Abstract
Cystogenesis and tubulogenesis are basic building blocks for many epithelial organs, including the kidney. Most researchers have used two-dimensional (2D) cell culture to investigate signaling pathways downstream of hepatocyte growth factor (HGF). We hypothesize that three-dimensional (3D) collagen-grown Madin-Darby canine kidney (MDCK) cells, which form cysts and then tubulate in response to HGF, are a much more in vivo-like system for the identification of novel tubulogenes. With the use of a canine microarray containing over 20,000 genes, 2,417 genes were identified as potential tubulogenes that were differentially regulated, exclusively in 3D-grown MDCK cells. Among these, 840 were dependent on MAPK signaling. Importantly, this work shows that many putative tubulogenes, previously identified via microarray analysis of 2D cultures, including by us, do not change in 3D culture and vice versa. The use of a 3D-culture system allowed for the identification of novel MAPK-dependent and -independent genes that regulate early renal tubulogenesis in vitro, e.g., matrix metalloproteinase 1 (MMP1). Knockdown of MMP1 led to defects in cystogenesis and tubulogenesis in 3D-grown MDCK cells, most likely due to problems establishing normal polarity. We suggest that data obtained from 2D cultures, even those using MDCK cells treated with HGF, should not be automatically extrapolated to factors important for cystogenesis and tubulogenesis. Instead, 3D culture, which more closely replicates the biological environment and is therefore a more accurate model for identifying tubulogenes, is preferred. Results from the present analysis will be used to build a more accurate model of the signaling pathways that control cystogenesis and tubulogenesis.
Keywords: mitogen-activated protein kinase, matrix metalloproteinase, development, kidney, tubulogenesis
epithelial organs, such as the kidney, mammary gland, lung, and pancreas, originate from two basic types of building blocks: cysts and tubules (14, 21, 35, 37, 38, 44). Cysts are spherical (whereas tubules are cylindrical) monolayers of epithelial cells that enclose a central lumen (33). The epithelial cells in a cyst/tubule are well polarized, with an apical surface facing the central lumen, a lateral surface contacting adjacent cells, and a basal surface adhering to the underlying basement membrane—a specialized form of extracellular matrix (46). Cystogenesis is critical for early kidney development, resulting in the formation of the renal vesicle. Growth-factor induction of the renal vesicle then gives rise to all nephron tubule segments, with the exception of the collecting ducts (11, 22).
The understanding of the developmental programs that control cyst and tubule formation and repair is crucial for the development of therapies for diseases that affect tubule-based organs, such as the kidney. For example, autosomal-dominant polycystic kidney disease (ADPKD) is the most common, potentially lethal genetic disease in humans (43), and acute tubular necrosis of the kidney, with the subsequent need for tubule regeneration, is one of the leading causes of acute kidney injury, with mortality rates in affected patients as high as 50–70% (9). Therefore, the identification of genes involved in the regulation of these processes could lay the foundation for the discovery of novel therapeutics. Unfortunately, due to the complexity of organogenesis (the human kidney is composed of >20 cell types and 1 million nephrons) (33, 41) and the transitory nature of tubule formation, it is exceedingly difficult to study these processes in vivo.
Classic, two-dimensional (2D) cell-culture models, where cells are grown as a monolayer on plastic or on a Transwell filter, are easy to use and inexpensive. However, given that 2D growth is not what occurs in vivo, we hypothesize that 2D-culture systems are incomplete models for the study of cystogenesis and tubulogenesis. In fact, differences have been demonstrated for individual genes and their protein products in cells grown in 2D vs. three-dimensional (3D) culture. For example, galectin-3 is secreted apically in monolayers (20, 40) but basolaterally in 3D cysts (5, 6). 3D-culture systems, where cells are grown in biological substrates, such as collagen, for the study of tubule formation, are therefore more likely to capture what is actually occurring in living animals. The Madin-Darby Canine Kidney (MDCK) cell line is one of the most commonly used cells for the study of epithelial cell biology in vitro (42) and has been used successfully in 3D culture by us and others to study various aspects of tubulogenesis (7, 10, 21, 36, 39).
MDCK cells grown in type I collagen form multicellular structures (18, 19) that are characterized by a polarized epithelium surrounding a fluid-filled space, apical microvilli, a solitary cilium, and apical, tight junctions (28, 29), meeting the most rigorous definition of “cysts” (27). Subsequent exposure to hepatocyte growth factor (HGF) causes these cysts to develop branching tubules (30) in a process that resembles renal tubulogenesis in vivo (41). Cells in the MDCK cysts exposed to HGF initially send out extensions of their basolateral surface. Short chains are then formed, which lack apicobasolateral polarity and are surrounded by a basolateral surface. Thus these cells are said to undergo a partial epithelial-to-mesenchymal transition (pEMT) that lasts for 24 h. Further cell migration and/or division over the next 48 h lead to the formation of cords of cells, with nascent lumina appearing between the cells as they begin to repolarize, eventually forming mature tubules in the “redifferentiation” phase (35). This suggests that the transient loss and subsequent restoration of cell polarity are crucial for tubulogenesis and are logical, as cells that are highly polarized and tightly adherent would be unable to migrate out to form the organs necessary for development (33, 46).
Although studies using 3D-grown MDCK cells have allowed for the identification of complex interactions that take place during tubulogenesis, the signaling pathways that regulate this process remain unclear. Here, we sought to study changes in gene expression associated with early tubulogenesis in vitro using MDCK cells and DNA microarray analysis. We previously showed defects in tubulogenesis in MDCK cells using short hairpin RNA (shRNA)-mediated knockdown (KD) of putative tubulogenes, identified via microarray analysis of 2D-cultured MDCK cells (16). However, KD of these previously identified tubulogenes presented with variable defects (16), suggesting that although the genes identified via microarray analysis of 2D-grown MDCK cells were important for some aspects of tubulogenesis, not all tubulogenes could be identified using this approach.
In the present study, we identified differentially regulated genes involved in early kidney tubulogenesis that were missed in microarray analysis of 2D-cultured MDCK cells (16). Additionally, similar to our previous work (16), we investigated which of our newly identified, putative tubulogenes were dependent on MAPK signaling by performing microarray analysis in the presence and absence of HGF and two independent MAPK pathway inhibitors: U0126 and PD098059. This analysis led to the identification of 2,417 novel, 3D-specific tubulogenes, 840 of which are MAPK dependent.
