Abstract
The prefrontal cortex exerts top-down influences on several aspects of higher-order cognition by functioning as a filtering mechanism that biases bottom-up sensory information toward a response that is optimal in context. However, research also indicates that not all aspects of complex cognition benefit from prefrontal regulation. Here we review and synthesize this research with an emphasis on the domains of learning and creative cognition, and outline how the appropriate level of cognitive control in a given situation can vary depending on the organism's goals and the characteristics of the given task. We offer a Matched Filter Hypothesis for cognitive control, which proposes that the optimal level of cognitive control is task-dependent, with high levels of cognitive control best suited to tasks that are explicit, rule-based, verbal or abstract, and can be accomplished given the capacity limits of working memory and with low levels of cognitive control best suited to tasks that are implicit, reward-based, non-verbal or intuitive, and which can be accomplished irrespective of working memory limitations. Our approach promotes a view of cognitive control as a tool adapted to a subset of common challenges, rather than an all-purpose optimization system suited to every problem the organism might encounter.
Keywords: cognitive control, prefrontal cortex, dynamic filtering, learning, creativity
Although our elaborate sensory and motor systems provide detailed information about the external world and make available a large repertoire of actions, this introduces greater potential for interference and confusion. (...) To deal with this multitude of possibilities and to curtail confusion, we have evolved mechanisms that coordinate lower-level sensory and motor processes along a common theme, an internal goal. This ability for cognitive control no doubt involves neural circuitry that extends over much of the brain, but it is commonly held that the prefrontal cortex (PFC) is particularly important (Miller & Cohen, 2001, pp. 167–168).
Introduction
The prefrontal cortex (PFC) is commonly believed to underlie the most complex of cognitive functions, including language, memory, attention, problem solving, and decision-making. The PFC appears to exert top-down influences on cognition and behavior by biasing competing representations of bottom-up input from the external environment and internal states toward the optimal alternative within a given context (Banich, 2009; Miller & Cohen, 2001; Thompson-Schill, Bedny, & Goldberg, 2005). Over the past two decades, many studies have implicated this region in shielding working memory from interference from recently presented information, overriding prepotent responses, following rules, preventing distraction, and switching attention between cognitively demanding tasks. We refer to these functions collectively, and not altogether precisely, as “cognitive control.” Although the prefrontal cortex is unarguably essential for the regulation of complex behavior, a growing body of research suggests that not all aspects of complex cognition benefit from cognitive control. Here, we review and synthesize this research with a view toward characterizing the circumstances under which limited cognitive control is advantageous, focusing on the domains of learning and creative cognition. In light of this evidence, we propose that tradeoffs between prefrontal and other brain regions exert the necessary level of cognitive control over bottom-up, sensory information that is optimal for performance in common cognitive tasks.
In particular, cognitive control has been described as a process of filtering, in which certain information—in the best case, useless information—is attenuated or discarded (Shimamura, 2000). In signal processing theory, a matched filter is one that optimally extracts signal from noise in a given context. By loose analogy, here we combine these two notions to advance a Matched Filter Hypothesis (MFH) for cognitive control: Task performance is optimized not simply by the application of high levels of cognitive control, but by a good match between the level of control exerted and the degree to which the task requires filtering of available low-level information. Although the precise function of the neural systems supporting this optimization mechanism exceeds the scope of the current MFH, we postulate that the organism's attainment of the optimal level of cognitive control is influenced by competitive interactions between PFC and posterior or subcortical brain systems, and the outcome of those interactions is influenced by factors including the stage of development of the organism, the health of its brain, and individual differences in its neurophysiology. In the next section, we will motivate and articulate the MFH in more detail.
A Matched Filter Hypothesis for Cognitive Control
Cognitive development is marked by remarkable advances in children's mental abilities. For example, as children grow into adult speakers they become very proficient at extracting meaning from language in the face of semantically irrelevant phonetic variations such as accents (Evans & Iverson, 2004; Maye, Aslin, & Tenenhaus, 2008; see Cristia, Seidl, Vaughn, Schmale, Bradlow, & Foccia, 2012, for a review). On the other hand, these advances in language comprehension come at a cost for language learning: Infants and young children are capable of perceiving phonetic distinctions that do not occur as phonological contrasts in their native language; in contrast, adults have trouble perceiving such distinctions (Best, McRoberts, & Goodell, 2001; MacKain, Best, & Strange, 1981; Trehub, 1976; Werker, Gilbert, Humphrey, & Tees, 1981). Such developmental differences between children and adults are not limited to language learning. On the contrary, these tradeoffs might be the rule rather than the exception in cognitive development. Our focus here is on tradeoffs that accompany the development of cognitive control (Thompson-Schill, Ramscar, & Chrysikou, 2009; Munakata, Snyder, & Chatham, in press). We aim to demonstrate that the costs and benefits of cognitive control are recapitulated at many levels of cognition, from simple cue-outcome associative learning to the unexpected associations that kindle creativity.
The ability for cognitive control develops incrementally during childhood and young adulthood, in parallel with the development of the prefrontal cortex (Cragg & Nation, 2010; Huttenlocher & Dabholkar, 1997; Khanna & Boland, 2010; cf. Davidson et al., 2006). Because their frontal lobes are not yet fully developed, children may be characterized as hypofrontal1. The majority of research on the development of cognitive control has focused on children's cognitive control deficits (e.g., Diamond, 2009; Diamond et al., 2007; Diamond & Doar, 1989). For example, young children find it hard to swiftly adopt a new strategy even after the demands of the task have changed, whereas this kind of flexibility characterizes adult performance in similar tasks (e.g., Kirkham, Cruess, & Diamond, 2003; Kirham & Diamond, 2003; Zelazo, Frye, & Rapus, 1996). However, there is mounting evidence that children outperform adults in certain situations. One such situation is a probabilistic choice task in which one alternative is on average more rewarding than another. Adults will match (i.e., if the best alternative confers a reward 75% of the time, they will choose it 75% of the time, leading to an expected success rate of 62.5%), whereas children employ the superior strategy of maximizing (choosing that same alternative 100% of the time, once the probabilities are known, leading to an expected success rate of 75%; Derks & Paclisanu, 1967). Recent research has suggested that certain kinds of probability matching may involve executive function (e.g., Gaissmaier & Schooler, 2008; Koehler & James, 2009; Otto, Taylor, & Markman, 2011); thus, in this instance, deploying cognitive control, perhaps paradoxically, impairs adult performance on this task.
In recognition of the costs of cognitive control, it has been suggested that the benefits of cognition without control might be rooted in neurocognitive development (Thompson-Schill, Ramscar, & Chrysikou, 2009; Munakata et al., in press). It is well known that the human brain develops heterochronously, with prefrontal cortices maturing many years after sensory cortices (Chugani & Phelps, 1986; Shaw et al., 2008). Thompson-Schill et al. (2009) assert that this pattern of brain maturation plays a critical role in neurocognitive development: Children's prolonged period of hypofrontality, and the attendant lack of cognitive control, allows them to master evolutionarily important faculties, such as language, with remarkable efficacy. In general, during periods in which evolutionary pressures have placed a premium on learning over task execution, it may be beneficial for the organism to limit the filtering of information by reducing PFC activity.
The approach offered by Thompson-Schill et al. (2009) emphasizes the advantages of hypofrontality in light of the evolution of neural development. Beyond the phylogenetic scale of evolution and the ontogenetic scale of human development, though, periods of hypofrontality may benefit an organism even on a scale of hours or moments, depending on the specific characteristics of the task in question. In particular, an extended body of research supports the involvement of PFC in rule-driven and typically explicit tasks that require a higher level of abstraction of concepts or rules and can be adequately represented in working memory (for a compelling computational hypothesis about these features of PFC function, see O'Reilly, Mozer, Munakata, & Miyake, 1999). In contrast, automatic, habitual, and largely implicit tasks that do not involve abstraction of information and, thus, exceed the representational capacity of working memory may benefit from lower PFC activity and increased involvement of posterior or subcortical networks (e.g., sensorimotor cortex, basal ganglia). Not only do these tasks not require cognitive control, but in fact, we argue that the deployment of PFC-mediated cognitive control will indeed hurt performance when posterior or subcortical brain systems are best suited to the task.
Here, we combine these two accounts of organism and task influences on complex cognition to offer the Matched Filter Hypothesis (MFH) for cognitive control. Our approach draws on the guided activation framework of Miller and Cohen (2001), which argues that the function of cognitive control is to facilitate the appropriate response in a given task context, whether that response is dominant or not. We further build on the dynamic filter theory for prefrontally-mediated cognitive control offered by Shimamura (2000), which proposes that the PFC supports different aspects of cognitive control (including selecting, maintaining, updating, and rerouting of information) by acting as a dynamic filtering mechanism that maintains task-relevant information while gating task-irrelevant information available in posterior brain regions. The MFH extends these proposals by suggesting that the intervention of PFC-mediated regulatory filtering mechanisms comes at a cost of certain functions of posterior and subcortical brain systems. Whether this cost yields a net benefit in performance depends on individual objectives and task demands. Specifically, in the face of a wealth of low-level information available to the organism from the external environment or internal states, the MFH proposes that optimal performance is determined by whether the degree, scope, and type of regulatory filtering of this information matches the organism's current goals, as well as the requirements of the task at hand. For present purposes, we set aside the trenchant question of how the organism might determine a situationally appropriate level of cognitive control, a function likely influenced by the locus coeruleus and the norepinephrine system (cf. Aston-Jones & Cohen, 2005; see also O'Reilly et al., 1999; Shenhav, Botvinick, & Cohen, 2013). Instead, we focus on how task factors influence the need for cognitive control, and how competitive interactions between PFC and posterior and subcortical brain systems enable the brain to meet, or prevent it from meeting, that need.
Specifically, the MFH predicts that PFC-mediated cognitive control improves performance through extensive filtering of raw input to the extent that the task is top-down, rule-based or goal-directed, requires an abstract understanding of concepts and rules, and can be accomplished by maintenance and manipulation of explicit representations; such tasks are not well suited to hypofrontal brain states. In contrast, hypofrontality improves performance by limiting regulatory filtering of information when the task is bottom-up or stimulus-driven, not amenable to abstraction, and the complexity of the necessary representations exceeds the capacity of working memory; such tasks are not well suited to brain states marked by strong prefrontal regulation. A summary of the MFH model of cognitive control along these (and other) dimensions is provided in Figure 1. Critically, the degree of matching between organism- and task-specific factors may be influenced by individual variation including developmental stage, genotype, disruption in brain function (either long-term, as a result of brain damage or psychopathology, or short-term, as a result of dual-task conditions or noninvasive brain stimulation), and other factors.
Figure 1.
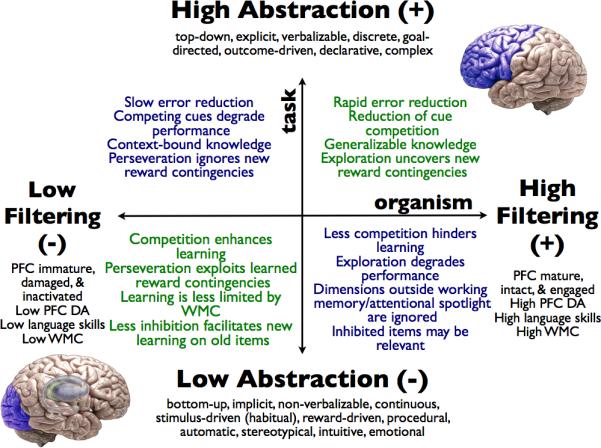
Dimensions of the Matched Filter Hypothesis model for cognitive control.
In other words, under certain circumstances and within the context of a given task, an organism's filter may dynamically match task requirements for optimal performance, whereas at other times it may not; moreover, successful matching may occur at different rates across different organisms and different tasks. Although, in some cases, negotiating optimal levels of regulatory filtering is restricted by biological constraints (e.g., PFC maturation, brain damage), in others it might be possible for the organism to adjust dynamically its filtering mechanisms to meet task requirements, by increasing or decreasing cognitive control—for example, as a result of training, neural stimulation, pharmacological agents, and so on.
We first proceed to situate our approach in the context of other influential models of cognitive control. Thereafter, we adduce evidence for the MFH in the domains of learning and creative cognition.
