Abstract
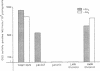
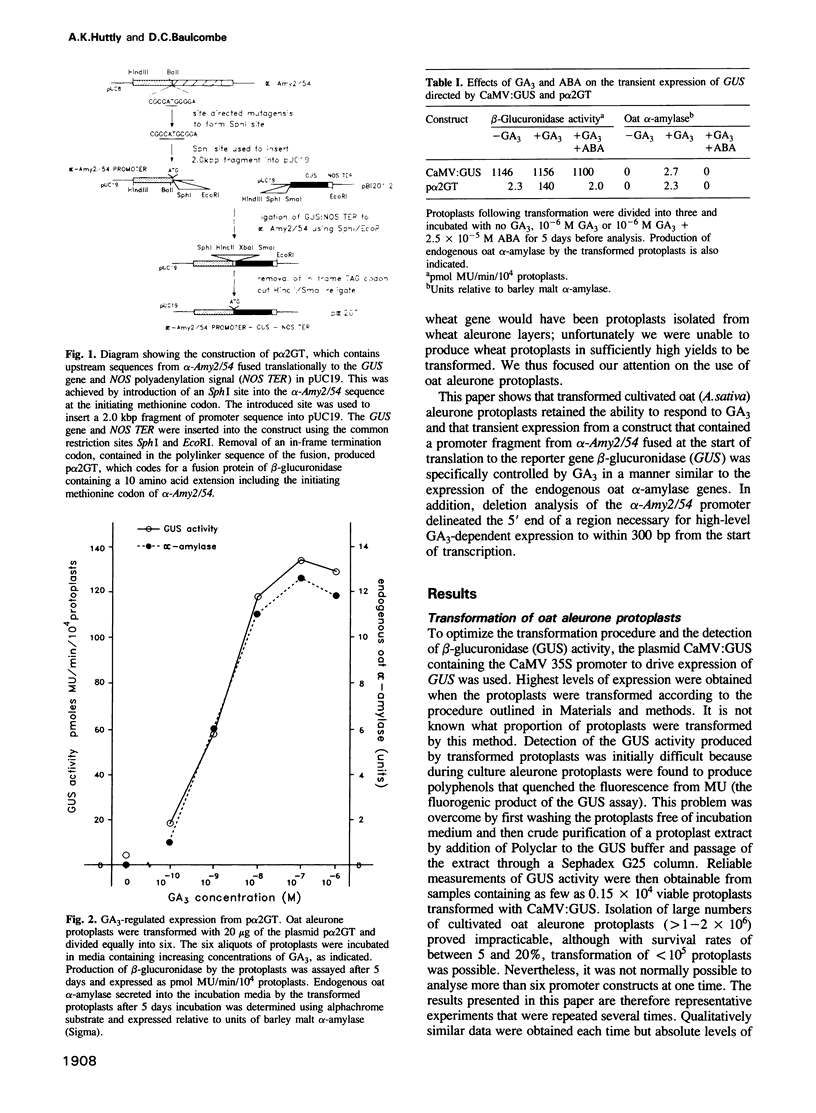
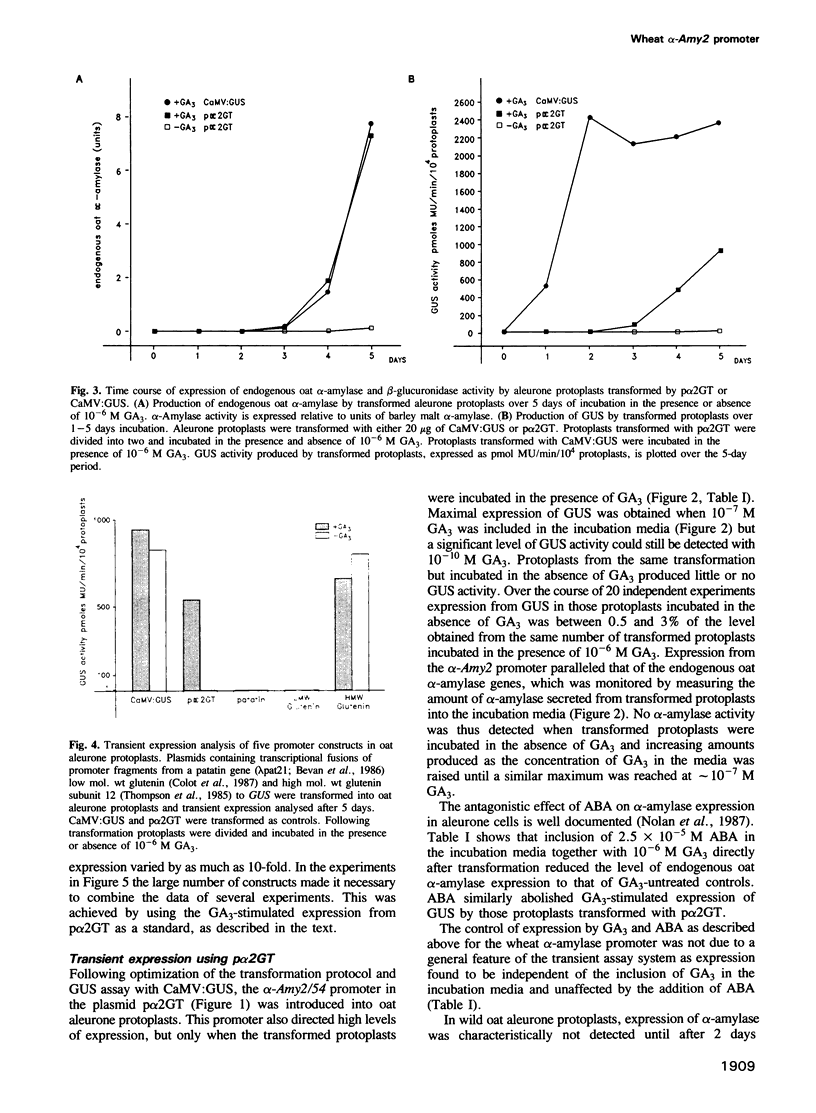
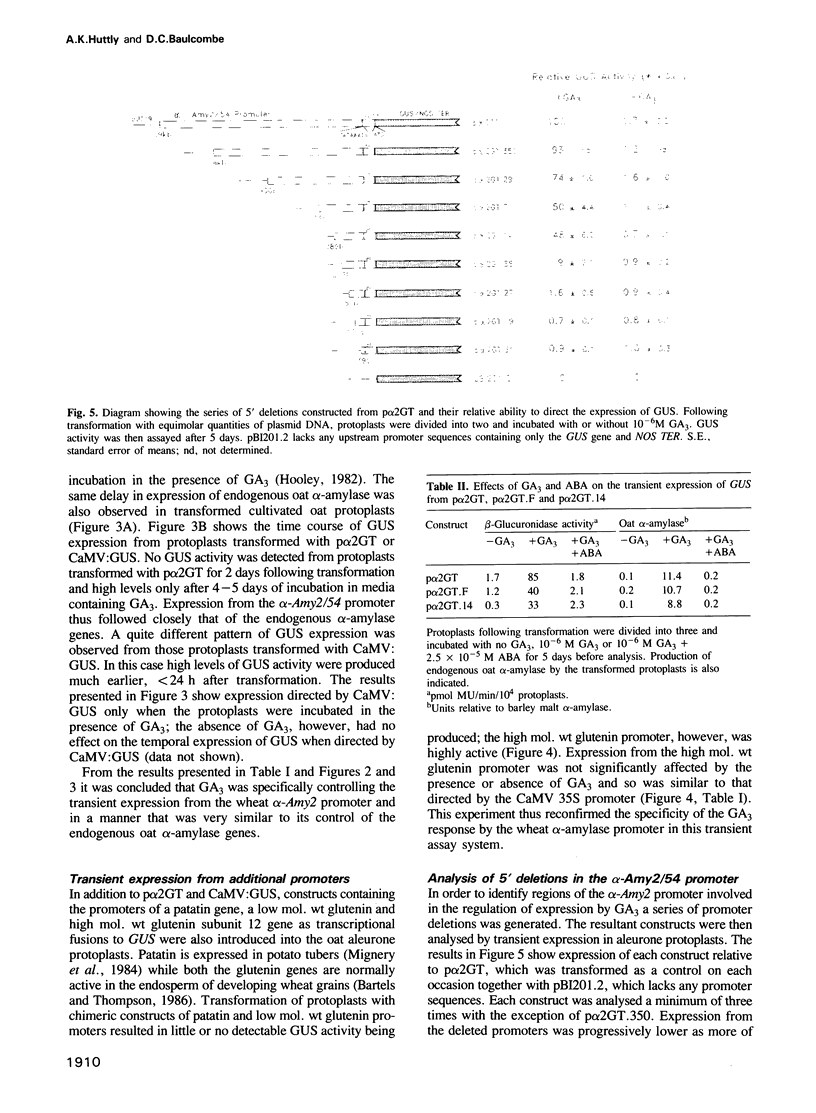
Gibberellin (GA3)-responsive aleurone protoplasts isolated from Avena sativa have been successfully used as a transient expression system to analyse promoter fusions between the wheat α-amylase gene α-Amy2/54 and the reporter gene GUS. Following PEG-mediated uptake of plasmid DNA, transient expression directed by the α-Amy2/54 promoter was found to be regulated in the same way as the endogenous oat α-amylase genes. Expression was thus dependent on the inclusion of GA3 in the protoplast incubation media, could not be detected before a lag phase of 2 days following transformation and was inhibited by simultaneous addition of abscisic acid (ABA) with GA3 to the media. In contrast, expression from the CaMV 35S promoter in the same system was not affected by GA3 or ABA and could be detected 1 day after transformation. Introduction of a further three different promoters into the aleurone protoplasts confirmed that GA3 specifically controlled transient expression from the α-Amy2/54 promoter only. Promoter deletions of the α-Amy2/54: GUS fusion demonstrated that sequences within 300 bp of the start of transcription of the gene were sufficient to direct high-level expression that was regulated by GA3 and ABA.