Consistent with our previous work (16) and supporting the idea that interactions with the extracellular environment are critical for tubulogenesis, we identified the secreted collagenase matrix metalloproteinase 1 (MMP1) as a novel tubulogene, which is dependent on the MAPK pathway for expression. shRNA-mediated KD of MMP1 led to defects in 3D cell polarization, cystogenesis, and tubulogenesis.
In summary, this study allowed for the identification of novel, MAPK-dependent and -independent tubulogenes, such as MMP1, previously not identified using 2D-cultured cells, highlighting the importance of complementing traditional 2D-culture models with 3D cultures for the study of complex morphogenetic processes, such as cystogenesis and tubulogenesis.
MATERIALS AND METHODS
MDCK culture, HGF treatment, and RNA isolation.
Low-passage type II MDCK cells were obtained from Keith Mostov (University of California, San Francisco, CA) and used as described previously (3, 4). These cells were originally cloned by Daniel Louvard at the European Molecular Biology Laboratory (Heidelberg, Germany) and came to Keith Mostov via Karl Matlin (University of Chicago, Chicago, IL). Cells were cultured in modified MEM (11095; Gibco, Life Technologies, Carlsbad, CA), containing Earle's balanced salt solution (including 26.19 mM sodium bicarbonate) and l-glutamine, which was supplemented with 5% fetal calf serum, 100 units/ml penicillin, and 100 μg/ml streptomycin.
2D culture.
Type II MDCK cells were seeded on 24-mm Transwell filter units (Costar, Cambridge, MA). Pore size on all filters was 0.4 μm. Cell monolayers were confluent, as determined by the ability to maintain fluid levels in the apical chamber (24), and used for experiments after 6–7 days of culture with daily changes in medium. Recombinant HGF at 10 or 100 ng/ml was added to the basolateral compartment of MDCK cell monolayers for HGF-treated 2D cells. Media were changed daily for 2D-cultured cells.
3D culture.
Type II MDCK cells were plated in a 3D type I collagen matrix, as described previously (2, 16, 42), and allowed to mature into hollow, cystic structures over the course of 2 wk. Cysts were then exposed to HGF or untreated medium at a concentration of 10 ng/ml (for imaging experiments) (1) or 100 ng/ml (for all other experiments) for 24–72 h, and HGF-containing media were changed daily. MDCK cell cysts, subjected to HGF at 10 or 100 ng/ml for 24 h, have been shown to induce pEMT, the initial stage of tubulogenesis, in the vast majority of MDCK cell cysts (15, 21, 42). Recombinant human HGF was very generously provided by the late Ralph Schwall (Genentech, South San Francisco, CA).
RNA isolation.
Total RNA was obtained from MDCK cell monolayers using the RNeasy Mini Protocol (Qiagen, Germantown, MD) for the isolation of total RNA from animal cells. For the microarray studies, RNA samples were visualized by agarose RNA electrophoresis. RNA concentration and purity were measured by determining the 260:280 nm ratio. All ratios were >1.8.
Microarray analysis.
For our experiments, all protocols were conducted as described in the GeneChip Expression Analysis Technical Manual (Affymetrix, Santa Clara, CA). All conditions were performed in quadruplicate, except where otherwise stated. Total RNA (15 μg), collected from MDCK cells grown for 6 days on Transwell filters for the 2D group or MDCK cells grown for 10 days in type I collagen matrix for the 3D group and exposed to 0 or 24 h of HGF (100 ng/ml), was converted to first-strand cDNA using Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI). Second-strand cDNA synthesis was followed by in vitro transcription for linear amplification and probe hybridization onto GeneChip Canine Genome 2.0 Arrays and were performed as described previously (16). A complete microarray expression dataset has been submitted to the National Center for Biotechnology Information's Gene Expression Omnibus database (#GSE49518). Analysis of microarray data was performed with the assistance of the Bioinformatics Group of the Molecular Profiling Facility at the University of Pennsylvania (Philadelphia, PA) as follows: the Affymetrix MAS5 algorithm, as implemented in Expression Console Software (v1.1; Affymetrix), was applied with default parameters to yield absent (A), marginal (M), or present (P) flags for each probeset in each sample. Affymetrix .CEL files, containing probe intensities, were also imported into GeneSpring (version 7.3; Agilent Technologies, Santa Clara, CA), where GC-Robust Multi-array Average (GCRMA) was applied to calculate log2-transformed signal intensities. To assess global intersample relationships, we filtered the probesets on the array for those present in at least three of 29 samples. The filtered dataset was imported into Partek Genomics Suite (v6.6; Partek, St. Louis, MO), where hierarchical clustering (Fig. 1), Principal Components Analysis (PCA; see Fig. 3), and differential expression analysis were performed. PCA is a standard technique for visualizing variation within a multidimensional dataset. Using orthogonal transformations, a dataset containing possibly correlated data is converted into linearly uncorrelated variables called Principal Components (PC). This transformation is defined in such a way that the first PC (PC1) has the highest variance possible (accounting for most of the variability), while each successive PC (PC2-3) contains the next highest possible variance. A three-way ANOVA with pairwise contrasts was applied, with each contrast yielding a P value and a fold change. All P values were corrected for multiple testing [false discovery rate (FDR)], with the Benjamini-Hochberg step-up method as implemented in Partek. We identified differentially expressed genes for six pairwise comparisons: 1) 2D + HGF vs. no HGF control, 2) 2D + HGF + U0126 vs. 2D + HGF, 3) 2D + HGF + PD098059 vs. 2D + HGF, 4) 3D + HGF vs. no HGF control, 5) 3D + HGF + U0126 vs. 3D + HGF, and 6) 3D + HGF + PD098059 vs. 3D + HGF. For each comparison, cutoffs of ± twofold, with a FDR of ≤5%, were used to identify differentially expressed genes.
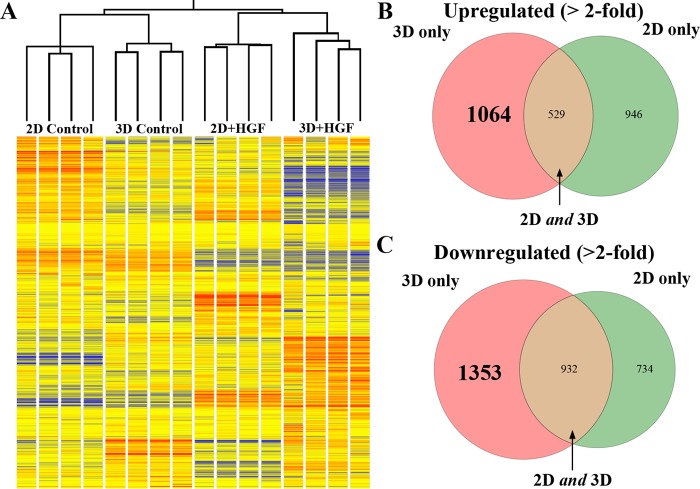
Fig. 1.
