doi: 10.1007/s10555-012-9377-5. [DOI] [PubMed] [Google Scholar]
- 7.Yates CC, Shepard CR, Stolz DB, Wells A. Co-culturing human prostate carcinoma cells with hepatocytes leads to increased expression of E-cadherin. Br J Cancer. 2007;96(8):1246–1252. doi: 10.1038/sj.bjc.6603700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kassis J, Lauffenburger DA, Turner T, Wells A. Tumor invasion as dysregulated cell motility. Semin Cancer Biol. 2001;11(2):105–117. doi: 10.1006/scbi.2000.0362. [DOI] [PubMed] [Google Scholar]
- 9.Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci USA. 2011;108(29):11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chao Y, Wu Q, Acquafondata M, Dhir R, Wells A. Partial mesenchymal to epithelial reverting transition in breast and prostate cancer metastases. Cancer Microenviron. 2012;5(1):19–28. doi: 10.1007/s12307-011-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kowalski PJ, Rubin MA, Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003;5(6):R217–R222. doi: 10.1186/bcr651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao Y, Wu Q, Shepard C, Wells A. Hepatocyte induced re-expression of E-cadherin in breast and prostate cancer cells increases chemoresistance. Clin Exp Metastasis. 2012;29(1):39–50. doi: 10.1007/s10585-011-9427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wendt MK, Taylor MA, Schiemann BJ, Schiemann WP. Down-regulation of epithelial cadherin is required to initiate metastatic outgrowth of breast cancer. Mol Biol Cell. 2011;22(14):2423–2435. doi: 10.1091/mbc.E11-04-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naumov GN, MacDonald IC, Weinmeister PM, Kerkvliet N, Nadkarni KV, Wilson SM, Morris VL, Groom AC, Chambers AF. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res. 2002;62(7):2162–2168. [PubMed] [Google Scholar]
- 15.Kmiec Z. Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol. 2001;161:III–XIII. 1–151. doi: 10.1007/978-3-642-56553-3. [DOI] [PubMed] [Google Scholar]
- 16.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213(2):286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5(10):836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 18.Michalopoulos G, Cianciulli HD, Novotny AR, Kligerman AD, Strom SC, Jirtle RL. Liver regeneration studies with rat hepatocytes in primary culture. Cancer Res. 1982;42(11):4673–4682. [PubMed] [Google Scholar]
- 19.Vlodavsky I, Fuks Z, Bar-Ner M, Ariav Y, Schirrmacher V. Lymphoma cell-mediated degradation of sulfated proteoglycans in the subendothelial extracellular matrix: relationship to tumor cell metastasis. Cancer Res. 1983;43(6):2704–2711. [PubMed] [Google Scholar]
- 20.Matsumura T, Takesue M, Westerman KA, Okitsu T, Sakaguchi M, Fukazawa T, Totsugawa T, Noguchi H, Yamamoto S, Stolz DB, Tanaka N, Leboulch P, Kobayashi N. Establishment of an immortalized human-liver endothelial cell line with SV40T and hTERT. Transplantation. 2004;77(9):1357–1365. doi: 10.1097/01.tp.0000124286.82961.7e. [DOI] [PubMed] [Google Scholar]
- 21.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Investig Dermatol. 1992;99(6):683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 22.Coulthard LR, White DE, Jones DL, McDermott MF, Burchill SA. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol Med. 2009;15(8):369–379. doi: 10.1016/j.molmed.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aguirre-Ghiso JA, Bragado P, Sosa MS. Metastasis awakening: targeting dormant cancer. Nat Med. 2013;19(3):276–277. doi: 10.1038/nm.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ranganathan AC, Adam AP, Zhang L, Aguirre-Ghiso JA. Tumor cell dormancy induced by p38SAPK and ER-stress signaling: an adaptive advantage for metastatic cells? Cancer Biol Ther. 2006;5(7):729–735. doi: 10.4161/cbt.5.7.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong X, Ming X, Deng P, Jiang Y. Mechanisms regulating the nuclear translocation of p38 MAP kinase. J Cell Biochem. 2010;110(6):1420–1429. doi: 10.1002/jcb.22675. [DOI] [PubMed] [Google Scholar]
- 26.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25(3):409–416. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 27.De Wever O, Nguyen QD, Van Hoorde L, Bracke M, Bruyneel E, Gespach C, Mareel M. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J. 2004;18(9):1016–1018. doi: 10.1096/fj.03-1110fje. [DOI] [PubMed] [Google Scholar]
- 28.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 29.Rubio N, Espana L, Fernandez Y, Blanco J, Sierra A. Metastatic behavior of human breast carcinomas overexpressing the Bcl-x(L) gene: a role in dormancy and organospecificity. Lab Investig. 2001;81(5):725–734. doi: 10.1038/labinvest.3780281. [DOI] [PubMed] [Google Scholar]
- 30.Shepard CR, Kassis J, Whaley DL, Kim HG, Wells A. PLC gamma contributes to metastasis of in situ-occurring mammary and prostate tumors. Oncogene. 2007;26(21):3020–3026. doi: 10.1038/sj.onc.1210115. [DOI] [PubMed] [Google Scholar]
- 31.Davidson NE, Visvanathan K, Emens L. New findings about endocrine therapy for breast cancer. Breast. 2003;12(6):368–372. doi: 10.1016/s0960-9776(03)00138-3. [DOI] [PubMed] [Google Scholar]