Keywords: GA3-regulated, transient expression, oat aleurone protoplasts, α-amylase, β-glucuronidase
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baulcombe D C, Huttly A K, Martienssen R A, Barker R F, Jarvis M G. A novel wheat alpha-amylase gene (alpha-Amy3). Mol Gen Genet. 1987 Aug;209(1):33–40. doi: 10.1007/BF00329833. [DOI] [PubMed] [Google Scholar]
- Baulcombe D. C., Barker R. F., Jarvis M. G. A gibberellin responsive wheat gene has homology to yeast carboxypeptidase Y. J Biol Chem. 1987 Oct 5;262(28):13726–13735. [PubMed] [Google Scholar]
- Bevan M., Barker R., Goldsbrough A., Jarvis M., Kavanagh T., Iturriaga G. The structure and transcription start site of a major potato tuber protein gene. Nucleic Acids Res. 1986 Jun 11;14(11):4625–4638. doi: 10.1093/nar/14.11.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colot V., Robert L. S., Kavanagh T. A., Bevan M. W., Thompson R. D. Localization of sequences in wheat endosperm protein genes which confer tissue-specific expression in tobacco. EMBO J. 1987;6(12):3559–3564. doi: 10.1002/j.1460-2075.1987.tb02685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard J. M., Herbomel P., Ott M. O., Mottura-Rollier A., Weiss M., Yaniv M. Determinants of rat albumin promoter tissue specificity analyzed by an improved transient expression system. Mol Cell Biol. 1987 Jul;7(7):2425–2434. doi: 10.1128/mcb.7.7.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttly A. K., Martienssen R. A., Baulcombe D. C. Sequence heterogeneity and differential expression of the alpha-Amy2 gene family in wheat. Mol Gen Genet. 1988 Oct;214(2):232–240. doi: 10.1007/BF00337716. [DOI] [PubMed] [Google Scholar]
- Jacobsen J. V., Higgins T. J. Characterization of the alpha-Amylases Synthesized by Aleurone Layers of Himalaya Barley in Response to Gibberellic Acid. Plant Physiol. 1982 Dec;70(6):1647–1653. doi: 10.1104/pp.70.6.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamppa G., Nagy F., Chua N. H. Light-regulated and organ-specific expression of a wheat Cab gene in transgenic tobacco. Nature. 1985 Aug 22;316(6030):750–752. doi: 10.1038/316750a0. [DOI] [PubMed] [Google Scholar]
- McCleary B. V. New chromogenic substrates for the assay of alpha-amylase and (1 leads to 4)-beta-D-glucanase. Carbohydr Res. 1980 Nov 1;86(1):97–104. doi: 10.1016/s0008-6215(00)84584-x. [DOI] [PubMed] [Google Scholar]
- Mignery G. A., Pikaard C. S., Hannapel D. J., Park W. D. Isolation and sequence analysis of cDNAs for the major potato tuber protein, patatin. Nucleic Acids Res. 1984 Nov 12;12(21):7987–8000. doi: 10.1093/nar/12.21.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy J., Hejgaard J., Hansen A., Hallgren L., Jorgensen K. G., Munck L. Differential synthesis in vitro of barley aleurone and starchy endosperm proteins. Plant Physiol. 1986 Jun;81(2):630–636. doi: 10.1104/pp.81.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Kavanagh T. Speeding-up the sequencing of double-stranded DNA. Nucleic Acids Res. 1988 Jun 10;16(11):5198–5198. doi: 10.1093/nar/16.11.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou-Lee T. M., Turgeon R., Wu R. Interaction of a gibberellin-induced factor with the upstream region of an alpha-amylase gene in rice aleurone tissue. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6366–6369. doi: 10.1073/pnas.85.17.6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Rogers J. C., Dean D., Heck G. R. Aleurain: a barley thiol protease closely related to mammalian cathepsin H. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6512–6516. doi: 10.1073/pnas.82.19.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. D., Bartels D., Harberd N. P. Nucleotide sequence of a gene from chromosome 1D of wheat encoding a HMW-glutenin subunit. Nucleic Acids Res. 1985 Oct 11;13(19):6833–6846. doi: 10.1093/nar/13.19.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittier R. F., Dean D. A., Rogers J. C. Nucleotide sequence analysis of alpha-amylase and thiol protease genes that are hormonally regulated in barley aleurone cells. Nucleic Acids Res. 1987 Mar 25;15(6):2515–2535. doi: 10.1093/nar/15.6.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwar J. A., Hooley R. Hormonal Regulation of alpha-Amylase Gene Transcription in Wild Oat (Avena fatua L.) Aleurone Protoplasts. Plant Physiol. 1986 Feb;80(2):459–463. doi: 10.1104/pp.80.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]