Differential gene expression observed between 2- and 3-dimensional (2D and 3D)-cultured Madin-Darby canine kidney (MDCK) cells, as assessed by microarray analysis. A: hierarchical clustering of samples using Pearson correlation with average linkage. Dendrogram and heat map show replicate group similarity and distinguishable and reproducible gene-expression patterns between treatments. Gene-expression levels are relative to the median across all samples (red, above median; blue, below median; yellow, median). B: Venn diagram of genes upregulated in 2D and 3D after hepatocyte growth factor (HGF) treatment. There are 1,064 genes upregulated exclusively in 3D (pink), 529 genes upregulated in 2D and 3D (brown), and 946 genes upregulated only in 2D (green). C: Venn diagram of genes downregulated in 2D and 3D after HGF treatment. There are 1,353 genes downregulated only in 3D (pink), 932 genes downregulated in 2D and 3D (brown), and 734 genes downregulated only in 2D (green).
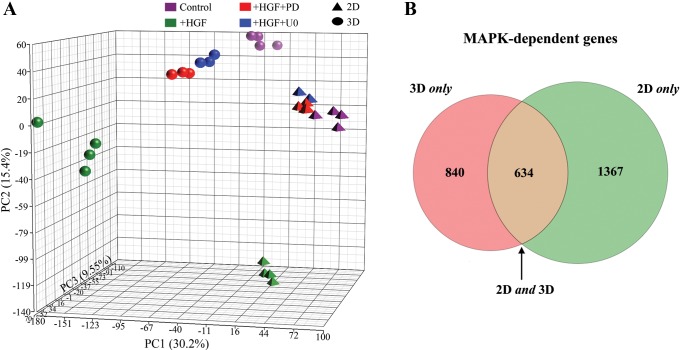
Fig. 3.
Subtraction pathway microarray analysis was used for identification of MAPK-dependent gene expression. A: Principal Components Analysis (PCA) was used to plot the treatment groups. Each axis is a linear combination of all 20,000 transcripts, each with its own coefficient chosen in such a way as to best show the sample variation. Each axis is assigned a percentage, indicating the fraction of total variation captured. The axes are built, such that this fraction is greatest in x > y > z. Note that HGF + inhibitor-treated groups [red, PD098059 (PD); blue, U0126 (U0)] are closer to the control cells (purple) in the PCA plot than the HGF-treated cells (green). B: Venn diagram of 2D and 3D MAPK-dependent gene expression. A total of 2,841 genes was identified as MAPK dependent in the subtraction microarray: 840 (30%) changed only in 3D (pink), 634 (22%) changed in 2D and 3D (brown), and 1,367 (48%) changed only in 2D (green).
Quantitative real-time PCR.
Total RNA was collected from type II MDCK cells, grown in 2D or 3D conditions, exposed to 0 or 24 h of 100 ng/ml HGF, and converted to first-strand cDNA. cDNA and the TaqMan primer/probe system, individualized for each mRNA, were used in conjunction with the 7700 PRISM sequence detection instrument (both Applied Biosystems, Life Technologies, Carlsbad, CA), as described in the Applied Biosystems Technical Manual. When the reaction product amplification exceeded the threshold value, the corresponding cycle number was termed the threshold cycle (CT). Fold change between conditions was calculated through an exponential function of the observed difference in CT, as described previously (25). The values were normalized to a control mRNA—the 18S ribosome—and all real-time PCR studies were performed at least three times in triplicate.
Western blot.
Western blot analysis was performed as described previously (23). For MMP1 analysis, nitrocellulose membranes (Invitrogen, Carlsbad, CA) were probed with goat anti-MMP1 at a concentration of 1:1,000 (AF901; R&D Systems, Minneapolis, MN). Membranes were then probed with horseradish peroxidase-labeled bovine anti-goat antibody at 1:1,000–1:2,000 dilution. Membranes were developed using SuperSignal West Pico or Supersignal West Femto maximum sensitivity substrate kit (both from Pierce, Rockford, IL) and visualized on film. Western blots were quantified by scanning films in an Epson Perfection 3490 scanner at high resolution (at least 300 pixels/inch) and saving them as .TIFF files. Images were subsequently subjected to densitometry analysis in Fiji Is Just ImageJ software. Densitometry analysis of Western blots was performed using the semiautomatic Analyze > Gels function in Fiji/ImageJ (for most blots) or by measuring the signal area (for blot in Fig. 2D). All samples were normalized to GAPDH as loading control.
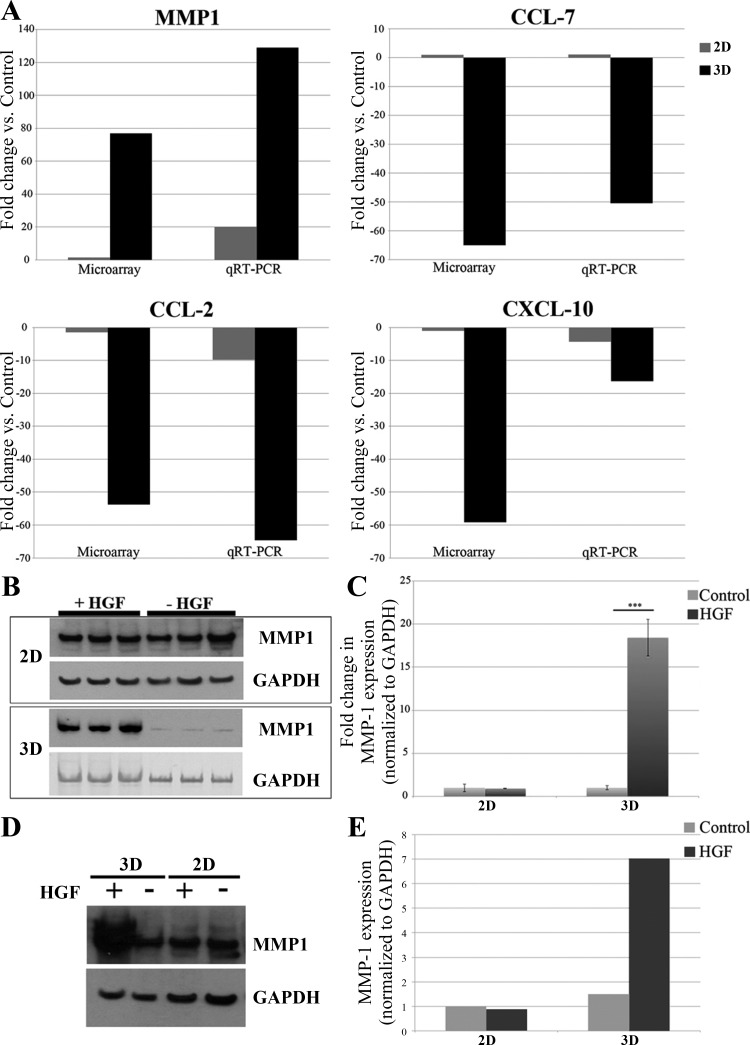
Fig. 2.