Relationship to Earlier Work
The MFH shares some similarities with the influential Supervisory Attentional System (SAS) model of goal-driven action proposed by Norman and Shallice (1986; see also Cooper & Shallice, 2000), which offers a distinction between automatically-performed actions that may occur outside of awareness and actions that require cognitive control. According to this framework, information about familiar actions is stored in a hierarchy of abstract scripts and more specific object-based schemas (e.g., how to use a fork, how to drive a car, etc.), which can be activated by the environment or the organism's current goals. A contention scheduling mechanism selects which schema will be implemented based on the relative strength of activation among several competing schemas, thus allowing for the control of action depending on context. Under this model, familiar actions are performed automatically with limited SAS involvement, whereas novel actions require significantly more intervention from SAS mechanisms, presumed to be supported by PFC. Subsequent iterations of the model (e.g., Shallice & Burgess, 1996) included additional modules focusing on goal maintenance, performance monitoring, and evaluation, each assumed to be supported by distinct regions within PFC. Similar to the SAS model, our account discusses a balance between bottom-up and top-down influences in the control of behavior. However, the SAS model emphasizes the need for cognitive control in novel behavior. This need is unarguable when the behavior in question is well specified—for example, in the Stroop task, where cognitive control must override a well-learned schema for interaction with words in order to support the relatively novel task of color naming. However, it is not clear that cognitive control is always useful when the organism does not know what to do—for example, in insight problems, where the required behavior often hinges on exploiting a feature of an object unrelated to its function; or in complex categorization tasks, where the categorization rule is initially unknown and difficult to specify explicitly. As we show later, cognitive control often poses an active impediment to these tasks, although it can come in useful later in evaluating performance (Goldman-Rakic, 1996; Miller & Cohen, 2001). In this sense, the MFH extends the SAS model by proposing that the execution of unfamiliar actions can benefit from both unfiltered information to support novel solutions, and top-down PFC guidance to evaluate their effectiveness.
Our account is further compatible with certain models of attention, which we consider broadly as the process of orienting selectively toward an endogenous state or an exogenous perceptual stimulus. Of particular relevance to the MFH is the framework proposed by Corbetta and Shulman (2002), who discuss two interactive systems for top-down (cognitive) and bottom-up (sensory) attentional control: a bilateral dorsal system (intraparietal sulcus and dorsal frontal cortex [frontal eye fields]) implicated in the endogenous or voluntary guidance of attention toward an anticipated stimulus, and a right-hemisphere ventral system (temporoparietal and inferior frontal cortex) that acts as a “circuit breaker” for the dorsal system, implicated in the exogenous or bottom-up guidance of attention towards salient, unanticipated stimuli. In parallel with the MFH, Corbetta and Shulman suggest that dorsal frontal regions support preparatory signals toward a particular task goal, whereas left dorsal posterior parietal cortex is involved in the selection of appropriate stimulus-response associations, with a special role of this region in humans for simple or well-learned tasks. The MFH affords a slightly different view of these interactions. Although the dorsal system is described as “top-down” and the ventral as “bottom-up,” both systems incorporate actively maintained representations: The frontal eye fields are responsible for orienting attention consistent with a goal, whereas the inferior frontal cortex is responsible for orienting attention to stimuli that are relevant or unusual in context. Consistent with the MFH, either frontal region may interfere with the operations of the intraparietal sulcus, which allocates attention as a learned response to stimuli, without recourse to actively maintained representations such as goals and expectations.
In the following sections we review research from the domains of learning and creative cognition that offers support for the MFH. The work we discuss underscores the tradeoffs between PFC-mediated cognitive control and hypofrontality for different aspects of complex cognition, as well as highlights the significance of individual differences in determining the organism's ability to match the level of cognitive control demanded by the task.
MFH and Learning
As discussed in the previous section, the MFH predicts that hypofrontality will limit input filtering and improve learning in some circumstances, notably when the domain cannot be learned well by explicit strategies, when the rules governing the domain exceed working memory capacity, or when to-be-tested information is attended away from or otherwise suppressed. Direct support for this prediction in adults is sparse but intriguing. Dual-task conditions help subjects learn nonverbalizable morphological rules, motor sequences, probabilistic classification rules, and decision boundaries (Brown & Robertson, 2007; Cochran et al., 1999; Foerde et al., 2007; Filoteo et al., 2010); and, disruption of activity in the dorsolateral prefrontal cortex via transcranial magnetic stimulation has been linked to improvements in implicit motor learning (Galea et al., 2010). A similar dynamic arises in retrieval-induced forgetting (RIF), in which participants are trained on a set of category-exemplar pairs (e.g., Fruit-Orange, Fruit-Banana, Fish-Catfish, Fish-Herring), and then tested on their memory of half the pairs in half the categories via stem-completion (e.g., complete Fruit-Or__, but not the other three). In a subsequent recall test, recall of the untested pairs in tested categories is reduced relative to the control pairs in untested categories, suggesting that those items have been suppressed in support of recalling the tested pairs (Anderson et al., 1994; Table 1). However, this forgetting effect is diminished when prefrontal resources are taxed with a secondary task (Roman et al., 2009). Overall, these studies suggest that hypofrontality benefits learning when the domain is procedural or implicit, or when the task invites suppression of information that will later become relevant.
Table 1.
Structure and results of retrieval-induced forgetting experiment
| Acquisition | Retrieval test | Delayed recall | Recall result |
|---|---|---|---|
| Fruit-Orange | Fruit-Or___? | Fruit-? | Better than control pairs (Fish-*) |
| Fruit-Banana | No practice | Fruit-? | Worse than control pairs (Fish-*) |
| Fish-Catfish | No practice | Fish-? | |
| Fish-Herring | No practice | Fish-? |
Note: In retrieval-induced forgetting, subjects learn category-exemplar pairs and are then administered a retrieval test on half the exemplars from half the categories. Relative to exemplars of untested categories, the retrieval test improves subsequent recall for tested exemplars of tested categories, but reduces recall for untested exemplars of tested categories.
The above examples concern hypofrontality induced by dual-task manipulations or noninvasive brain stimulation, but variation in PFC function can also result from endogenous influences, including genetic variation. Indeed, some studies of individual differences in genetic regulation of prefrontal dopamine (DA) report improved learning in populations with reduced PFC DA. However, a broader view of this literature emphasizes the importance of understanding the relationship between brain systems, cognitive function, and task demands. In general, PFC-mediated cognitive control seems to be governed by a balance between activation of D1 and D2 DA receptors: D1 activation promotes stability (e.g., maintenance of working memory representations over distraction), but also perseveration, and D2 activation promotes flexibility (e.g., low task-switching costs), but also distractibility (Durstewitz & Seamans, 2008). The D1 receptor seems to have greater affinity for DA, meaning that populations with higher PFC DA have more D1 activation and thus greater cognitive stability (Slifstein et al., 2008; Farrell, Tunbridge, Braeutigam, & Harrison, 2012; Nolan, Bilder, Lachman, & Volavka, 2004; Colzato, Waszak, Nieuwenhuis, Posthuma, & Hommel, 2010). These variations are consequential for learning. Consistent with PFC DA promoting cognitive stability, subjects with a genetic polymorphism that increases PFC DA are outperformed by their lower-DA counterparts on reversal learning tasks (Krugel et al., 2009). However, subjects with higher PFC DA are more likely to use lose-shift strategies during learning (Frank et al., 2007) and explore response options with highly uncertain outcomes (Frank et al., 2009). These behaviors involve both maintenance and flexibility, making the effect of changes in PFC DA difficult to predict. In general, understanding the effect of PFC DA on a task requires understanding the degree to which the task in question relies on the cognitive functions (maintenance and switching) supported by different, and antagonistic, DA receptor pathways in the PFC.
Individual differences in age and executive function have also shown some advantages for hypofrontal learners. Aslan and Bäuml (2011) showed that RIF is positively correlated with working memory capacity, such that greater working memory is associated with greater inhibition of non-practiced pairs in practiced categories relative to control categories. In line with this finding, RIF is also reduced in children, who have underdeveloped working memory (Aslan & Bäuml, 2010). Additionally, Campbell and colleagues (2012) showed that older adults, whose prefrontal function is diminished, can learn the statistical structure of a stream of unattended images, whereas previous work shows that college students do not (Toro et al., 2005; Turk-Browne et al., 2005; Gallistel, 2009). In the absence of more precisely targeted experiments, juvenile advantages in learning cannot be uniquely attributed to juvenile hypofrontality; it is important to consider other differences between children and adults, such as knowledge, learned strategies, and the maturity of brain regions other than the PFC. However, these results nonetheless raise the possibility that limited filtering through reduced cognitive control might be advantageous for learning when initially irrelevant information (e.g., non-practiced pairs, unattended images) later becomes relevant.
How exactly hypofrontality and the consequent lack of input filtering might improve learning remains an open question. Here we consider the issue in several domains. First we turn to the attentional phenomena of blocking and highlighting, both cases in which regulatory filtering rapidly reduces errors at the cost of distorting the learner's representations of information in the environment. Next, we examine the role of cognitive control in language learning. Finally, we sketch an account of competition between learning systems embodied in different corticostriatal loops. A common theme in these three sections will be tradeoffs—between different learning systems, between learning and performance, and between immediate and long-term performance. Throughout, we emphasize the simple insight that the system best adapted for learning a domain might not be the system best adapted for putting that learning into practice, and that evolutionary success might hinge on an organism's ability to implement either system at different developmental stages or within the context of different tasks.
Attention and learning
Thompson-Schill et al. (2009) frame the tension between learning and cognitive control as a conceptual tradeoff: The suppression of competitive interactions by cognitive control is helpful for performance but detrimental to learning (see also Ramscar & Gitcho, 2007). Several computational models of memory and language leverage cue competition to improve learning (e.g., Norman et al., 2005; Ramscar & Yarlett, 2007; Ramscar et al., 2010), and in each case it is plausible that the attenuation of competition through prefrontally mediated processes might impair learning (Ramscar & McClure, 2011). One direct and detailed investigation of this possibility is provided by Kruschke and colleagues in several behavioral and computational studies of attentional learning, which demonstrate that various associative learning phenomena are best explained by subjects learning to orient their attention differentially to different stimuli in order to reduce errors quickly; this redistribution of attention then gates cue-response learning (see Kruschke, 2003, for a partial review and synthesis).
Specifically, Kruschke and colleagues have investigated the attentional basis of learning phenomena such as blocking and highlighting (Table 2). In blocking, an earlier-learned association prevents learning of one presented later (Kamin, 1969). In an example paradigm, a subject might learn that stimulus A predicts outcome X (A.X), then learn that cues A and B presented simultaneously predict outcome X (AB.X), and likewise CD.Y. Although AB.X and CD.Y are presented equally often, preconditioning with A.X leads subjects to choose response Y over X when confronted with the new compounds BC and BD—that is, it limits the strength of the association between B and X, or blocks B. In highlighting, a later-learned association is learned more strongly. For example, subjects might first learn AB.X and then learn AC.Y; although B and C both perfectly cue their respective responses, subjects confronted with the new compound BC, again, overwhelmingly choose Y over X. These effects are not explained by differences in the frequency or reliability of the earlier- and later-learned associations (Kruschke, 2001, 2003; Denton & Kruschke, 2006), hence our use of Kruschke's term ‘highlighting’ rather than the earlier ‘inverse base rate effect’ (Medin & Edelson, 1988). Rather, the effects are explained by changes in the allocation of attention: Subjects learn to attend away from blocked cues (in blocking) and the shared cue (in highlighting). This redistribution of attention helps subjects respond quickly and accurately by diverting attention from an uninformative cue (in the case of blocking) or a cue associated with the wrong response (in highlighting); however, it also attenuates learning about that cue, leading to the observed distortion in association strengths. Eye movement data are consistent with this attentional account of cue competition, with subjects selectively looking away from blocked cues and toward highlighted cues (Kruschke, Kappenman, & Hetrick, 2005); additionally, learning about blocked cues, and even new cues that share features with blocked cues, is attenuated, suggesting a learned redistribution of attention away from blocked cues and their features (Kruschke & Blair, 2000; Kruschke, 2005; Kaminski, Heckler, & Sloutsky, 2008). These attentional dynamics preserve already-learned associations while responding accurately to new ones, a strategy well motivated by the goal of rapid error reduction (Kruschke & Blair, 2000; Kruschke, 2003).
Table 2.
Structure of blocking and highlighting experiments
| Blocking | Highlighting | |||
|---|---|---|---|---|
| Phase | Cues | Response | Cues | Response |
| Early | A | X | AB | X |
| Late | AB | X | AC | Y |
| CD | Y | |||
| Test | BD | ? | A | ? |
| BC | ? | |||
| Observed | BD | Y | A | X |
| BC | Y | |||
Note: Blocking and highlighting are response biases caused by learning associations that are unevenly distributed in time. One plausible account of these phenomena appeals to selective attention in the service of error reduction.
