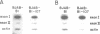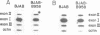Abstract
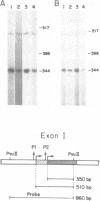
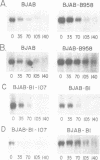
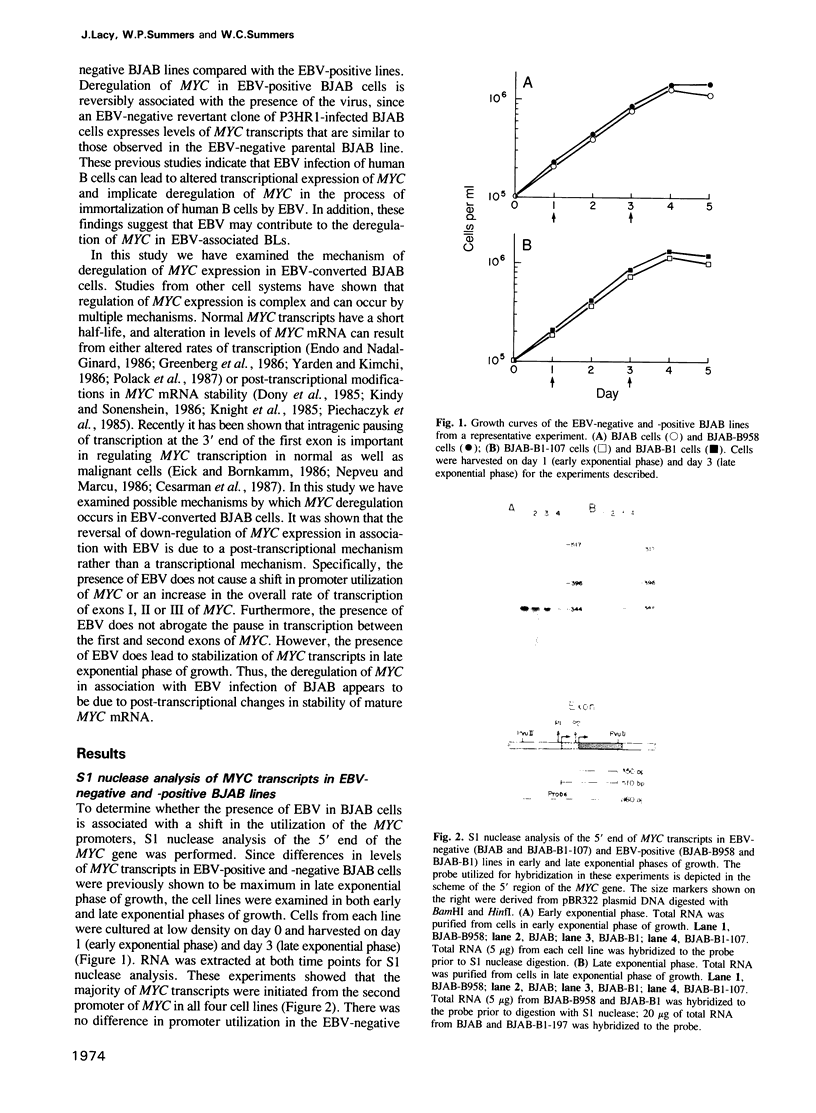
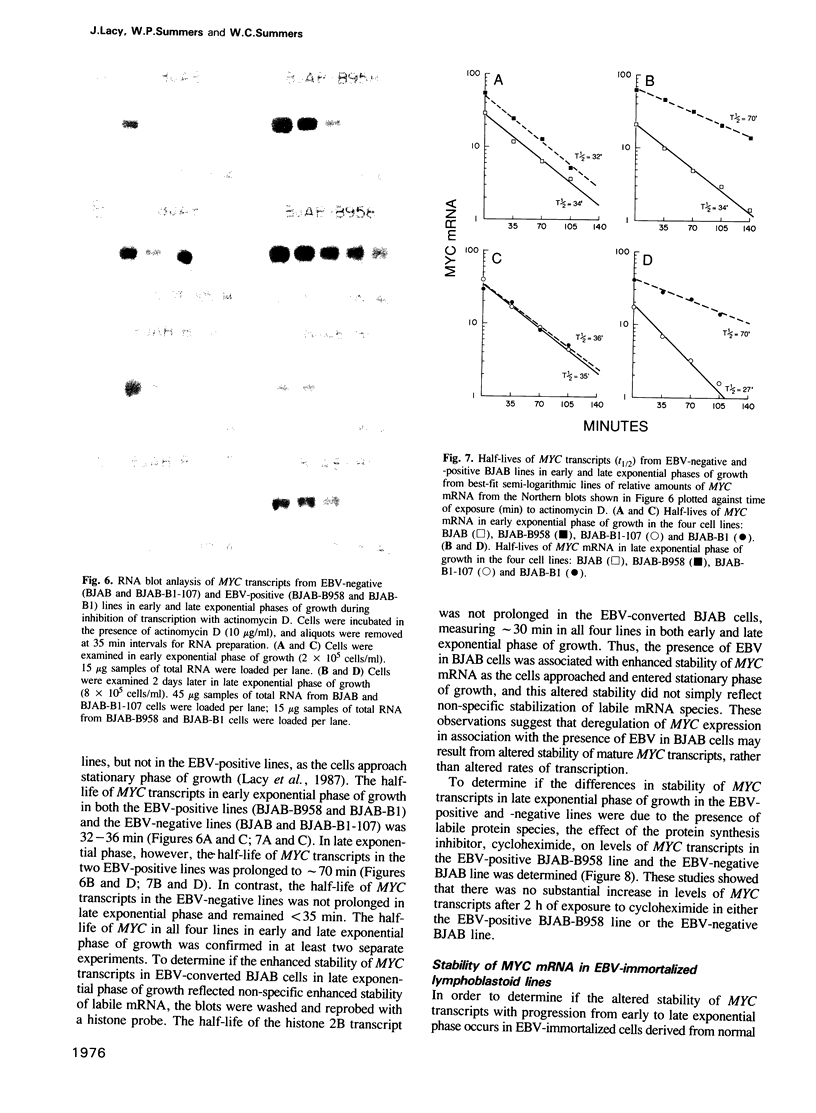
By utilizing an Epstein-Barr virus (EBV)-negative Burkitt's lymphoma line (BJAB) and several EBV-positive sub-lines derived from it by in vitro infection, it has been shown previously that the presence of the EBV genome in this B cell line is reversibly associated with deregulation of MYC expression. Specifically, the decline in the level of MYC transcripts observed in the EBV-negative BJAB line as cells approach stationary phase of growth is abrogated in the presence of the virus. In the studies described herein, the mechanism of deregulation of MYC in EBV-converted BJAB cells was examined. It was shown that the presence of EBV in BJAB cells was not associated with changes in the rate of transcription from exons I, II or III of MYC as cells approached stationary phase of growth. In contrast, the stability of MYC mRNA was altered in EBV-positive BJAB cells. Specifically, the half-life of MYC mRNA increased from less than 36 to greater than 70 min in EBV-positive BJAB lines as cells progressed from early to late exponential phase of growth. This alteration in stability of MYC transcripts was reversibly associated with the presence of the virus, since an EBV-negative revertant BJAB line did not display an increase in stability of MYC transcripts in late exponential phase of growth. In addition, progression from early to late exponential phase of growth in two EBV-immortalized lymphoblastoid lines derived from normal human B cells was also associated with prolongation of the half-life of MYC.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battey J., Moulding C., Taub R., Murphy W., Stewart T., Potter H., Lenoir G., Leder P. The human c-myc oncogene: structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell. 1983 Oct;34(3):779–787. doi: 10.1016/0092-8674(83)90534-2. [DOI] [PubMed] [Google Scholar]
- Calender A., Billaud M., Aubry J. P., Banchereau J., Vuillaume M., Lenoir G. M. Epstein-Barr virus (EBV) induces expression of B-cell activation markers on in vitro infection of EBV-negative B-lymphoma cells. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8060–8064. doi: 10.1073/pnas.84.22.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Cesarman E., Dalla-Favera R., Bentley D., Groudine M. Mutations in the first exon are associated with altered transcription of c-myc in Burkitt lymphoma. Science. 1987 Nov 27;238(4831):1272–1275. doi: 10.1126/science.3685977. [DOI] [PubMed] [Google Scholar]
- Cheah M. S., Ley T. J., Tronick S. R., Robbins K. C. fgr proto-oncogene mRNA induced in B lymphocytes by Epstein-Barr virus infection. Nature. 1986 Jan 16;319(6050):238–240. doi: 10.1038/319238a0. [DOI] [PubMed] [Google Scholar]