Matrix metalloproteinase 1 (MMP1) is upregulated in 3D but not in 2D cells. A: validation of microarray results by quantitative real-time PCR (qRT-PCR): MMP1 fold change in gene expression after HGF treatment in 2D vs. 3D. Both microarray analysis and qRT-PCR indicate that MMP1 gene expression after HGF treatment increases more in 3D (black) than in 2D (gray); C-C motif chemokine ligand (CCL)-7 fold change in gene expression after HGF treatment in 2D vs. 3D. Microarray analysis and qRT-PCR show that CCL-7 does not change in 2D (gray) but is downregulated in 3D (black); CCL-2 fold change in gene expression after HGF treatment in 2D vs. 3D. Both microarray analysis and qRT-PCR indicate that CCL-2 gene expression after HGF treatment is downregulated more in 3D (black) than in 2D (gray); C-X-C motif chemokine ligand (CXCL)-10 fold change in gene expression after HGF treatment in 2D vs. 3D. Both microarray analysis and qRT-PCR indicate that CXCL-10 gene expression after HGF treatment is downregulated more in 3D (black) than in 2D (gray). B: Western blot analysis of MMP1 expression in 2D and 3D MDCK cells ± HGF. MMP1 protein expression (top) significantly increases in 3D + HGF but not in 2D + HGF. GAPDH (bottom) is shown as a loading control. Please note that 2D and 3D Western blots were run on separate gels and should not be compared directly with each other. C: quantification of MMP1 protein expression in 2D and 3D MDCK cells ± HGF. All blots were normalized to GAPDH expression. ***P < 0.005 (Student's t-test). D: from the same Western blot. MDCK cells express MMP1 at slightly higher levels when cultured in 3D than when cultured in 2D, even before the addition of HGF. To detect MMP1 in lysates from 2D-cultured cells, protein loading was greater than that needed to detect MMP1 in lysates from 3D-cultured cells. E: Western blot quantification of MMP1 expression in 2D and 3D MDCK cells ± HGF. The degree of MMP1 expression in each condition was determined by calculating the area of the band, as the density of the bands was relatively similar in this exposure.
Creation of MDCK shRNA cell lines.
The pGFP-V-RS shRNA vector from Origene (Rockville, MD) was used to generate all shRNA-containing vectors in this paper. The sequence selected for MMP1 shRNA KD was 5′-AACAGTGACTTCAGAGACACAGGAGCAAG-3′, which is located in exon 1 of the predicted sequence for canine MMP1 (XM_546546.3). Both MMP1 shRNA and scrambled shRNA vectors were purchased from Origene. Type II MDCK cells were transfected with shRNA plasmids, containing green fluorescent protein (GFP), using Lipofectamine LTX (Invitrogen), followed by fluorescence-activated cell sorting (FACS) at the University of Pennsylvania Flow Cytometry Core Facility and plating of GFP-positive cells. Stable clones were isolated following growth in media containing 5 μg/ml puromycin. MMP1 KD was confirmed by quantitative real-time PCR (qRT-PCR) and by Western blotting from whole-cell MDCK lysate.
Immunofluorescence and confocal microscopy.
MDCK cells were stained using antibodies against E-cadherin (1:100; a kind gift from Dr. W. James Nelson, Stanford University, Stanford, CA) (26); GP-135 (1:100; a generous gift from Dr. George Ojakian, State University of New York, Brooklyn, NY) (34); and cleaved caspase-3 (1:500; Cell Signaling Technology, Danvers, MA). AF555-Phalloidin (Invitrogen) was used at 1:1,000 to visualize actin. Cells were imaged using a Leica TSC SP8 confocal microscope at the University of Pennsylvania Cell and Developmental Biology Microscopy Core. Gray-scale confocal micrograph images were processed (false colored and scale bars added) in Fiji/ImageJ software, and composite figures with multiple panels were constructed using Adobe Photoshop CS5 (San Jose, CA).
RESULTS
Gene expression in response to HGF is differentially regulated in 2D- and 3D-cultured MDCK cells.
We used the GeneChip Canine Genome 2.0 Array to determine the expression profiles of control and HGF-treated MDCK cells grown in 2D and 3D-culture conditions. Consistent with the hypothesis that MDCK cells respond differently to their environment when grown in 2D and 3D cultures, the gene-expression profiles segregate in distinct patterns on the dendrogram between treatment groups but are highly consistent within each biological group (Fig. 1A). To examine further the relationship between differentially regulated genes in 2D vs. 3D MDCK cells, we ranked all genes by fold change in control vs. HGF-treated cells within each group (i.e., 2D or 3D). We used twofold change with a 5% false discovery rate (FDR) as the threshold in gene-expression change when comparing any conditions with the control group. We found that 2,539 genes are upregulated after HGF treatment; out of these, 1,064 (42%) are upregulated in 3D + HGF vs. 3D control but not in 2D + HGF vs. 2D control, 529 (21%) are upregulated in both 3D + HGF vs. 3D control and 2D + HGF vs. 2D control, whereas 946 (37%) are upregulated only in 2D (Fig. 1B). Additionally, we found that 3,019 genes are downregulated after HGF treatment; out of these, 1,353 (45%) are downregulated in 3D + HGF vs. 3D control but not in 2D + HGF vs. 2D control, 932 (31%) are downregulated in 3D + HGF vs. 3D control and 2D + HGF vs. 2D control, whereas 734 (24%) are downregulated only in 2D + HGF vs. 2D control (Fig. 1C). These results, consistent with our hypothesis that gene expression is differentially regulated in 2D vs. 3D conditions, show that gene-expression changes, in response to HGF, are quite different between 2D- and 3D-grown MDCK cells.