One consequence of hypofrontality is difficulty controlling the allocation of attention. For example, monkeys with unilateral PFC lesions are impaired in attentionally demanding visual search tasks, but not in undemanding pop-out tasks, when the cue changes frequently (Rossi et al., 2007), and patients with unilateral PFC lesions show attentional deficits selective to the contralesional visual field (Voytek & Knight, 2010). Children also have difficulty allocating their attention (Hanania & Smith, 2010). Kruschke and colleagues’ framework of attentional learning thus fits well with the MFH: Mature learners apply cognitive control to reduce errors quickly but at the cost of distorting their representations of association strengths, whereas immature learners without cognitive control make graver errors for a longer time but ultimately learn the domain veridically. Developmental evidence is consistent with this view: 3-year-olds are less prone to blocking than 4-year-olds, and 8- and 9-year-olds show less highlighting than adults (Sobel et al., 2004; Winman et al., 2005). Corbetta and Shulman (2002) assign the ventral PFC a “circuit-breaking” response to novel stimuli, which may be at work here: When hypofrontal subjects are confronted with new cue combinations, attenuated signals from ventral PFC lead to weaker reorienting, less error reduction, and ultimately less distortion in the association strengths. The right middle and inferior frontal gyri respond to prediction errors in a manner that could serve to signal novelty. Activation in these regions corresponds well to the prediction error signal generated by the learning rule of Rescorla and Wagner (1972), and is evoked in proportion to prediction error by various learning manipulations, including backward blocking (Fletcher et al., 2001; Turner et al., 2004; Corlett et al., 2001). However, direct evidence of the role of prefrontal activation in cue competition is limited; further investigation will be required to cement this hypothesis.
A similar argument can be sketched in other domains of learning where attention is relevant. In the case of retrieval-induced forgetting, discussed earlier, performance on the retrieval test can be improved by suppressing the competing untested associations, but that suppression impairs long-term learning of the complete stimulus set. Likewise, in the visual statistical learning study by Campbell and colleagues (2012), subjects were familiarized with the input through a one-back task incorporating a stream of red images interleaved with a stream of green; subjects monitored one color for repeated images while ignoring the other. This selective attention comes at the cost of learning the statistical structure in those stimuli. In other words, when voluntary, top-down attentional systems (which typically engage prefrontal cortex) are involved in the task, learning of unattended information (that is implicit and secondary to the main task) is impaired, providing support for possible competitive interactions between these systems.
Based on these results, we suggest that in the cases of cue competition, retrieval-induced forgetting, and visual statistical learning, the application of cognitive control produces a short-term benefit to accuracy at a long-term cost—respectively, to the veridicality of learned association strengths, the ability to remember unretrieved competitors to retrieved items, or the implicit learning of statistical structure.
Language learning
The inability to filter input has the consequence of rendering learning more homogeneous across learners—a learner with fully developed cognitive control functions may direct its attention where it chooses, but a learner with reduced cognitive control will have difficulty attending away from the most salient aspects of the stimulus. This means that learning in the absence of cognitive control may be especially well adapted for domains that benefit from being learned the same way by different learners, such as language. Specifically, limited filtering of linguistic input may allow children to learn the most frequent patterns they hear instead of deliberating about probabilistic rules (Chrysikou, Novick, Trueswell, & Thompson-Schill, 2011; Singleton & Newport, 2004). For example, when adults learn an artificial language from sparse and inconsistent input, their language production reflects the noise of the input; in contrast, children only produce the most frequent form, insulating their linguistic output from their teachers’ errors, thus outperforming adults on this task (Hudson Kam & Newport, 2005, 2009). Consistent with this view, young children are more hesitant than older children and adults to generalize rules to new lexical items, which may help them learn linguistic conventions like irregular verbs and plurals (Boyd & Goldberg, 2011). In contrast, adult language learning is characterized by top-down probabilistic rules, which tend to vary across learners, potentially undermining learning of linguistic conventions (Ramscar & Yarlett, 2007; Ramscar et al., 2010).
For similar reasons, limitations in working memory may promote language learning in young children by forcing them to focus on the components of larger structures—words rather than phrases, or morphemes rather than whole words. Indeed, adults’ superior working memory capacity may allow them to learn morphologically complex words as independent wholes, rather than focusing on the words’ internal structure. As a result, adult second-language learners often show “frozen” language, failing to adjust the morphology of a complex word to the syntactic context (Newport, 1988, 1990; Singleton & Newport, 2004). This tendency is ameliorated when adults learn morphologically complex words during a concurrent task that taxes working memory capacity (Cochran et al., 1999). Likewise, young children regularize noisy linguistic input by producing only the most frequent forms emitted by their teachers (Newport, 1999; Singleton & Newport, 2004). However, adults do so only when the inconsistencies are highly complex—for example, when a language contains one dominant form of a determiner and 16 less frequent forms, but not one dominant and 8 less frequent forms (Hudson Kam & Newport, 2005, 2009). In other words, adults’ superior memory for the infrequent variants may lead them to regularize only under circumstances of extreme variability. These results suggest that the ability to keep large linguistic structures in mind may impair learning by offering the learner an intractably large inventory of items. Thus, adult language learners may benefit from focusing on the components of language more than complex linguistic structures (Newport, 1988; Elman, 1993; Ramey, Chrysikou, & Reilly, 2013; but see Rohde & Plaut, 1999). In support of this conjecture, languages spoken by large communities, and thus by large proportions of adult learners, have simpler inflectional morphology than those spoken by small communities, and thus principally by young learners (Lupyan & Dale, 2010).
Learning and inference
Categorizing objects is useful for at least two reasons: It supports generalization to new exemplars, and it supports cognitive economy for tasks made on the category level, because the only features that demand attention are the features relevant to categorization (e.g., fur color can be ignored in determining whether a given animal barks, but not whether a given dog is your neighbor's). However, that cognitive economy is achieved at the cost of the categorization-irrelevant information, which may not be irrelevant in other contexts (Hoffman & Rehder, 2010; Yim, Best, & Sloutsky, 2011). This tension is elucidated in the work of Sloutsky and Fisher (2004), who compared children and adults’ memory for exemplars after different preconditioning procedures. When children and adults were simply directed to remember a set of pictures of cats, bears, and birds, adults outperformed children substantially on the memory test. However, when both adults and children were preconditioned with a category-based induction task, where they were required to learn that cats but not bears and birds had “beta cells” in their bodies, children lost no accuracy, but adults’ memory for the pictures plummeted to chance levels. Sloutsky and Fisher (2004) further report that explicitly training children to do category-based induction like adults reduced their performance to chance levels after the induction preconditioning, confirming the relevance of the categorization process in generating the disadvantage for induction.
In general, adults’ focus on category labels appears to be a learned disposition; children tend to treat category labels as a feature. This makes it difficult for children to attend away from features that are salient but category-irrelevant (Yao & Sloutsky, 2010; Deng & Sloutsky 2010, 2012), and reveals a disposition to perform induction on the basis of similarity rather than category membership (Sloutsky & Fisher, 2004; Sheya & Smith, 2006). This is not to say that children are insensitive to labels; on the contrary, even very young children are quite capable of using labels to guide and enhance learning (Smith et al., 2002; Xu, 2002; Yoshida & Smith, 2005; Rakison & Lupyan, 2008). However, category labels do not enjoy the same privileged influence on attention and inference in children that they do in adults—likely due to children's difficulty in suppressing perceptual information that competes with categorization rules.
There is reason to believe that the capacity for categorization relies, at least in part, on the PFC. Miller and colleagues have repeatedly demonstrated that neurons in the PFC of the macaque code category identity, even over changes in the categorization rule and the behavioral relevance of the categorization (Freedman, Riesenhuber, Poggio, & Miller, 2001, 2002, 2003; Meyers, Freedman, Kreiman, Miller, & Poggio, 2008; Roy, Riesenhuber, Poggio, & Miller, 2010). The lateral PFC may also support generalization of learned information to unseen category members, a key function of categorization (Pan, Sawa, Tsuda, Tsukada, & Sakagami, 2008). In humans, rule-based categorization engages the caudate during early learning, then the ventrolateral PFC, and finally premotor cortex (Hélie, Roeder, & Ashby, 2011; Soto, Waldschmidt, Hélie, & Ashby, 2013). Computational modeling suggests that, with experience, the PFC can self-organize connections to stimulus and task representations that support rule-based categorization and generalization (Rougier & O'Reilly, 2002; Rougier, Noelle, Braver, Cohen, & O'Reilly, 2005). The lack of a strong PFC signal and well-organized connections to bias representations of the stimulus and task context may lead children to default to similarity-based categorization.
Independent of categorization, hypofrontality may also increase sensitivity to correlations. The sampling distribution of correlations is negatively skewed when the true correlation is positive, and the skewness increases with sample size, such that very small samples are likely to exhibit a correlation much higher than the true correlation (Kareev, 1995; Kareev, Lieberman, & Lev, 1997; Kareev, 2000). Limited working memory capacity may allow children to consider fewer items at once, inflating the strength of positive correlations and thus, presumably, making them more salient to the learner.
Learning in competing corticostriatal loops
The work reviewed in the previous subsections elucidates influences of cognitive control on learning that are essentially computational: Learning is impaired by cognitive control processes that suppress or extinguish aspects of the input information (as in cue competition, retrieval-induced forgetting, and attentional suppression of statistical learning), that allow the learner to try to fit noise (as in frozen language and underregularization), or that rectify noisy input based on possibly deficient knowledge (as in mediated priming). However, there is a wealth of evidence for multiple learning systems in the brain, the gist of which is that prefrontal cortex is involved in declarative learning, as of explicitly defined rules or categories, whereas the striatum supports procedural learning (Ashby et al., 1998; Cohen & Eichenbaum, 1993; Daw et al., 2005; Shohamy et al., 2008). In fact, the striatum is functionally divided in this respect, with neuroanatomical (Alexander et al., 1986; Joel & Weiner, 2000), neuropsychological (Ashby et al., 2003; Filoteo et al., 2004; Reber & Squire, 1999), and neuroimaging (Filoteo et al., 2005; Di Martino et al., 2008; Seger & Cincotta, 2005; Cincotta & Seger, 2007) evidence implicating the caudate nucleus, or at least its head, in the declarative system.
Work by Yin and colleagues (Yin et al., 2005; Yin & Knowlton, 2006; Yin et al., 2009) has shown a different but plausibly related dissociation in the functions of rodent striatal substructures, with the dorsomedial striatum (the rat homologue to the caudate) encoding action-outcome associations (goal-oriented learning; Dickinson, 1985) and the dorsolateral striatum or putamen encoding stimulus-response associations (habitual learning; ibid.). Goal-oriented learning occurs early in the learning process and is sensitive to variation in the value of the action, as well as the contingency of the outcome on the action; habitual learning occurs with overtraining and is insensitive to such variation. This pattern suggests a relationship to the declarative-procedural divide: Declarative knowledge is flexibly deployed in pursuit of explicitly represented goals, whereas procedural knowledge is bound strongly to the learning context (Foerde et al., 2006).
There is evidence that these dissociable learning systems are also competitive. The results of Yin and colleagues suggest competition between caudate- and putamen-based learning systems, as activity in the caudate falls during learning even as activity in the putamen rises. Daw and colleagues (2005) advance a model for uncertainty-based competition between prefrontal/caudate and dorsolateral striatal (i.e., putamen) reinforcement learning, with the former implementing a model-based learning system reliant on working memory and the latter implementing a model-free system with minimal memory demands; likewise, the COVIS category learning model of Ashby et al. (1998, inter alia) incorporates competition between a prefrontal hypothesis-testing system and a striatal procedural system. Consistent with such an architecture, learners with low working memory capacity are superior to their high-capacity counterparts at learning nonverbalizable decision boundaries (DeCaro, Thomas, & Beilock, 2008). Additionally, Ashby and Crossley (2010) have shown that humans cannot learn “hybrid” decision boundaries where one part of the boundary is verbalizable and another part is not, suggesting that either declarative or procedural learning systems are active at any given time. Work on declarative and procedural memory in probabilistic learning offers a similar distinction. Declarative memory relying on the medial temporal lobes appears to compete with procedural memory relying on the striatum (Poldrack, Prabhakaran, Seger, & Gabrieli, 1999; Seger, Prabhakaran, Poldrack, & Gabrieli, 2000; see Poldrack & Packard, 2003, for a review). These studies do not distinguish the contributions of the caudate and the putamen to striatal activation, but later work implicates the head of the caudate in the declarative rather than the procedural system, as described earlier.
But why should these systems be competitive, and why should children and adults be biased to use different systems? Yin and colleagues’ work on outcome devaluation and contingency degradation in goal-oriented and habitual learning may shed some light on the first matter. Habitual (stimulus-response) learning is only useful given a reliable reinforcement history for the response; thus, it would make sense for goal-directed learning mechanisms to suppress habitual learning mechanisms early in learning, in order to avoid creating a habitual association to a stimulus whose value is unreliable. Considerations of cognitive economy might also suggest mutual exclusion of declarative and procedural learning systems; for example, there is little point in exercising the declarative system to learn a set of rules that is difficult to represent explicitly or too complex to hold in working memory. The question of why children and adults might be biased to use different systems is harder to answer, but it is interesting to speculate on the relative distribution of learning problems across the lifespan. Adults, who were once required to range across their environment frequently in search of food, may have encountered more varied and volatile situations in which new associations had to be quickly learned and then unlearned, with considerable stakes. Children, by comparison, stayed close to home and devoted their efforts to learning domains such as language and social conventions, which require extensive training and could be expected to remain invariant for their whole lives. Thus, the PFC's trajectory of maturation might be adapted to support the kinds of learning and behavior that are most beneficial to humans at different points throughout the lifespan.