- Chisholm G. E., Summers W. C. The promoter for the late gene encoding Vp5 of herpes simplex virus type 1 is recognized by cell extracts derived from uninfected cells. J Virol. 1986 Nov;60(2):620–625. doi: 10.1128/jvi.60.2.620-625.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dony C., Kessel M., Gruss P. Post-transcriptional control of myc and p53 expression during differentiation of the embryonal carcinoma cell line F9. Nature. 1985 Oct 17;317(6038):636–639. doi: 10.1038/317636a0. [DOI] [PubMed] [Google Scholar]
- Eick D., Bornkamm G. W. Transcriptional arrest within the first exon is a fast control mechanism in c-myc gene expression. Nucleic Acids Res. 1986 Nov 11;14(21):8331–8346. doi: 10.1093/nar/14.21.8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T., Nadal-Ginard B. Transcriptional and posttranscriptional control of c-myc during myogenesis: its mRNA remains inducible in differentiated cells and does not suppress the differentiated phenotype. Mol Cell Biol. 1986 May;6(5):1412–1421. doi: 10.1128/mcb.6.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fresen K. O., Hausen H. Establishment of EBNA-expressing cell lines by infection of Epstein-Barr virus (EBV)-genome-negative human lymphoma cells with different EBV strains. Int J Cancer. 1976 Feb 15;17(2):161–166. doi: 10.1002/ijc.2910170203. [DOI] [PubMed] [Google Scholar]
- Fresen K. O., Merkt B., Bornkamm G. W., Hausen H. Heterogeneity of Epstein-Barr virus originating from P3HR-1 cells. I. Studies on EBNA induction. Int J Cancer. 1977 Mar 15;19(3):317–323. doi: 10.1002/ijc.2910190306. [DOI] [PubMed] [Google Scholar]
- Fulton R., Birnie G. D., Knowler J. T. Post-transcriptional regulation of rat liver gene expression by glucocorticoids. Nucleic Acids Res. 1985 Sep 25;13(18):6467–6482. doi: 10.1093/nar/13.18.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kelly K., Siebenlist U. The regulation and expression of c-myc in normal and malignant cells. Annu Rev Immunol. 1986;4:317–338. doi: 10.1146/annurev.iy.04.040186.001533. [DOI] [PubMed] [Google Scholar]
- Kindy M. S., Sonenshein G. E. Regulation of oncogene expression in cultured aortic smooth muscle cells. Post-transcriptional control of c-myc mRNA. J Biol Chem. 1986 Sep 25;261(27):12865–12868. [PubMed] [Google Scholar]
- Klein G., Lindahl T., Jondal M., Leibold W., Menézes J., Nilsson K., Sundström C. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3283–3286. doi: 10.1073/pnas.71.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight E., Jr, Anton E. D., Fahey D., Friedland B. K., Jonak G. J. Interferon regulates c-myc gene expression in Daudi cells at the post-transcriptional level. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1151–1154. doi: 10.1073/pnas.82.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy J., Sarkar S. N., Summers W. C. Induction of c-myc expression in human B lymphocytes by B-cell growth factor and anti-immunoglobulin. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1458–1462. doi: 10.1073/pnas.83.5.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy J., Summers W. P., Watson M., Glazer P. M., Summers W. C. Amplification and deregulation of MYC following Epstein-Barr virus infection of a human B-cell line. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5838–5842. doi: 10.1073/pnas.84.16.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinbach S. S., Summers W. C. Herpes simplex virus type 1 infection of isogenic Epstein-Barr virus genome-negative and -positive Burkitt's lymphoma-derived cell lines. J Virol. 1979 Apr;30(1):248–254. doi: 10.1128/jvi.30.1.248-254.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys E. J., Crouse G. F., Kellems R. E. Dihydrofolate reductase gene expression in cultured mouse cells is regulated by transcript stabilization in the nucleus. J Cell Biol. 1984 Jul;99(1 Pt 1):180–187. doi: 10.1083/jcb.99.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Gunderson N., Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985 Dec 6;230(4730):1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G., Clements G. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine. 1975 Jul;22(4):276–284. [PubMed] [Google Scholar]
- Nepveu A., Marcu K. B. Intragenic pausing and anti-sense transcription within the murine c-myc locus. EMBO J. 1986 Nov;5(11):2859–2865. doi: 10.1002/j.1460-2075.1986.tb04580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechaczyk M., Yang J. Q., Blanchard J. M., Jeanteur P., Marcu K. B. Posttranscriptional mechanisms are responsible for accumulation of truncated c-myc RNAs in murine plasma cell tumors. Cell. 1985 Sep;42(2):589–597. doi: 10.1016/0092-8674(85)90116-3. [DOI] [PubMed] [Google Scholar]
- Polack A., Eick D., Koch E., Bornkamm G. W. Truncation does not abrogate transcriptional downregulation of the c-myc gene by sodium butyrate in Burkitt's lymphoma cells. EMBO J. 1987 Oct;6(10):2959–2964. doi: 10.1002/j.1460-2075.1987.tb02601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinitz M., Klein G. Comparison between growth characteristics of an Epstein--Barr virus (EBV)-genome-negative lymphoma line and its EBV-converted subline in vitro. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3518–3520. doi: 10.1073/pnas.72.9.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinitz M., Klein G. Epstein-Barr virus (EBV)-induced change in the saturation sensitivity and serum dependence of established, EBV-negative lymphoma lines in vitro. Virology. 1976 Apr;70(2):570–573. doi: 10.1016/0042-6822(76)90302-0. [DOI] [PubMed] [Google Scholar]
- Studzinski G. P., Brelvi Z. S., Feldman S. C., Watt R. A. Participation of c-myc protein in DNA synthesis of human cells. Science. 1986 Oct 24;234(4775):467–470. doi: 10.1126/science.3532322. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennborg A., Aman P., Saranath D., Pear W., Sümegi J., Klein G. Conversion of the lymphoma line "BJAB" by Epstein-Barr virus into phenotypically altered sublines is accompanied by increased c-myc mRNA levels. Int J Cancer. 1987 Aug 15;40(2):202–206. doi: 10.1002/ijc.2910400213. [DOI] [PubMed] [Google Scholar]
- Yarden A., Kimchi A. Tumor necrosis factor reduces c-myc expression and cooperates with interferon-gamma in HeLa cells. Science. 1986 Dec 12;234(4782):1419–1421. doi: 10.1126/science.3097823. [DOI] [PubMed] [Google Scholar]