Gene-expression trends from microarray analysis are validated by real-time PCR.
The top 50 genes down- and upregulated in 3D + HGF but not in 2D + HGF, identified in the microarray analysis, are listed in Tables 1 and 2, respectively. The “MAPK 3D” columns indicate whether each listed gene was found to be dependent on MAPK in 3D-cultured cells (see “subtraction microarray” description in Fig. 3 legend). Previously, we validated DNA microarray results extensively, using the GeneChip Canine Genome 2.0 Array (16) and other arrays (1). The following genes were chosen for validation of our current microarray results by qRT-PCR (Fig. 2A): MMP1, C-C motif chemokine ligand (CCL)-7, CCL-2, and C-X-C motif chemokine ligand 10 (CXCL-10). Consistent with the trends observed in the microarray data, MMP1 expression increases much more robustly in 3D + HGF than in 2D + HGF (Fig. 2A). It should also be noted that in 2D culture, the transcript levels of MMP1 were extremely low; e.g., the MMP1 qRT-PCR reactions required 100–1,000 times more starting mRNA/cDNA than any other reaction group to be able to detect the MMP1 transcript in 2D-cultured cells. A very low baseline level of MMP1 mRNA may, in part, account for the fact that there was a detectable increase in MMP1 transcript levels in 2D + HGF vs. 2D control. Most importantly, however, results for MMP1 mRNA expression are completely consistent with the microarray data (Fig. 2A). Additionally, all three genes downregulated in 3D + HGF but not 2D + HGF—chosen for validation of the microarray by qRT-PCR (Fig. 2A), namely, CCL-7, CCL-2, and CXCL-10—are more highly downregulated in 3D + HGF than in 2D + HGF. For example, CCL-7 mRNA expression changes upon HGF treatment were as follows: 1.9-fold increase in 2D + HGF and 50-fold decrease in 3D + HGF (vs. microarray data: 1.02-fold increase in 2D + HGF and 65-fold decrease in 3D + HGF; Fig. 2A).
Table 1.
Top 50 genes downregulated in 3D + HGF but not in 2D + HGF
| Gene Symbol | Affymetrix Probeset ID | Fold Change in 2D + HGF | Fold Change in 3D + HGF | MAPK 3D | |
|---|---|---|---|---|---|
| 1 | IL8 | Cfa.3510.1.S2_at | −1.01077 | −235.476 | Yes |
| 2 | CCL7 | Cfa.16337.1.S1_s_at | 1.0226 | −65.0118 | Yes |
| 3 | CXCL10 | Cfa.16590.1.S2_at | −1.03483 | −59.1649 | No |
| 4 | CCL2 | Cfa.3851.1.S1_s_at | −1.41702 | −53.8085 | Yes |
| 5 | KLHL24 | Cfa.1578.1.S1_at | −1.24551 | −48.1247 | Yes |
| 6 | Cfa.382.1.S1_at | −1.62398 | −43.6817 | Yes | |
| 7 | ACTA2 | CfaAffx.24064.1.S1_at | 1.00428 | −43.5689 | Yes |
| 8 | Cfa.12151.1.A1_at | −1.66839 | −36.735 | Yes | |
| 9 | IDO1 | CfaAffx.9573.1.S1_at | −1.06122 | −34.3352 | Yes |
| 10 | FILIP1L | Cfa.12291.1.A1_at | −1.11281 | −33.5399 | Yes |
| 11 | DAO | CfaAffx.17496.1.S1_s_at | −1.15505 | −31.333 | Yes |
| 12 | Cfa.14008.1.A1_at | −1.45647 | −25.9029 | Yes | |
| 13 | AGPHD1 | Cfa.2465.1.A1_at | −1.92466 | −24.6451 | Yes |
| 14 | TAGLN | CfaAffx.20384.1.S1_at | 1.07404 | −21.6368 | Yes |
| 15 | TMEM234 | CfaAffx.16726.1.S1_at | −1.90083 | −19.818 | Yes |
| 16 | LRRC19 | CfaAffx.3462.1.S1_at | −1.72585 | −19.0976 | Yes |
| 17 | HBP1 | CfaAffx.6846.1.S1_at | −1.66142 | −18.8617 | Yes |
| 18 | PARP9 | CfaAffx.18544.1.S1_s_at | −1.67553 | −18.6741 | Yes |
| 19 | STAP2 | CfaAffx.29150.1.S1_at | 1.08093 | −17.7161 | Yes |
| 20 | TTC30A/TTC30B | CfaAffx.20940.1.S1_at | −1.94454 | −17.5377 | Yes |
| 21 | GBP1 | Cfa.18819.1.S1_at | −1.13765 | −16.6679 | Yes |
| 22 | ENOSF1 | Cfa.18734.1.S1_at | −1.09466 | −15.7093 | Yes |
| 23 | HLA-DMA | Cfa.18297.1.S1_at | −1.55155 | −14.9005 | Yes |
| 24 | HLA-DMA | CfaAffx.2197.1.S1_s_at | −1.67813 | −14.2987 | Yes |
| 25 | LAMB2 | Cfa.20623.1.S1_at | 1.18279 | −13.8203 | Yes |
| 26 | TMEM37 | CfaAffx.8275.1.S1_at | −1.91704 | −13.5176 | Yes |
| 27 | VLDLR | Cfa.9396.1.A1_s_at | −1.51316 | −13.5103 | Yes |
| 28 | ACTA1 | Cfa.307.3.S1_at | −1.00364 | −13.3361 | Yes |
| 29 | PCMTD2 | Cfa.21242.1.S1_at | −1.483 | −12.974 | Yes |
| 30 | CCNG2 | CfaAffx.13729.1.S1_at | 1.09686 | −12.3896 | Yes |
| 31 | ANKRD1 | Cfa.16041.1.S1_at | 1.70275 | −12.3206 | No |
| 32 | Cfa.14049.1.A1_at | −1.69276 | −11.8271 | Yes | |
| 33 | IDO1 | CfaAffx.9573.1.S1_s_at | 1.04883 | −11.8065 | Yes |
| 34 | CCNG2 | CfaAffx.13734.1.S1_s_at | 1.14545 | −11.7528 | Yes |
| 35 | DAO | Cfa.15688.1.A1_at | −1.06773 | −11.752 | Yes |
| 36 | HBP1 | Cfa.16872.1.S1_s_at | −1.82799 | −11.7115 | Yes |
| 37 | Cfa.4114.1.S1_at | −1.87317 | −11.2273 | Yes | |
| 38 | ACTA1/ACTA2 | CfaAffx.18955.1.S1_s_at | −1.05663 | −11.212 | Yes |
| 39 | DTX3L | CfaAffx.18558.1.S1_at | −1.87712 | −11.2001 | Yes |
| 40 | KLHL24 | CfaAffx.18456.1.S1_s_at | −1.50436 | −11.1903 | Yes |
| 41 | LOC100856683 | CfaAffx.22409.1.S1_at | −1.06832 | −10.9963 | Yes |
| 42 | Cfa.9097.1.A1_at | 1.53325 | −10.9001 | Yes | |
| 43 | Cfa.2835.1.A1_at | −1.79756 | −10.869 | Yes | |
| 44 | Cfa.9524.1.A1_at | −1.00196 | −10.3906 | No | |
| 45 | FYCO1 | Cfa.10325.1.A1_at | −1.79487 | −10.3473 | Yes |
| 46 | EDN1 | Cfa.125.1.S1_s_at | −1.38134 | −10.1535 | Yes |
| 47 | TAGLN | Cfa.12238.1.A1_a_at | 1.05448 | −9.65693 | Yes |
| 48 | SRPX | Cfa.12650.1.S1_at | −1.00325 | −9.6031 | Yes |
| 49 | TAGLN | CfaAffx.20384.1.S1_s_at | 1.05297 | −9.55568 | Yes |
| 50 | PDCD4 | CfaAffx.16895.1.S1_s_at | −1.81865 | −9.54413 | Yes |
3D, three-dimensional; HGF, hepatocyte growth factor; 2D, two-dimensional.