The idea of opposing prefrontal and striatal learning systems is foreshadowed by, and related to, the complementary learning systems (CLS) framework for hippocampus and neocortex advanced by McClelland, McNaughton, and O'Reilly (1995; see also O'Reilly & Norman, 2002; Norman & O'Reilly, 2003). The main argument of the CLS framework for learning and memory is that the cortex (generally described in terms that characterize sensorimotor cortex, although PFC is not explicitly excluded) learns slowly, making it well-suited to discovering persistent structure in the environment but ill-suited to rapid adjustment of behavior by swiftly learning new associations. In contrast, the hippocampus can rapidly bind arbitrary elements of the environment together into new associations, but those associations decay rapidly without repetition. There is, thus, a schematic relationship between the CLS and MFH frameworks, with the hippocampal system of the CLS similar to our prefrontal system in its ability to encode arbitrary associations rapidly, and the neocortical system of the CLS similar to our posterior/subcortical system in its ability to absorb enormous amounts of information about the persistent structure of the environment. However, the computational concerns motivating the two frameworks are somewhat different. The CLS framework aims to describe the computational factors that underlie when and whether information is stored in memory. The MFH, in contrast, is primarily interested in what information is processed and what suppressed (although this can manifest in timing effects, as in cue competition and RIF). Further theoretical work will require integration of these concerns. A naïve approach would be to view the prefrontal/posterior interactions of the MFH as prior to the hippocampal/neocortical interactions described by the CLS framework; regulatory filtering reshapes the information that enters the system, and the interactions between the hippocampal and neocortical systems determine the fate of that reshaped information. This is likely a useful start, but at a minimum, the striatum's known role in memory encoding is sure to complicate the story (e.g., Ashby & Crossley, 2011; Crossley, Ashby, & Maddox, 2012).
In summary, the mature prefrontal cortex may bias learners toward the use of a declarative, goal-oriented learning system, even when that system is not well adapted to the problem at hand; this tendency is well documented by Ashby and colleagues (Filoteo et al., 2010; Ashby & Crossley, 2010, inter alia). There is evidence for competition between brain systems for declarative and procedural learning, implying that prefrontal influence tends to take the procedural, habitual learning system offline by default. The balance of competition between declarative/goal-oriented and procedural/habitual systems may reflect the goals of the learner at its particular stage in development: Children may benefit more from having the procedural/habitual system released from inhibition, whereas adults may find more use for declarative/goal-oriented learning and cognitive control.
MFH and Creative Cognition
The review of the literature on learning discussed above reveals complex tradeoffs among PFC and striatal subregions that allow for different types of learning depending on context and task goals. Critically, not all aspects of learning benefit similarly from PFC regulation: instead, a careful match between the degree of filtering of perceptual input and individual aims within a given learning context determines successful outcomes for different learner profiles (e.g., children vs. adults). Beyond learning, similar competitive interactions between PFC and sensorimotor brain regions may determine other domains of cognitive performance, for example, tasks involving creative thinking. Specifically, the MFH proposes that a hypofrontal cognitive state may prove advantageous for certain aspects of creative cognition to the extent that optimal performance in some creativity tasks requires availability of unfiltered low-level perceptual input. Conversely, for such tasks the application of prefrontally-mediated regulatory filtering of perceptual data will be associated with performance costs, as important information present in the low-level input is inhibited and discarded.
What types of creativity tasks would benefit from increased or decreased availability of raw perceptual information? An extended body of research supports the notion that a particular region of the PFC, the left ventrolateral prefrontal cortex, is implicated in tasks that require participants to tap their memory about the world (e.g., retrieving a verb associated with an object [e.g., dog-bark] or performing similarity judgments among items based on a particular property, like an object's color or function [e.g., is a hammer more similar to a hairdryer or a wrench?]; Thompson-Schill, et al., 2005; Thompson-Schill, D'Esposito, Aguirre, & Farah, 1997). A distinctive feature of all such tasks is that they are guided by a set of explicit rules and that they require one correct response, the form of which is typically known to the participants. To be able to identify that response, an individual would have to evaluate critically and select from among the available data the optimal alternative, while suppressing all irrelevant information. As discussed above, this process is possible through the involvement of the PFC and it is critical for some aspects of everyday problem solving. For example, if one needs to insert a nail to the wall one needs to succinctly represent this goal as well as attend to an abstract understanding of nails and hammers and the relationship between them to identify the right tool for the task. At the same time, low-level information that is irrelevant to the goals of the task will be filtered to optimize performance (e.g., the color of the nail, its material and consistency, etc. will be suppressed).
On the other hand, for some aspects of everyday problem solving that are frequently associated with the generation of novel ideas there is no obvious single response and the tasks seem to have multiple, equally likely solutions (e.g., writing an essay, sketching a drawing, finding alternative uses for old kitchen utensils, or inserting a nail into the wall in an emergency when a hammer is unavailable). In particular, such tasks are not guided by the implementation of an explicit rule, but rather they may require access to low-level perceptual data (e.g., shapes, sounds, materials) to dictate performance. In such data-driven tasks, the MFH proposes that a reduction in PFC-guided thought will limit input filtering which might facilitate the initiation of alternative possibilities prior to the identification of the optimal solution2.
Possible competitive interactions between two distinct brain systems would further suggest that, in contrast to goal-driven tasks, certain aspects of data-driven tasks might benefit from limited involvement of regions implicated in rule-based processing (i.e., prefrontal cortex) and the increased contribution of regions involved in object processing, particularly processing of object attributes or features (e.g., visual cortex; Dietrich, 2004; Thompson-Schill et al., 2009). As mentioned earlier, activity in these distinct brain regions may be associated with different types of thought, namely rule-driven or goal-driven (top-down) thinking and stimulus-driven or data-driven (bottom-up) thinking, respectively. Specifically, the prefrontal cortex may support the construction of rules and regularities about the world, abstracting away from low-level, ‘raw’ environmental data (e.g., learning that hammers are used for hammering regardless of their particular shape, size, or color; Wolford, Miller, & Gazzaniga, 2000). In contrast, focusing on low-level, ‘raw’ perceptual information in the environment (e.g., sounds, shapes, colors, materials) may involve increased activity in sensorimotor brain regions (e.g., occipitotemporal cortex). According to the MFH, depending on whether the task at hand (or a task subcomponent) requires access to low-level perceptual data, an individual may benefit from either top-down or bottom-up thinking for optimal performance, as supported by relative activity in these distinct brain regions.
In support of this prediction, recent work has suggested that hypofrontal cognitive states are associated with enhanced perceptual processing. For example, children with autism exhibit reduced sensitivity in discrimination between color categories, but better memory for unlabeled color stimuli relative to typically developing children (Heaton, Ludlow, & Roberson, 2008; see also Franklin et al., 2010). Indeed, the suboptimal prefrontal functioning in autism may increase the availability of bottom-up, environmentally-driven information in these individuals, which may allow some of them to become musical, mathematical, or artistic savants (Snyder, 2009). Finally, similar effects of hypofrontality on perceptual processing have also been observed in neuro-typical subjects: temporarily disrupting left prefrontal cortex activity using rapid transcranial magnetic stimulation (rTMS) can improve absolute pitch perception and number estimation in normal subjects (Snyder et al., 2003, 2006). These findings are in line with reports of preferential processing of absolute pitch patterns in continuous tone sequences in infants; interestingly, 8-month olds recognize and remember absolute pitch better than adults, who are only able to attend to the relationships between the notes (e.g., Saffran, 2003; Saffran et al., 2005). Although the tasks employed in these studies are not creative production tasks and should not be interpreted as reflective of superior creative production in these populations, the findings, overall, strongly support the increased availability of perceptual, bottom-up information in the context of diminished PFC function.
Accordingly, the MFH proposes that the generation of ideas within the context of a data-driven task (e.g., a creative design task) might involve a temporary distancing from knowledge-driven (top-down) thought—as guided by the prefrontal cortex—and a focus, instead, on data-driven (bottom-up) thought, as supported by sensorimotor brain regions (for a related model see Hélie & Sun, 2010). Indeed, evidence from studies with neuropsychological patients would support this hypothesis. Recent research has suggested that a hypofrontal state resulting from disease or injury may enhance one's ability for bottom-up cognitive processing in the context of complex data-driven tasks. For instance, patients with progressive aphasia, a neurodegenerative disease that targets selectively the patient's left frontal and temporal cortices, have been reported to exhibit increased levels of visual ability in spontaneous drawing or painting that they did not possess prior to their disease (Miller et al., 2000; Seeley et al., 2008; Shamay-Tsoory, Adler, Aharon-Peretz, Perry, Mayseless, 2011), although this tendency is not uniform across patients (Palmiero, Di Giacomo, & Passafiume, 2012). Likewise, patients with focal strokes in the left prefrontal cortex have been shown to outperform normal participants in creative problem solving tasks that require breaking away from rule-based thinking (Reverberi et al., 2005), although the results of these investigations are not consistent (see de Souza et al., 2010).
Specifically with regards to certain aspects of creative thinking such as idea generation, tasks that require broad conceptual associations have been linked to highly complex electroencephalogram (EEG) patterns across the entire brain but also reduced activity in frontal brain areas (Mölle et al., 1999), though these patterns may depend on the exact nature and duration of the creative task (see Fink et al., 2009, 2011). A study that employed functional magnetic resonance imaging (fMRI) has shown hypofrontal neural profiles in professional musicians during jazz improvisation, but not during the reproduction of well-practiced musical sequences (Limb & Brown, 2008; see also Liu et al., 2012). Similarly, in a recent fMRI experiment healthy adults appeared to benefit from a tradeoff between perceptually-based and rule-based thought for optimal performance: When generating creative uses for common objects (e.g., using a belt as a tourniquet), participants exhibited lower PFC activity, reflecting reduced cognitive control or filtering of low-level data (e.g., the shape or materials of the objects that would support a novel use), and increased activity in perceptual (visual object processing) regions, compared to participants who generated typical uses for the objects (Chrysikou & Thompson-Schill, 2011). Moreover, inhibitory transcranial direct current stimulation (tDCS) over left PFC, relative to inhibitory tDCS over right PFC or sham stimulation, increased the speed in which participants generated creative uses for common objects as well as the number of responses generated, an effect which was specific to creative use generation and not other control tasks (Chrysikou, Hamilton, Coslett, Datta, Bikson, & Thompson-Schill, 2013). Thus, under the demands of a data-driven creative thinking task, during which regulatory filtering of perceptual information would be associated with performance costs, healthy adults may benefit from a state of lower cognitive control.
Further support for this idea comes from observed differences between children and adults in tasks that may lead to functional fixedness. Functional fixedness is an example wherein adult participants are excessively inflexible; for example, when challenged to mount a candle on a wall given a book of matches and a box of tacks, they think of the box as a container for the tacks, thus failing to use it as a platform for the candle (Duncker, 1945). In contrast, under similar conditions, children appear resistant to functional fixedness effects and solve these sorts of problems more readily than adults (German & Defeyter, 2000; Defeyter & German, 2003). Under functional fixedness, it is likely that adult participants generate the intended use for the materials based on an abstract understanding of function in the context of artifact use and constrain their search for a solution based on that intended use; in contrast, young children do not generate the intended use as strongly and, thus, are able to organize their search around physical features of the materials rather than intended uses (Defeyter & German, 2003). These findings are in line with the MFH, although no current research has linked these changes directly to the development of PFC.
Beyond the influences exerted by specific task characteristics that require the use of low-level perceptual data in various degrees, the effects of cognitive control on creative thought may also be determined by trait-level variation. In particular, de Manzano and colleagues (2010) have recently shown that healthy individuals with decreased thalamic D2 densities (as measured by PET) had higher scores in a battery of data-driven creativity tasks. The authors propose that decreased D2 receptor densities in the thalamus may increase the flow of information in corticothalamic circuits by lowering thalamic gating thresholds, thus leading to advantages for data-driven tasks. On the other hand, Takeuchi and colleagues (2010) employed voxel-based morphometry to demonstrate that performance in a set of similar data-driven tests was associated with increased regional gray matter volumes in cortico-striatal dopaminergic regions, including the DLPFC and bilateral basal ganglia. Critically, Chermahini and Hommel (2010) demonstrated that spontaneous eyeblink rate (EBR), a clinical marker of striatal dopaminergic production, differentially predicted participants’ performance in data-driven and rule-driven tasks (the alternative uses task and the remote associates task, respectively), both of which are assumed to capture different aspects of creative thought. Specifically, EBRs and data-driven, bottom-up thinking were related in a non-linear fashion, such that individuals with medium EBRs (i.e., medium striatal dopamine levels) showed the greatest cognitive flexibility in the data-driven task. In contrast, a linear relationship between EBRs and rule-driven, top-down thinking revealed that individuals with low EBRs (i.e., decreased striatal dopamine levels) showed the best performance in the rule-driven task. Taken together, the above results reveal a possible sensitive balance between the amount of prefrontally mediated cognitive control (as directed by thalamic and striatal dopaminergic systems) for optimal performance in different components of creativity (e.g., data- vs. rule-driven thinking), although much future work is required to identify the exact nature of these relationships.
Overall, these findings offer support for the MFH according to which reduced prefrontal cortex activity limits filtering of low-level perceptual data and may facilitate certain aspects of perceptual processing by shifting the individual's focus from abstract, knowledge-based thinking to bottom-up, data-driven thinking. Although the effects of hypofrontality may promote the generation of novel solutions in an open-ended task, other aspects of creative thought likely depend on the involvement of rule-based prefrontal regulatory circuits. For example, the evaluation of the appropriateness and projected effectiveness of a generated solution for the achievement of a given goal likely involves a rapid reversal from a hypofrontal to a frontally-guided cognitive state, with the cycle repeating many times until the optimal solution is achieved. As an example of this process, in their computational model Hélie and Sun (2010) describe such an iterative (and possibly bidirectional) processing involving both explicit and implicit knowledge, which is hypothesized to be implicated simultaneously in the majority of problem solving and other cognitive tasks. Thus, creativity might be best viewed as the result of multiple cognitive processes (see Dietrich & Kanso, 2010), some of which may benefit whereas others might be compromised by a hypofrontal state. In consequence, among the challenges for the MFH is to determine the exact circumstances under which hypofrontality promotes creativity, as well as to identify the brain mechanisms allowing for the rapid transitions between hypofrontal and rule-based thinking during creative production.
Challenges and Future Directions
Scholars of cognitive control sometimes forget how capable an organism can be without much, or any, prefrontal cortex (for welcome exceptions, see, e.g., Shimamura, Gershberg, Jurica, Mangels, & Knight, 1992; Sylvester & Shimamura, 2002; Uretzky & Gilboa, 2010). Although it is our prefrontally-mediated cognitive functions that allow us to excel in many aspects of higher-order cognitive tasks, here we have proposed that tradeoffs between PFC-mediated regulatory mechanisms and the function of sensorimotor and subcortical brain systems may promote performance depending on individual objectives, evolutionary and developmental priorities, and specific task demands. We have offered a Matched Filter Hypothesis for cognitive control, which predicts that PFC-mediated regulatory filtering of low-level sensory information improves performance for top-down, rule-based, or goal-directed tasks that depend on the maintenance and manipulation of explicit representations. In contrast, hypofrontal states that restrict filtering of sensory input improve performance for bottom-up or stimulus-driven tasks for which the complexity of the necessary representations exceeds working memory limits3.
This proposal extends prior frameworks (e.g., Banich, 2009; Miller & Cohen, 2001; Shimamura, 2000) by suggesting not only that the function of cognitive control is to facilitate the appropriate response in a given task context, but also that a lack of cognitive control may be advantageous for data-driven tasks optimally supported by sensorimotor and subcortical brain systems, the activity of which is typically biased and reshaped by PFC-mediated interventions. We have presented evidence from the domains of learning and creative cognition that offers support for this proposal, as well as emphasized the central role of individual differences (e.g., age, genetic variation, brain damage) that can moderate the relationships between organism- and task-specific factors. Although we have hinted at the possible brain systems likely involved in this dynamic filtering process, a major challenge for the MFH is to specify the exact neural mechanisms under which cognitive control matches the type of filter applied to low-level data to the aims of the organism and the requirements of the task in question.
For example, it is clear that release from prefrontal regulation sometimes improves learning. A challenge for the MFH is to elucidate the circumstances under which this is true and the neurobiological mechanisms by which it occurs. For example, some level of prefrontal function is critical for reversal learning, as testified by neuroimaging (Cools et al., 2002, 2007b; Clark, Cools, & Robbins, 2004) and neuropsychology (Iversen & Mishkin, 1970; Dias, Robbins, & Roberts, 1996; Fellows & Farah, 2005), suggesting that reduction in prefrontal function does not linearly improve reversal learning—a finding recapitulated in studies of COMT and PFC DA, which generally find an inverted-U-shaped relationship between DA levels and task performance. Additionally, the relationship between declarative and goal-oriented learning is not logically necessary and has yet to be confirmed. Likewise, although a few studies have examined the relationship between hypofrontality and declarative learning (Filoteo et al., 2010; Foerde et al., 2006), it is not clear whether hypofrontality biases the brain toward habitual learning, as we hypothesize it does. Additionally, the relationship between hypofrontality and attentional learning is suggested by child studies (Sobel et al., 2004; Winman et al., 2005) but requires confirmation in adults. Computational modeling can support refining and testing hypotheses about the interaction between learning and cognitive control.
Convergent investigations of hypofrontality and learning via brain stimulation, functional neuroimaging, and genotyping will speak more clearly to questions of mechanism and provide a finer-grained perspective on the roles of different prefrontal subregions—for example, are the benefits of hypofrontality to procedural learning driven principally by the inactivity of regions supporting maintenance and conflict resolution, such as the ventrolateral PFC; of those supporting working memory, such as the dorsolateral PFC; or of those underlying attentional allocation, such as the frontal and supplementary eye fields? What are the roles of orbitofrontal cortex, which is richly connected to the ventral striatum, and posterior parietal cortex, which is functionally connected to PFC and whose role in the allocation of attention is well documented? Although extant work on cognitive control and learning has provided grist for interesting suppositions on these questions, concerted application of behavioral, computational, and cognitive neuroscience approaches is required to verify those conjectures. The MFH provides a conceptual framework for such investigations.
Overall, a high level of cognitive control provides obvious benefits for learning. It is very useful to be able to select, maintain, and manipulate information, amplify relevant differences, and abstract away from irrelevant ones. What unifies the results on learning discussed here is the simple insight that transforming the input comes at a cost: A particular transformation may be useful given one set of reinforcement contingencies and harmful given another. At least in some contexts, learning strategies that prize rapid error reduction pay for it over the long term in the fidelity and completeness of the learned information. The MFH can help generate hypotheses to guide multi-level methodological approaches incorporating neuroimaging, behavioral, computational, and individual differences (due to genetic variation, psychopathology, brain damage, or other factors) assessments to explore tradeoffs between PFC-mediated mechanisms and subcortical systems in different types of learning tasks.
Relative to the work in learning, studies of creative cognition are admittedly few in number, but nonetheless there are some intriguing findings indicating that aspects of creative thought that depend on bottom-up, stimulus driven idea generation may benefit from hypofrontal cognitive states. On the other hand, PFC-mediated cognitive control might be required for the evaluation of the appropriateness of these ideas in a given task context. Further experimental examinations of performance under these distinct task circumstances in individuals that exhibit different hypofrontality profiles due to age, brain damage, genetic variation, behavioral, non-invasive brain stimulation, or other interventions (both in terms of independent and compound influences of these factors) is required to further substantiate the predictions of the MFH. Moreover, research has suggested that different components of creative thought may depend on complex interhemispheric interactions particularly within lateral PFC, in addition to possible differential contributions of medial PFC regions bilaterally (e.g., Shamay-Tsoory et al., 2011). Whether a hypofrontal profile in all or a selective subset of these regions is beneficial or detrimental to different components of creative thought, in line with the predictions of the MFH, requires extensive additional empirical work. Importantly, successful performance in complex creative thinking tasks depends on the combined influences of both top-down (possibly mediated by PFC regulatory systems) and bottom-up (possibly mediated by sensorimotor cortical areas) processes (see Chrysikou, in press). It is important for future research using multi-method approaches to identify individual neurobiological factors that determine one's ability to increase or decrease intentionally the regulatory contributions of PFC-mediated cognitive control depending on the requirements of the task in hand, as such differences may account for the extent of individual variability in performance in creative cognition tasks (e.g., Akinola & Mendes, 2008; Cools, Roberts, & Robbins, 2007; Cools, Sheridan, Jacobs, & D'Esposito, 2007).
Even though our examples were heavily drawn from these two research areas, the main suppositions of the MFH pertain to numerous other domains beyond learning and creative thinking. For example, children's limited ability to filter random input and their bias toward most salient responses paradoxically benefits performance in probabilistic reinforcement tasks (Derks & Paclisanu, 1967; Estes, 1964, 1976). Furthermore, in line with the predictions of the MFH— and contrary to common assumptions—recent research in decision making has shown that working memory capacity limits the utility of conscious deliberation in complex, multidimensional domains: Although conscious deliberation is beneficial for relatively simple decisions, unconscious processing leads to better decisions when the number of relevant features far exceeds working memory capacity (e.g., Dijksterhuis, Bos, Nordgren, & Van Baaren, 2006a, 2006b; Dijksterhuis & Nordgren, 2006). The predictions of the MFH are also strongly relevant to research on emotion regulation (e.g., Bartz, Zaki, Bolger, & Ochsner, 2011; Gross, 2013; McRae et al., 2012; Ochsner, Silvers, & Buhle, 2012). Although we have largely examined bottom-up, data-driven responses in the context of sensorimotor systems, optimal regulatory filtering of low-level emotional responses as subserved by PFC mechanisms and for specific tasks may also subscribe to the principles outlined in the MFH.
Conclusion
Overall, the Matched Filter Hypothesis for cognitive control embraces a view of cognitive control as a complex and powerful system for fitting behavior to the situation, capable of producing dramatically different behaviors in subtly different contexts. Our goal is to respect that complexity and power while advancing a mechanistic understanding of cognitive control, specifically by exposing the advantageous consequences of its failure in the domains of learning and creative cognition. We hope this approach will foster an appreciation for cognitive control as a tool adapted to a subset of common challenges, rather than an all-purpose optimization system suited to every problem the organism might encounter.
Acknowledgments
This research was supported by NIH grants R01-DC009209 and R21-MH083029 to S.T.S.
Footnotes
PFC is not, of course, the only neural system implicated in cognitive control. It is likely that regions such as the anterior cingulate cortex (ACC) cooperate with the PFC to modulate filtering depending on the specific demands for conflict resolution (e.g., Botvinick et al., 2001; Braver, 2012; Friedman & Miyake, 2004; Milham & Banich, et al., 2001; Milham, Banich, Klaus, & Cohen, 2003; Kerns et al., 2004; Milham & Banich, 2005; Miyake, Friedman, Emerson, Witzki, Howerter, & Wager, 2000; Liu, Banich, Jacobson, & Tanabe, 2006; van Veen et al., 2001). For example, developmental studies using electroencephalography (EEG) have shown that the error related negativity (ERN), thought to originate in the ACC, matures relatively late during adolescence and into mid-adulthood (Davies et al., 2004a, 2004b; Ladouceur et al., 2007). Neuroimaging evidence further suggests that adults demonstrate increased activation in anterior cingulate gyrus relative to children and adolescents during inhibition failures in an individually adjusted stop task (Rubia, Smith, Taylor, & Brammer, 2007). Overall, these and other studies (e.g., Bunge et al., 2002; Rubia et al., 2000; Shaw et al., 2008) suggest that different brain systems that coordinate with PFC to implement cognitive control may show similar developmental trajectories. Here, in any case, we focus on PFC and use the term ‘hypofrontal’ as shorthand for low function in the various brain systems that support cognitive control.
We note that filtering can apply to various task-relevant attributes. For example, when looking for an object to insert a nail into a wall when a hammer is not available, reduced filtering can promote accessibility of low-level perceptual attributes of other items within the same class (e.g., other heavy tools in one's toolbox) or even items outside of that class (e.g., a sturdy shoe).
Although it is commonly held that all aspects of complex behavior demand prefrontally-guided regulatory mechanisms, the reviewed literature brings attention to the fact that the notion of task complexity is multifaceted and can take on different meanings depending on the circumstances. For instance, a task can be complex due to its increased requirements for abstraction or its high demands on working memory; at the same time, task complexity can be determined by the need to represent large amounts of unfiltered low-level information that exceed the capacity of working memory and consideration of which is necessary for optimal performance in certain domains.