Table 2.
Top 50 genes upregulated in 3D + HGF but not in 2D + HGF
| Gene Symbol | Affymetrix Probeset ID | Fold Change in 2D + HGF | Fold Change in 3D + HGF | MAPK 3D | |
|---|---|---|---|---|---|
| 1 | MMP1 | CfaAffx.23166.1.S1_s_at | 1.7815 | 74.6253 | Yes |
| 2 | MAP3K5 | Cfa.14238.1.A1_s_at | −1.3043 | 24.5208 | Yes |
| 3 | TXNRD1 | Cfa.8894.1.A1_s_at | −1.20665 | 19.466 | No |
| 4 | WDR37 | Cfa.7195.1.A1_at | 1.69907 | 18.4215 | Yes |
| 5 | LRRC59 | CfaAffx.26063.1.S1_at | 1.70389 | 16.8271 | Yes |
| 6 | GPC3 | Cfa.2467.1.S1_at | −1.27932 | 13.7171 | Yes |
| 7 | DKC1 | Cfa.15181.1.A1_s_at | −1.09159 | 12.1819 | No |
| 8 | FANCM | CfaAffx.21714.1.S1_s_at | 1.91544 | 11.6979 | Yes |
| 9 | DKC1 | Cfa.19618.1.S1_s_at | 1.12708 | 10.488 | No |
| 10 | CCDC99 | Cfa.18573.1.S1_at | 1.3448 | 10.4055 | Yes |
| 11 | FARSA | CfaAffx.26158.1.S1_at | −1.19907 | 10.3604 | No |
| 12 | DKC1 | Cfa.15181.1.A1_at | −1.00987 | 9.78106 | No |
| 13 | SACS | Cfa.9009.1.A1_s_at | 1.92716 | 8.952 | Yes |
| 14 | SLC25A32 | CfaAffx.1910.1.S1_s_at | 1.14517 | 8.36223 | Yes |
| 15 | Cfa.10817.1.A1_at | 1.64832 | 8.14561 | Yes | |
| 16 | LOC100856757 | CfaAffx.12913.1.S1_at | 1.17254 | 7.92665 | Yes |
| 17 | STK4 | Cfa.11970.1.A1_s_at | 1.45166 | 7.68948 | Yes |
| 18 | PDK4 | Cfa.2282.1.S1_at | 1.44404 | 7.5243 | Yes |
| 19 | LOC100856610 | CfaAffx.7874.1.S1_at | 1.04913 | 7.36905 | Yes |
| 20 | TOMM40 | CfaAffx.7881.1.S1_at | 1.09175 | 7.22431 | Yes |
| 21 | MPP6 | Cfa.19439.1.S1_s_at | −1.06586 | 7.00514 | No |
| 22 | TPMT | Cfa.13501.1.S1_at | −1.26947 | 6.75627 | Yes |
| 23 | Cfa.5147.1.A1_at | −1.00366 | 6.7445 | No | |
| 24 | DDX27 | Cfa.16395.1.S1_s_at | −1.2651 | 6.72183 | Yes |
| 25 | GNL3 | Cfa.728.1.S1_at | −1.06477 | 6.69788 | Yes |
| 26 | ATAD3B | CfaAffx.29389.1.S1_s_at | 1.07357 | 6.67137 | Yes |
| 27 | PTER | CfaAffx.7837.1.S1_at | 1.14624 | 6.43559 | Yes |
| 28 | Cfa.11219.1.A1_at | 1.80816 | 6.28498 | Yes | |
| 29 | ECE2 | CfaAffx.19432.1.S1_at | −1.22996 | 6.12386 | No |
| 30 | SEPT7 | Cfa.19142.1.S1_s_at | −1.66533 | 6.05097 | No |
| 31 | DOHH | Cfa.16291.1.S1_at | 1.57353 | 6.00274 | Yes |
| 32 | ZMYND19 | CfaAffx.29651.1.S1_at | −1.32081 | 5.99707 | Yes |
| 33 | FAM114A2 | Cfa.20692.1.S1_s_at | −1.351 | 5.94761 | Yes |
| 34 | Cfa.14137.1.A1_at | 1.24951 | 5.91518 | Yes | |
| 35 | RBM28 | CfaAffx.3410.1.S1_at | 1.18868 | 5.74236 | No |
| 36 | Cfa.5850.1.A1_at | 1.80063 | 5.65197 | Yes | |
| 37 | Cfa.532.1.S1_at | 1.13559 | 5.65041 | Yes | |
| 38 | SYNCRIP | Cfa.21357.1.S1_s_at | −1.10952 | 5.64882 | No |
| 39 | VASH2 | Cfa.10389.1.S1_at | −1.0515 | 5.64609 | Yes |
| 40 | DNTTIP2 | Cfa.20735.1.S1_s_at | −1.78273 | 5.62785 | No |
| 41 | CS | Cfa.17251.1.S1_s_at | −1.60963 | 5.59711 | Yes |
| 42 | Cfa.2317.1.A1_at | 1.91658 | 5.50254 | Yes | |
| 43 | BLM | Cfa.10357.1.A1_at | 1.84429 | 5.4452 | No |
| 44 | C27H12orf5 | Cfa.18758.1.S1_s_at | −1.36122 | 5.4441 | No |
| 45 | MPP6 | CfaAffx.5143.1.S1_s_at | 1.1381 | 5.29845 | No |
| 46 | DDX18 | CfaAffx.8348.1.S1_s_at | −1.29815 | 5.28791 | No |
| 47 | PAM16 | Cfa.6309.1.A1_x_at | −1.00851 | 5.24061 | No |
| 48 | SDAD1 | Cfa.9228.1.A1_at | −1.05421 | 5.2358 | Yes |
| 49 | ITGB1 | CfaAffx.6707.1.S1_s_at | 1.21637 | 5.17063 | Yes |
| 50 | IMMT | Cfa.3180.2.S1_s_at | −1.96215 | 5.13259 | No |
Protein expression of a novel candidate, MAPK-dependent tubulogene MMP1, is consistent with microarray results.