References
- Akinola M, Mendes WB. The dark side of creativity: Biological vulnerability and negative emotions lead to greater artistic creativity. Personality and Social Psychology Bulletin. 2008;34:1677–1688. doi: 10.1177/0146167208323933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Reviews Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Bjork RA, Bjork EL. Remembering can cause forgetting: Retrieval dynamics in long-term memory. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1994;20:1063–1087. doi: 10.1037//0278-7393.20.5.1063. [DOI] [PubMed] [Google Scholar]
- Aslan A, Bäuml K-HT. Retrieval-induced forgetting in children. Psychonomic Bulletin & Review. 2010;17:704–709. doi: 10.3758/PBR.17.5.704. [DOI] [PubMed] [Google Scholar]
- Aslan A, Bäuml K-HT. Individual differences in working memory capacity predict retrieval-induced forgetting. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2011;37:264–269. doi: 10.1037/a0021324. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Crossley MJ. Interactions between declarative and procedural-learning categorization systems. Neurobiology of Learning and Memory. 2010;94:1–12. doi: 10.1016/j.nlm.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby FG, Crossley MJ. A computational model of how cholinergic interneurons protect striatal-dependent learning. Journal of Cognitive Neuroscience. 2011;23:1549–1566. doi: 10.1162/jocn.2010.21523. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Alfonso-Reese LA, Turken U, Waldron EM. A neuropsychological theory of multiple systems in category learning. Psychological Review. 1998;105:442–481. doi: 10.1037/0033-295x.105.3.442. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Noble S, Filoteo J, Waldron EM, Ell SW. Category learning deficits in Parkinson's disease. Neuropsychology. 2003;17:115–124. [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. doi:10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Banich MT. Executive function: The search for an integrated account. Current Directions in Psychological Science. 2009;18:89–94. [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011 2011 Jul;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Best CT, McRoberts GW, Goodell E. Discrimination of non-native consonant contrasts varying in perceptual assimilation to the listener's native phonological system. Journal of the Acoustical Society of America. 2001;109:775–794. doi: 10.1121/1.1332378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Boyd JK, Goldberg AE. Younger children fail to fully generalize a novel argument structure construction when exposed to the same input as older learners. Journal of Child Language. 2011 doi: 10.1017/S0305000911000079. DOI:10.1017/S0305000911000079. [DOI] [PubMed] [Google Scholar]
- Braver TS. The variable nature of cognitive control: A dual mechanisms framework. Trends in Cogntive Sciences. 2012;16:106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Robertson EM. Inducing motor skill improvements with a declarative task. Nature Neuroscience. 2007;10:148–149. doi: 10.1038/nn1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Zimerman S, Healey MK, Lee M, Hasher L. Age differences in visual statistical learning. Psychology and Aging. 2012;27:650. doi: 10.1037/a0026780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chermahini SA, Hommel B. The (b)link between creativity and dopamine: Spontaneous eye blink rates predict and dissociate divergent and convergent thinking. Cognition. 2010;115:458–465. doi: 10.1016/j.cognition.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Cristia A, Seidl A, Vaughn C, Schmale R, Bradlow A, Floccia C. Linguistic processing of accented speech across the lifespan. Frontiers in Psychology. 2012;3:479. doi: 10.3389/fpsyg.2012.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysikou EG. Creative states: A cognitive neuroscience approach to understanding and improving creativity in design. In: Gero J, editor. Proceedings of the workshop Visual and Spatial Reasoning for Design Creativity. Springer; New York, NY: (in press) [Google Scholar]
- Chrysikou EG, Hamilton RH, Coslett HB, Datta A, Bikson M, Thompson-Schill SL. Non-invasive transcranial direct current stimulation over the left prefrontal cortex facilitates cognitive flexibility in tool use. Cognitive Neuroscience. 2013;4:81–89. doi: 10.1080/17588928.2013.768221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysikou EG, Novick JM, Trueswell JC, Thompson-Schill SL. The other side of cognitive control: Can a lack of cognitive control benefit language and cognition? Topics in Cognitive Science. 2011;3:253–256. doi: 10.1111/j.1756-8765.2011.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysikou EG, Thompson-Schill SL. Dissociable brains states linked to common and creative object use. Human Brain Mapping. 2011;32:665–675. doi: 10.1002/hbm.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME. Maturational changes in cerebral function in infants determined by 18FDG positron emission tomography. Science. 1986;231:840–843. doi: 10.1126/science.3945811. [DOI] [PubMed] [Google Scholar]
- Cincotta CM, Seger CA. Dissociation between striatal regions while learning to categorize via feedback and via observation. Journal of Cognitive Neuroscience. 2007;19(2):249–265. doi: 10.1162/jocn.2007.19.2.249. [DOI] [PubMed] [Google Scholar]
- Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: Decision-making and reversal learning. Brain & Cognition. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- Cochran BP, McDonald JL, Parault SJ. Too smart for their own good: The disadvantage of a superior processing capacity for adult language learners. Journal of Memory and Language. 1999;41:30–58. [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. MIT Press; Cambridge, MA.: 1993. [Google Scholar]
- Colzato LS, Waszak F, Nieuwenhuis S, Posthuma D, Hommel B. The flexible mind is associated with the catechol-O-methyltransferase (COMT) Val158Met polymorphism: Evidence for a role of dopamine in the control of task-switching. Neuropsychologia. 2010;48(9):2764–2768. doi: 10.1016/j.neuropsychologia.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW. Defining the Neural Mechanisms of Probabilistic Reversal Learning Using Event-Related Functional Magnetic Resonance Imaging. Journal of Neuroscience. 2002;22(11):4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioral control processes. Trends in Cognitive Sciences. 2007;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D'Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. The Journal of Neuroscience. 2007;27:5506–5514. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R, Shallice T. Contention scheduling and the control of routine activities. Cognitive Neuropsychology. 2000;17:297–338. doi: 10.1080/026432900380427. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Aitken MR, Dickinson A, Shanks DR, Honey GD, Honey RA, Robbins TW, Bullmore ET, Fletcher PC. Prediction error during retrospective revaluation of causal associations in humans: fMRI evidence in favor of an associative model of learning. Neuron. 2004;44(5):877–888. doi: 10.1016/j.neuron.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Cragg N, Nation K. Language and the development of cognitive control. Topics in Cognitive Science. 2010;2:631–642. doi: 10.1111/j.1756-8765.2009.01080.x. [DOI] [PubMed] [Google Scholar]
- Crossley MJ, Ashby FG, Maddox WT. Erasing the Engram: The Unlearning of Procedural Skills. Journal of Experimental Psychology: General. 2012 doi: 10.1037/a0030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:215–229. doi: 10.1038/nrn755. doi:10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Cruess Anderson L, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of error monitoring ERPs in adolescents. Annals of the New York Academy of Sciences. 2004a;1021:324–328. doi: 10.1196/annals.1308.039. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of error monitoring ERPs in participants aged 7 to 25 years. Developmental Neuropsychology. 2004;25:355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nature Neuroscience. 2005;8:1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- De Manzano O, Cervenka S, Karabanov A, Fanke L, Ullen F. Thinking outside a less intact box: Thalamic dopamine D2 receptor densities are negatively related to psychometric creativity in healthy individuals. PLoS, ONE. 2010;5:E10670. doi: 10.1371/journal.pone.0010670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza LC, Volle E, Bertoux M, Czernecki V, Funkiewiez A, Allali G, et al. Poor creativity in frontotemporal dementia: A window into the neural bases of the creative mind. Neuropsychologia. 2010;48:3733–3742. doi: 10.1016/j.neuropsychologia.2010.09.010. [DOI] [PubMed] [Google Scholar]
- DeCaro MS, Thomas RD, Beilock SL. Individual differences in category learning: Sometimes less working memory capacity is better than more. Cognition. 2008;107(1):284–294. doi: 10.1016/j.cognition.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Defeyter M, German T. Acquiring an understanding of design: Evidence from children's insight problem solving. Cognition. 2003;89:133–155. doi: 10.1016/s0010-0277(03)00098-2. [DOI] [PubMed] [Google Scholar]
- Deng W, Sloutsky VM. The role of linguistic labels in categorization. In: Ohlsson S, Catrambone R, editors. Proceedings of the XXXII Annual Conference of the Cognitive Science Society. Erlbaum; Mahwah, NJ: 2010. pp. 230–235. [Google Scholar]
- Deng W, Sloutsky VM. Carrot-eaters and moving heads: Salient features provide greater support for inductive inference than category labels. Psychological Science. 2012;23:178–186. doi: 10.1177/0956797611429133. [DOI] [PubMed] [Google Scholar]
- Denton SE, Kruschke JK. Attention and salience in associative blocking. Learning & Behavior. 2006;34:285–304. doi: 10.3758/bf03192884. [DOI] [PubMed] [Google Scholar]
- Derks PL, Paclisanu MI. Simple strategies in binary prediction by children and adults. Journal of Experimental Psychology. 1967;73:278–285. [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: A resting state fMRI study. Cerebral Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Diamond A. The interplay of biology and the environment broadly defined. Developmental Psychology. 2009;45:1–8. doi: 10.1037/a0014601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Doar B. The performance of human infants on a measure of frontal cortex function, the delayed response task. Developmental Psychobiology. 1989;22:271–294. doi: 10.1002/dev.420220307. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dickinson A. Actions and habits: The development of behavioral autonomy. Philosophical Transactions of the Royal Society B. 1985;308:67–78. [Google Scholar]
- Dietrich A. The cognitive neuroscience of creativity. Psychonomic Bulletin and Review. 2004;11:1011–1026. doi: 10.3758/bf03196731. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kanso R. A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychological Bulletin. 2010;136:822–848. doi: 10.1037/a0019749. [DOI] [PubMed] [Google Scholar]
- Dijksterhuis A, Bos MW, Nordgren LF, van Baaren RB. On making the right choice: The deliberation-without-attention effect. Science. 2006a;311:1005–1007. doi: 10.1126/science.1121629. [DOI] [PubMed] [Google Scholar]
- Dijksterhuis A, Bos MW, Nordgren LF, van Baaren RB. Making choices without deliberation. Science. 2006b;312:1472. doi: 10.1126/science.1121629. [DOI] [PubMed] [Google Scholar]
- Dijksterhuis A, Nordgren LF. A theory of unconscious thought. Perspectives on Psychological Science. 2006;1:95–109. doi: 10.1111/j.1745-6916.2006.00007.x. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. The Dual-State Theory of Prefrontal Cortex Dopamine Function with Relevance to Catechol-O-Methyltransferase Genotypes and Schizophrenia. Biological Psychiatry. 2008;64(9):739–749. doi: 10.1016/j.biopsych.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Duncker K. On problem-solving. Psychological Monographs. 1945;58(5) Whole No.270. [Google Scholar]
- Elman JL. Learning and development in neural networks: The importance of starting small. Cognition. 1993;48:71–99. doi: 10.1016/0010-0277(93)90058-4. [DOI] [PubMed] [Google Scholar]
- Estes WK. Probability learning. In: Melton AW, editor. Categories of human learning. Academic; New York: 1964. pp. 89–128. [Google Scholar]
- Estes WK. The cognitive side of probability learning. Psychological Review. 1976;83:37–64. [Google Scholar]
- Evans B, Iverson P. Vowel normalization for accent: An investigation of best exemplar locations in northern and southern British English sentences. Journal of the Acoustical Society of America. 2004;115:352–361. doi: 10.1121/1.1635413. [DOI] [PubMed] [Google Scholar]
- Farrell SM, Tunbridge EM, Braeutigam S, Harrison PJ. COMT Val158Met Genotype Determines the Direction of Cognitive Effects Produced by Catechol-O-Methyltransferase Inhibition. Biological Psychiatry. 2012;71(6):538–544. doi: 10.1016/j.biopsych.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cerebral Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Filoteo JV, Lauritzen S, Maddox WT. Removing the frontal lobes: the effects of engaging executive functions on perceptual category learning. Psychological Science. 2010;21:415–423. doi: 10.1177/0956797610362646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoteo JV, Maddox WT, Salmon DP, Song DD. Information-integration category learning in patients with striatal dysfunction. Neuropsychology. 2004;19:212–222. doi: 10.1037/0894-4105.19.2.212. [DOI] [PubMed] [Google Scholar]
- Filoteo JV, Maddox WT, Simmons AN, Ing AD, Cagigas XE, Matthews S, Paulus MP. Cortical and subcortical brain regions involved in rule-based category learning. NeuroReport. 2005;16:111–115. doi: 10.1097/00001756-200502080-00007. [DOI] [PubMed] [Google Scholar]