MMP1 was one of the most highly upregulated, MAPK-dependent genes identified in this array. Consistent with the results from microarray analysis, MMP1 protein expression is very highly upregulated in 3D-cultured MDCK cells treated with HGF (18-fold increase vs. control) but not in 2D-cultured MDCK cells treated with HGF (0.9-fold vs. control; Fig. 2B). The increase in MMP1 protein expression in 3D + HGF is highly statistically significant (P < 0.005), as quantified in Fig. 2C. When run on the same gel, the levels of MMP1 are higher in 3D-, particularly 3D + HGF, than in 2D-cultured cells (Fig. 2D; quantified in Fig. 2E).
Identification of MAPK-dependent gene expression using subtraction microarray analysis.
To determine changes in response to MAPK signaling in HGF-induced gene expression, 2D and 3D MDCK cells treated with HGF were also treated with U0126 and PD098059, which are widely used MAPK inhibitors (both inhibit phosphorylation of MEK, which is directly upstream of ERK, the final protein in the MAPK phosphorylation cascade). We have demonstrated previously that this approach allows for the identification of MAPK-dependent gene expression in 2D filter-grown MDCK cells (16). In “subtraction pathway microarray analysis,” only genes regulated by the MAPK pathway are expected to change back toward baseline levels when comparing cells treated with HGF with cells in the HGF-plus-inhibitor group. Consistent with our hypothesis that the response to HGF is different in 2D- vs. 3D-cultured cells, HGF-treated 2D- and 3D-grown MDCK cells responded differently to the addition of MAPK inhibitors. First, we used Principal Components Analysis (PCA) to illustrate global gene-expression changes among the conditions (Fig. 3A). This plot shows that 2D and 3D cells treated with HGF and either inhibitor were much more similar to control than to HGF-treated cells. Taken together, these results indicate that we can define HGF-induced, MAPK-dependent gene expression.
With the comparison of the genes up/downregulated in 2D + HGF and 3D + HGF and subtracting genes that do not return toward baseline upon the addition of both MAPK inhibitors, we were able to identify a total of 2,841 MAPK-dependent genes. With the requirement of a return to baseline, using two independent MEK inhibitors, we increased our specificity. Out of the 2,841 MAPK-dependent genes, 2,001 change in 2D-, and 1,474 change in 3D-grown MDCK cells. Overall, we identified 1,367 MAPK-dependent genes that change in 2D only (48%), 634 (22%) that change in both 2D and 3D culture, and 840 (30%) that change in 3D only (Fig. 3B).
MMP1 KD inhibits cystogenesis and tubulogenesis in 3D-grown MDCK cells.
We reported previously that MMPs play a role in HGF-induced tubulogenesis in MDCK cells; however, we did not identify MMP1 in a microarray analysis of 2D + HGF MDCK cells (16). The present microarray and Western blot data (Figs. 1 and 2) show that MMP1 expression is very highly upregulated in 3D- but not 2D-cultured MDCK cells, following addition of HGF, suggesting that MMP1 is a novel candidate tubulogene. Additionally, our subtraction microarray analysis showed that MMP1 is dependent on MAPK signaling. To assess whether MMP1 is indeed a tubulogene (and therefore, required and/or sufficient for tubulogenesis), we examined whether KD of MMP1 affected HGF-induced tubulogenesis of 3D-grown MDCK.
To knock down MMP1 protein levels, we used shRNA targeting canine MMP1. Briefly, MDCK cells were transfected with shRNA plasmids coexpressing GFP, using Lipofectamine LTX, followed by FACS and plating of GFP-positive cells. Stable clones were isolated following growth in media containing 5 μg/ml puromycin. Multiple clones were then screened with qRT-PCR, and clone 2 was found to have a 92% reduction in MMP1 mRNA levels (Fig. 4A). To confirm MMP1 KD, we probed cell lysates from several MMP1-KD clones that had decreases in MMP1 mRNA levels with antibody against MMP1, normalized with antibody against GAPDH. Two clones had an ∼30% reduction in MMP1 protein expression in 3D culture (no HGF), whereas clone 2 had a 75% reduction in MMP1 protein expression (Fig. 4B). Clone 2 (MMP1 KD) was used in subsequent studies to assess the effects of MMP1 KD on cystogenesis and tubulogenesis in 3D-culture conditions. Similar, although milder, phenotypes were seen in the clones with less-robust MMP1 KD (data not shown).
Fig. 4.