- Fink A, Grabner RH, Benedek M, Reishofer G, Hauswirth V, Fally m., Neuper C, Ebner F, Neubauer AC. The creative brain: Investigation of brain activity during creative problem soliving by means of EEG and fMRI. Human Brain Mapping. 2009;30:734–748. doi: 10.1002/hbm.20538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink A, Schwab D, Papousek I. Sensitivity of EEG upper alpha activity to cognitive and affective creativity interventions. International Journal of Psychophysiology. 2011;82:233–289. doi: 10.1016/j.ijpsycho.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Anderson JM, Shanks DR, Honey R, Carpenter TA, Donovan T, Papadakis N, Bullmore ET. Responses of human frontal cortex to surprising events are predicted by formal associative learning theory. Nature Neuroscience. 2001;4(10):1043–1048. doi: 10.1038/nn733. [DOI] [PubMed] [Google Scholar]
- Foerde K, Knowlton BJ, Poldrack RA. Modulation of competing memory systems by distraction. Proceedings of the National Academy of Sciences USA. 2006;103:11778–11783. doi: 10.1073/pnas.0602659103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerde K, Poldrack RA, Knowlton BJ. Secondary-task effects on classification learning. Memory & Cognition. 2007;35:864–874. doi: 10.3758/bf03193461. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Moustafa AA, Haughey HM, Curran T, Hutchison KE. Genetic triple dissociation reveals multiple roles for dopamine in reinforcement learning. Proceedings of the National Academy of Sciences. 2007;104:16311–16316. doi: 10.1073/pnas.0706111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Doll BB, Oas-Terpstra J, Moreno F. Prefrontal and striatal dopaminergic genes predict individual differences in exploration and exploitation. Nature Neuroscience. 2009;12:1062–1068. doi: 10.1038/nn.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin A, Sowden P, Notman L, Gonzales-Dixon M, West D, Alexander I, Loveday S, White A. Reduced chromatic discrimination in children with Autism Spectrum Disorders. Developmental Science. 2010;13:188–200. doi: 10.1111/j.1467-7687.2009.00869.x. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291(5502):312–316. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Visual categorization and the primate prefrontal cortex: neurophysiology and behavior. Journal of Neurophysiology. 2002;88(2):929–941. doi: 10.1152/jn.2002.88.2.929. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. A comparison of primate prefrontal and inferior temporal cortices during visual categorization. The Journal of Neuroscience. 2003;23(12):5235–5246. doi: 10.1523/JNEUROSCI.23-12-05235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. The relations among inhibition and interference control functions: A latent-variable analysis. Journal of Experimental Psychology: General. 2004;133:101–135. doi: 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- Gaissmaier W, Schooler LJ. The smart potential behind probability-matching. Cognition. 2008;109(3):416–422. doi: 10.1016/j.cognition.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Galea JM, Albert NB, Ditye T, Miall RC. Disruption of the dorsolateral prefrontal cortex facilitates the consolidation of procedural skills. Journal of Cognitive Neuroscience. 2010;22:1158–1164. doi: 10.1162/jocn.2009.21259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR. The importance of proving the null. Psychological Review. 2009;116:439–445. doi: 10.1037/a0015251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German TP, Defeyter MA. Immunity to functional fixedness in young children. Psychonomic Bulletin & Review. 2000;7:707–712. doi: 10.3758/bf03213010. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proceedings of the National Academy of Sciences. 1996;93(24):13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Conceptualizing emotional labor: An emotion regulation perspective. In: Grandey A, Diefendorff J, Rupp D, editors. Emotional Labor in the 21st Century: Diverse Perspectives on Emotion Regulation at Work. Psychology Press; Routledge: NY: 2013. [Google Scholar]
- Hanania R, Smith LB. Selective attention and attention switching: towards a unified developmental approach. Developmental Science. 2010;13:622–635. doi: 10.1111/j.1467-7687.2009.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton P, Ludlow A, Roberson D. When less is more: poor discrimination but good colour memory in Autism. Research in Autism Spectrum Disorders. 2008;2:147–156. [Google Scholar]
- Hélie S, Sun R. Incubation, insight, and creative problem solving: A unified theory of a connectionist model. Psychological Review. 2010;117:994–1024. doi: 10.1037/a0019532. [DOI] [PubMed] [Google Scholar]
- Hoffman AB, Rehder B. The Costs of Supervised Classification: The Effect of Learning Task on Conceptual Flexibility. Journal of Experimental Psychology: General. 2010;139:319–340. doi: 10.1037/a0019042. [DOI] [PubMed] [Google Scholar]
- Hudson Kam CL, Newport EL. Regularizing unpredictable variation: The roles of adult and child learners in language formation and change. Language Learning and Development. 2005;1:151–195. [Google Scholar]
- Hudson Kam CL, Newport EL. Getting it right by getting it wrong: When learners change languages. Cognitive Psychology. 2009;59:30–66. doi: 10.1016/j.cogpsych.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hélie S, Roeder JL, Ashby FG. Evidence for cortical automaticity in rule-based categorization. Journal of Neuroscience. 2011;30:14225–14234. doi: 10.1523/JNEUROSCI.2393-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen S, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Experimental Brain Research. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451–474. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- Kamin LJ. Predictability, surprise, attention, and conditioning. In: Campbell BA, Church RM, editors. Punishment and aversive behavior. Appleton-Century-Crofts; New York: 1969. pp. 279–296. [Google Scholar]
- Kaminski JA, Sloutsky VM, Heckler AF. Blocking effects on dimensions: How attentional focus on values can spill over to the dimension level. In: Sloutsky VM, Love BC, McRae K, editors. Proceedings of the XXX Annual Conference of the Cognitive Science Society. Erlbaum; Mahwah, NJ: 2008. pp. 1075–1080. [Google Scholar]
- Kareev Y. Through a narrow window: Working memory capacity and the detection of covariation. Cognition. 1995;56(3):263–269. doi: 10.1016/0010-0277(95)92814-g. [DOI] [PubMed] [Google Scholar]
- Kareev Y, Lieberman I, Lev M. Through a narrow window: Sample size and the perception of correlation. Journal of Experimental Psychology: General. 1997;126(3):278–287. [Google Scholar]
- Kareev Y. Seven (indeed, plus or minus two) and the detection of correlations. Psychological Review. 2000;107(2):397–402. doi: 10.1037/0033-295x.107.2.397. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Khanna MM, Boland JE. The development of context-use for lexical ambiguity resolution. Quarterly Journal of Experimental Psychology. 2010;63:160–193. doi: 10.1080/17470210902866664. [DOI] [PubMed] [Google Scholar]
- Kirkham NZ, Diamond A. Sorting between theories of perseveration: Performance in conflict tasks requires memory, attention, and inhibition [Response]. Developmental Science. 2003;6:474–476. [Google Scholar]
- Kirkham NZ, Cruess L, Diamond A. Helping children apply their knowledge to their behavior on a dimension-switching task. Developmental Science. 2003;6:449–467. [Google Scholar]
- Koehler DJ, James G. Probability-matching in choice under uncertainty: Intuition versus deliberation. Cognition. 2009;113(1):123–127. doi: 10.1016/j.cognition.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Krugel LK, Biele G, Mohr PNC, Li S-C, Heekeren HR. Genetic variation in dopaminergic neuromodulation influences the ability to rapidly and flexibly adapt decisions. Proceedings of the National Academy of Sciences USA. 2009;106:17951–17956. doi: 10.1073/pnas.0905191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschke JK. Cue competition in function learning: Blocking and highlighting. The 3rd International Conference on Memory. 2001.
- Kruschke JK. Attention in learning. Current Directions in Psychological Science. 2003;12:171–175. [Google Scholar]
- Kruschke JK. Learning involves attention. In: Houghton G, editor. Connectionist Models in Cognitive Psychology. Psychology Press; Hove, East Sussex, UK: 2005. pp. 113–140. Ch. 4. [Google Scholar]
- Kruschke JK, Blair NJ. Blocking and backward blocking involve learned inattention. Psychonomic Bulletin & Review. 2000;7:636–645. doi: 10.3758/bf03213001. [DOI] [PubMed] [Google Scholar]
- Kruschke JK, Kappenman ES, Hetrick WP. Eye gaze and individual differences consistent with learned attention in associative blocking and highlighting. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2005;31:830–845. doi: 10.1037/0278-7393.31.5.830. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS. The development of action monitoring through adolescence into adulthood: ERP and source localization. Developmental Science. 2007;10:874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Limb CJ, Braun AR. Neural substrates of spontaneous musical performance: An fMRI study of jazz improvisation. PLoS ONE. 2008;32:e1679. doi: 10.1371/journal.pone.0001679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Chow HM, Xu Y, Erkkinen MG, Swett KE, Egle MW, Rizik-Baer DA, Braun AR. Neural correlates of lyrical improvisation: An fMRI study of freestyle rap. Scientific Reports. 2012;2:834. doi: 10.1038/srep00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Response and non-response related aspects of attentional selection as ascertained by fMRI. Cerebral Cortex. 2006;16:827–834. doi: 10.1093/cercor/bhj026. [DOI] [PubMed] [Google Scholar]
- Lupyan G, Dale R. Language structure is partly determined by social structure. PLoS ONE. 2010;5:e8559. doi: 10.1371/journal.pone.0008559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKain KS, Best CT, Strange W. Categorical perception of English /r/ and /1/ by Japanese bilinguals. Applied Psycholinguistics. 1981;2:369–390. [Google Scholar]
- Maye J, Aslin R, Tanenhaus M. The weckud wetch of the wast: Lexical adaptation to a novel accent. Cognitive Science. 2008;32:543–562. doi: 10.1080/03640210802035357. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Gabrieli JDE, Ochsner KN. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive and Affective Neuroscience. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medin DL, Edelson SM. Problem structure and the use of base-rate information from experience. Journal of Experimental Psychology: General. 1988;117:68–85. doi: 10.1037//0096-3445.117.1.68. [DOI] [PubMed] [Google Scholar]
- Meyers EM, Freedman DJ, Kreiman G, Miller EK, Poggio T. Dynamic population coding of category information in inferior temporal and prefrontal cortex. Journal of Neurophysiology. 2008;100(3):1407–19. doi: 10.1152/jn.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Banich MT. Anterior cingulate cortex: An fMRI analysis of conflict specificity & functional differentiation. Human Brain Mapping. 2005;25:328–335. doi: 10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Claus E, Cohen N. Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage. 2003;18:483–489. doi: 10.1016/s1053-8119(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cognitive Brain Research. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Miller BL, Boone K, Cummings JL, Read SL, Mishkin F. Functional correlates of musical and visual ability in frontotemporal dementia. British Journal of Psychiatry. 2000;176:458–63. doi: 10.1192/bjp.176.5.458. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager T. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mölle M, Marshall L, Wolf B, Fehm HL, Born J. EEG complexity and performance measures of creative thinking. Psychophysiology. 1999;36:95–104. doi: 10.1017/s0048577299961619. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Snyder HR, Chatham CH. Developing cognitive control: The costs and benefits of active, abstract representations. In: Zelazo P, Sera M, editors. Developing Cognitive Control Processes: Mechanisms, Implications, and Interventions. Erlbaum; Hillsdale, NJ: (in press) [Google Scholar]
- Newport EL. Constraints on learning and their role in language acquisition: Studies of the acquisition of American Sign Language. Language Sciences. 1988;10:147–172. [Google Scholar]
- Newport EL. Maturational constraints on language learning. Cognitive Science. 1990;14:11–28. [Google Scholar]
- Newport EL. Reduced input in the acquisition of signed languages: Contributions to the study of creolization. In: Degraff M, editor. Creolization, diachrony, and language acquisition. MIT Press; Cambridge, MA: 1999. pp. 161–178. [Google Scholar]
- Nolan KA, Bilder RM, Lachman HM, Volavka J. Catechol O-methyltransferase Val158Met polymorphism in schizophrenia: differential effects of Val and Met alleles on cognitive stability and flexibility. American Journal of Psychiatry. 2004;161(2):359–361. doi: 10.1176/appi.ajp.161.2.359. [DOI] [PubMed] [Google Scholar]
- Norman KA, O'Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychological Review. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Norman KA, Newman EL, Perotte AJ. Methods for reducing interference in the Complementary Learning Systems model: Oscillating inhibition and autonomous memory rehearsal. Neural Networks. 2005;18:1212–1228. doi: 10.1016/j.neunet.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Norman D, Shallice T. Attention to action: Willed and automatic control of behavior. In: Davidson R, Schwartz RG, Shapiro D, editors. Consciousness and self-regulation: Advances in research and theory. Plenum Press; New York: 1986. pp. 1–18. [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto AR, Taylor EG, Markman AB. There are at least two kinds of probability matching: Evidence from a secondary task. Cognition. 2011;118:274–279. doi: 10.1016/j.cognition.2010.11.009. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Mozer M, Munakata Y, Miyake A. The Second International Conference on Cognitive Science. Cognitive Science Society; Tokyo: Japanese: 1999. Discrete representations in working memory: A hypothesis and computational investigations. pp. 183–188. [Google Scholar]
- O'Reilly RC, Norman KA. Hippocampal and neocortical contributions to memory: Advances in the complementary learning systems framework. Trends in Cognitive Sciences. 2002;6:505–510. doi: 10.1016/s1364-6613(02)02005-3. [DOI] [PubMed] [Google Scholar]
- Palmiero M, Di Giacomo D, Passafiume D. Creativity and dementia: a review. Cognitive Processes. 2012;13:193–209. doi: 10.1007/s10339-012-0439-y. [DOI] [PubMed] [Google Scholar]
- Pan X, Sawa K, Tsuda I, Tsukada M, Sakagami M. Reward prediction based on stimulus categorization in primate lateral prefrontal cortex. Nature Neuroscience. 2008;11(6):703–712. doi: 10.1038/nn.2128. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition between memory systems: Converging evidence from animal and human studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Prabakharan V, Seger C, Gabrieli JDE. Striatal activation during cognitive skill learning. Neuropsychology. 1999;13:564–574. doi: 10.1037//0894-4105.13.4.564. [DOI] [PubMed] [Google Scholar]
- Rakison DH, Lupyan G. Developing object concepts in infancy: An associative learning perspective. Monographs of the Society for Research in Child Development. 2008;73:1–110. doi: 10.1111/j.1540-5834.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- Ramey CH, Chrysikou EG, Reilly J. Snapshots of children’s changing biases during language development: Differential weighting of perceptual and linguistic factors predicts noun age-of-acquisition. Journal of Cognition & Development. 2013;14:573–592. DOI:10.1080/15248372.2012.689386. [Google Scholar]
- Ramscar M, Gitcho N. Developmental change and the nature of learning in childhood. Trends in Cognitive Sciences. 2007;11:274–279. doi: 10.1016/j.tics.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Ramscar M, McClure SM. Manipulating information structure as a method of localizing information processing in the brain.. Poster presented at the 18th Annual Meeting of the Cognitive Neuroscience Society; April 2011.2011. [Google Scholar]
- Ramscar M, Yarlett D. Linguistic self-correction in the absence of feedback: A new approach to the logical problem of language acquisition. Cognitive Science. 2007;31:927–960. doi: 10.1080/03640210701703576. [DOI] [PubMed] [Google Scholar]
- Ramscar M, Yarlett D, Dye M, Denny K, Thorpe K. Feature-Label-Order effects and their implications for symbolic learning. Cognitive Science. 2010;34:909–957. doi: 10.1111/j.1551-6709.2009.01092.x. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Squire LR. Intact learning of artificial grammars and intact category learning by patients with Parkinson's disease. Behavioral Neuroscience. 1999;113:235–242. doi: 10.1037//0735-7044.113.2.235. [DOI] [PubMed] [Google Scholar]
- Reverberi C, Toraldo A, D'Agostini S, Skrap M. Better without (lateral) frontal cortex? Insight problems solved by frontal patients. Brain. 2005;128(Pt 12):2882–2890. doi: 10.1093/brain/awh577. [DOI] [PubMed] [Google Scholar]
- Rohde DLT, Plaut DC. Language acquisition in the absence of explicit negative evidence: How important is starting small? Cognition. 1999;72:67–109. doi: 10.1016/s0010-0277(99)00031-1. [DOI] [PubMed] [Google Scholar]
- Roman P, Soriano MF, Gomez-Ariza CJ, Bajo MT. Retrieval-induced forgetting and executive control. Psychological Science. 2009;20:1053–1058. doi: 10.1111/j.1467-9280.2009.02415.x. [DOI] [PubMed] [Google Scholar]
- Rossi AF, Bichot NP, Desimone R, Ungerleider LG. Top down attentional deficits in macaques with lesions of lateral prefrontal cortex. Journal of Neuroscience. 2007;27:11306–11314. doi: 10.1523/JNEUROSCI.2939-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier NP, O'Reilly RC. Learning representations in a gated prefrontal cortex model of dynamic task switching. Cognitive Science. 2002;26(4):503–520. [Google Scholar]
- Rougier NP, Noelle DC, Braver TS, Cohen JD, O'Reilly RC. Prefrontal cortex and flexible cognitive control: Rules without symbols. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(20):7338–7343. doi: 10.1073/pnas.0502455102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy JE, Riesenhuber M, Poggio T, Miller EK. Prefrontal cortex activity during flexible categorization. The Journal of Neuroscience. 2010;30(25):8519–8528. doi: 10.1523/JNEUROSCI.4837-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, Andrew C, Bullmore ET. Frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neuroscience Biobehavioural Review. 2000;24:13–21. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith A, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II. Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Saffran JR. Absolute pitch in infancy and adulthood: The role of tonal structure. Developmental Science. 2003;6:37–45. [Google Scholar]
- Saffran JR, Reeck K, Niehbur A, Wilson DP. Changing the tune: Absolute and relative pitch processing by adults and infants. Developmental Science. 2005;8:1–7. doi: 10.1111/j.1467-7687.2005.00387.x. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess P. The domain of supervisory processes and temporal organisation of behaviour. Philosophical Transactions of the Royal Society of London. 1996;B351:1405–1412. doi: 10.1098/rstb.1996.0124. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Matthews BR, Crawford RK, Gorno-Tempini ML, Foti D, Mackenzie IR, Miller BL. Unravelling Boléro: Progressive aphasia, transmodal creativity and the right posterior neocortex. Brain. 2008;131:39–49. doi: 10.1093/brain/awm270. [DOI] [PubMed] [Google Scholar]
- Seger CA, Cincotta CM. The roles of the caudate nucleus in human classification learning. Journal of Neuroscience. 2005;25:2941–2951. doi: 10.1523/JNEUROSCI.3401-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger CA, Prabhakaran V, Poldrack RA, Gabrieli JDE. Neural activity differs between explicit and implicit learning of artificial grammar strings: An fMRI Study. Psychobiology. 2000;28:283–292. [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The Expected Value of Control: An Integrative Theory of Anterior Cingulate Cortex Function. Neuron. 2013;79(2):217–240. doi: 10.1016/j.neuron.2013.07.007. doi:10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Adler N, Aharon-Peretz J, Perry D, Mayseless N. The origins of originality: The neural bases of creative thinking and originality. Neuropsychologia. 2011;49:178–185. doi: 10.1016/j.neuropsychologia.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheya A, Smith LB. Perceptual features and the development of conceptual knowledge. Journal of Cognition and Development. 2006;7:455–476. [Google Scholar]
- Shimamura AP. The role of the prefrontal cortex in dynamic filtering. Psychobiology. 2000;28:207–218. [Google Scholar]
- Shimamura AP, Gershberg FB, Jurica PJ, Mangels JA, Knight RT. Intact implicit memory in patients with frontal lobe lesions. Neuropsychologia. 1992;30:931–937. doi: 10.1016/0028-3932(92)90037-m. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Kalanithi J, Gluck MA. Basal ganglia and dopamine contributions to probabilistic category learning. Neuroscience and Behavioral Reviews. 2008;32:219–236. doi: 10.1016/j.neubiorev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton JL, Newport EL. When learners surpass their models: The acquisition of American Sign Language from inconsistent input. Cognitive Psychology. 2004;49:370–407. doi: 10.1016/j.cogpsych.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Slifstein M, Kolachana B, Simpson EH, Tabares P, Cheng B, Duvall M, Abi-Dargham A. COMT genotype predicts cortical-limbic D1 receptor availability measured with [11C]NNC112 and PET. Molecular Psychiatry. 2008;13(8):821–827. doi: 10.1038/mp.2008.19. [DOI] [PubMed] [Google Scholar]
- Sloutsky VM, Fisher AV. When Development and Learning Decrease Memory: Evidence against Category-Based Induction in Children. Psychological Science. 2004;15:553–558. doi: 10.1111/j.0956-7976.2004.00718.x. [DOI] [PubMed] [Google Scholar]
- Smith LB, Jones SS, Landau B, Gershkoff-Stowe L, Samuelson L. Object name learning provides on-the-job training for attention. Psychological Science. 2002;13:13–19. doi: 10.1111/1467-9280.00403. [DOI] [PubMed] [Google Scholar]
- Snyder A. Explaining and inducing savant skills: Privileged access to lower level, less-processed information. Philosophical Transactions of the Royal Society B. 2009;364:1399–1405. doi: 10.1098/rstb.2008.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A, Bahramali H, Hawker T, Mitchell DJ. Savant-like numerosity skills revealed in normal people by magnetic pulses. Perception. 2006;35:837–845. doi: 10.1068/p5539. [DOI] [PubMed] [Google Scholar]
- Snyder A, Mulcahy E, Taylor JL, Mitchell DJ, Sachdev P, Gandevia SC. Savant-like skills exposed in normal people by suppressing the left fronto-temporal lobe. Journal of Integrative Neuroscience. 2003;2:149–158. doi: 10.1142/s0219635203000287. [DOI] [PubMed] [Google Scholar]
- Sobel DM, Tenenbaum JB, Gopnik A. Children's causal inferences from indirect evidence: Backwards blocking and Bayesian reasoning in preschoolers. Cognitive Science. 2004;28:303–333. [Google Scholar]
- Soto FA, Waldschmidt JG, Hélie S, Ashby FG. Brain activity across the development of automatic categorization: A comparison of categorization tasks using multi-voxel pattern analysis. NeuroImage. 2013;71:284–297. doi: 10.1016/j.neuroimage.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester C-Y, Shimamura AP. Evidence for intact representations in patients with frontal lobe lesions. Neuropsychology. 2002;16:197–207. [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizumi H, Sekiguchi A, Fukushima A, Kawashima R. White matter structures associated with creativity: Evidence from diffusion tensor imaging. NeuroImage. 2010;51:11–18. doi: 10.1016/j.neuroimage.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Current Opinion in Neurobiology. 2005;15:219–224. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left prefrontal cortex in retrieval of semantic knowledge: A re-evaluation. Proceedings of the National Academy of Sciences USA. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Ramscar M, Chrysikou EG. Cognition without control: When a little frontal lobe goes a long way. Current Directions in Psychological Science. 2009;18:259–263. doi: 10.1111/j.1467-8721.2009.01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro JM, Sinnett S, Soto-Faraco S. Speech segmentation by statistical learning depends on attention. Cognition. 2005;97:B25–B34. doi: 10.1016/j.cognition.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Trehub SE. The discrimination of foreign speech contrasts by infants and adults. Child Development. 1976;47:466–472. [Google Scholar]
- Turk-Browne NB, Jungé JA, Scholl BJ. The automaticity of visual statistical learning. Journal of Experimental Psychology: General. 2005;134:552–564. doi: 10.1037/0096-3445.134.4.552. [DOI] [PubMed] [Google Scholar]
- Turner DC, Aitken MR, Shanks DR, Sahakian BJ, Robbins TW, Schwarzbauer C, Fletcher PC. The role of the lateral frontal cortex in causal associative learning: exploring preventative and super-learning. Cerebral Cortex. 2004;14(8):872–880. doi: 10.1093/cercor/bhh046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uretzky S, Gilboa A. Knowing your lines but missing your cue: Rostral prefrontal lesions impair prospective memory cue detection, but not action-intention superiority. Journal of Cognitive Neuroscience. 2010;22:2745–2757. doi: 10.1162/jocn.2010.21419. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. NeuroImage. 2001;14:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Voytek B, Knight RT. Prefrontal cortex and basal ganglia contributions to visual working memory. Proceedings of the National Academy of Sciences, USA. 2010;107:18167–18172. doi: 10.1073/pnas.1007277107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker JF, Gilbert JH, Humphrey K, Tees RC. Developmental aspects of cross-language speech perception. Child Development. 1981:349–355. [PubMed] [Google Scholar]
- Winman A, Wennerholm P, Juslin P, Shanks DR. Evidence for rule-based processes in the inverse base rate effect. Quarterly Journal of Experimental Psychology. 2005;58A(5):789–815. doi: 10.1080/02724980443000331. [DOI] [PubMed] [Google Scholar]
- Wolford G, Miller MB, Gazzaniga M. The left hemisphere's role in hypothesis formation. Journal of Neuroscience. 2000;20:1–4. doi: 10.1523/JNEUROSCI.20-06-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F. The role of language in acquiring object kind concepts in infancy. Cognition. 2002;85:223–250. doi: 10.1016/s0010-0277(02)00109-9. [DOI] [PubMed] [Google Scholar]
- Yao X, Sloutsky VM. Selective attention and development of categorization: An eye tracking study. In: Ohlsson S, Catrambone R, editors. Proceedings of the XXXII Annual Conference of the Cognitive Science Society. Erlbaum; Mahwah, NJ: 2010. pp. 1980–1985. [Google Scholar]
- Yim H, Best CB, Sloutsky VM. Cost of attention as an indicator of category learning. In: Carlson L, Hölscher Ch., Shipley T, editors. Proceedings of the XXXIII Annual Conference of the Cognitive Science Society. Erlbaum; Mahwah, NJ: 2011. pp. 1763–1769. [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature Reviews Neuroscience. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. European Journal of Neuroscience. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilario MRF, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nature Neuroscience. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Smith LB. Linguistic cues enhance the learning of perceptual cues. Psychological Science. 2005;16:90–95. doi: 10.1111/j.0956-7976.2005.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD, Frye D, Rapus T. An age-related dissociation between knowing rules and using them. Cognitive Development. 1996;11:37–63. [Google Scholar]