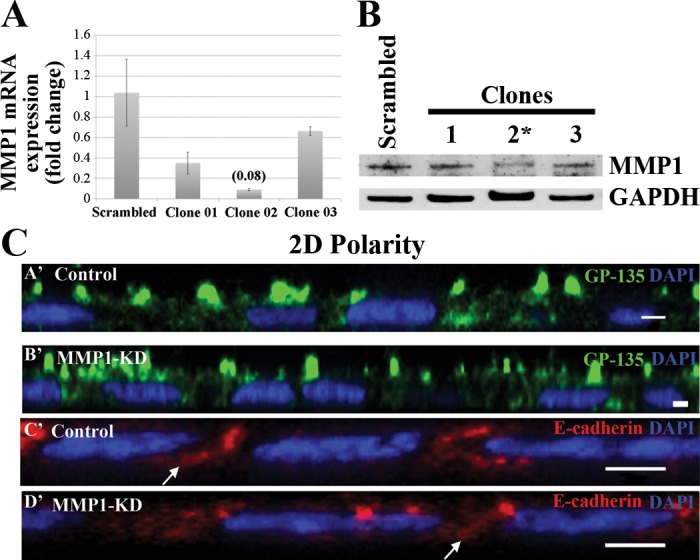
Normal apicobasal polarity is seen in 2D-cultured, MMP1-knockdown (KD) cells. MDCK cells were transfected using a MMP1-short hairpin RNA (shRNA) plasmid that coexpresses green fluorescent protein (GFP). Transfected cells were collected via flow cytometry, and clones were generated using antibiotic selection. A: MMP1 mRNA expression was assessed in 8 clones using qRT-PCR. MMP1-KD clone 2 had a 92% decrease in MMP1-mRNA expression levels compared with controls. B: the levels of MMP1 protein expression were assessed in several MMP1-KD clones that also had decreased MMP1 mRNA expression levels (although less than clone 2) via Western blot against MMP1 (normalized to GAPDH) in 3D-cultured MMP1-KD cells. Two MMP1-KD clones had an ∼30% reduction in MMP1 protein expression, with clone 2 having a 75% reduction in MMP1 expression (*, the clone that was used for the subsequent Figs. 5–7 and shown in images B′ and D′ in C). C: 2D-cultured MDCK cells were stained with the apical marker GP-135 (green; A′ and B′) or with the basolateral marker E-cadherin (red; C′ and D′). 4′,6-Diamidine-2′-phenylindole dihydrochloride (DAPI; blue) was used to label nuclei. The x, z section of confocal micrographs of control (A′ and C′) and MMP1-KD (B′ and D′) cells shows no difference in apicobasal polarity between both cell lines. In all cells, GP-135 is on the apical surface of the cell (A′ and B′), whereas E-cadherin is localized along the basolateral membrane (arrows; C′ and D′). Scale bars, 2 μm.
KD of MMP1 does not disrupt apicobasal polarity when the cells are cultured in 2D.
Both GP-135 and E-cadherin retain their normal localization patterns (apical and basolateral, respectively) when the MMP1-KD cells are cultured in 2D (Fig. 4C).
Depletion of MMP1 leads to disruptions in cyst formation and cell polarization, as well as increased apoptosis in 3D-cultured MDCK cells.
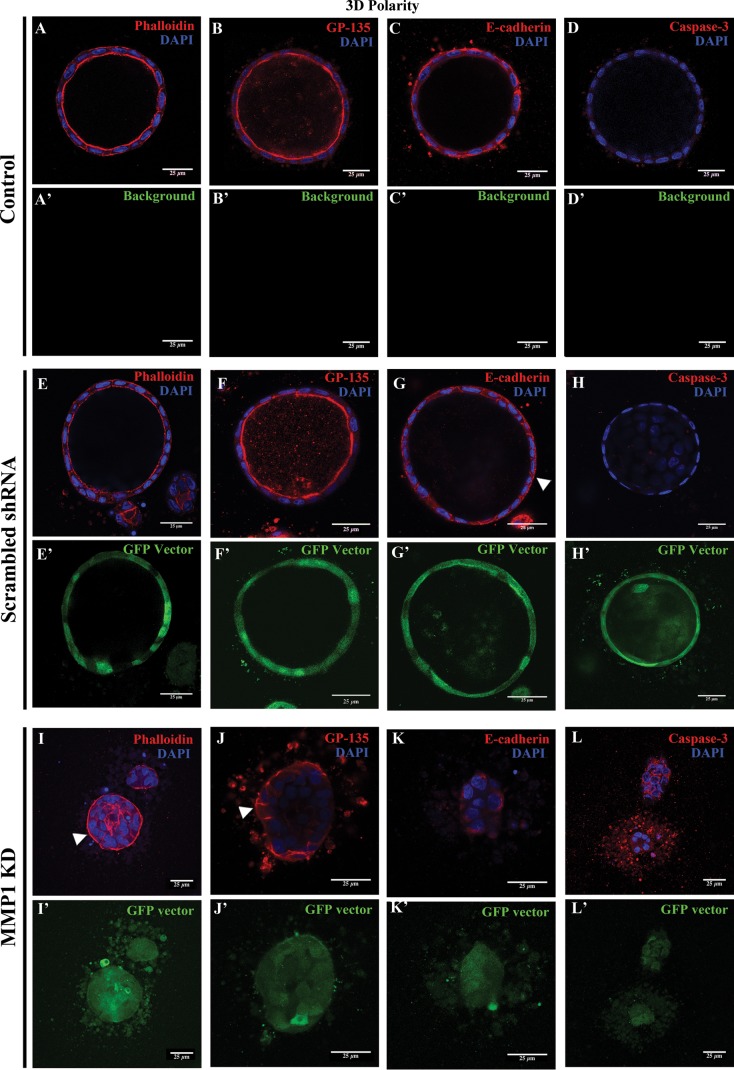
In contrast to control MDCK cells, which form hollow spheres when cultured in 3D, MMP1-KD MDCK cells fail to form cysts; instead, they form solid clusters without a lumen (Fig. 5). Interestingly, MMP1-KD cells had inverted apicobasal polarity. We confirmed this using cortical actin, GP-135, and E-cadherin as polarity markers. All three were disrupted in MMP1-KD cells. Actin and GP-135, which normally localize to the inner apical/lumenal surface in MDCK cells (Fig. 5, A and E, in control; Fig. 5, B and F, in scrambled shRNA cells), localized along the “outer” basal membrane and lateral junctions in the MMP1-KD cell clusters (Fig. 5, I and J, respectively). In control and scrambled shRNA cells, E-cadherin is normally distributed along the basolateral membrane of cells (Fig. 5, C and G), whereas in MMP1-KD cells, E-cadherin was mostly intracellular (Fig. 5K). The fact that apicobasal polarity is normal in 2D-cultured MMP1-KD cells but is reversed in 3D-cultured MMP1-KD cells suggests that MMP1 plays a specific role in 3D cystogenesis.
Fig. 5.
MMP1-KD cells have reversed apicobasal polarity and are unable to form cysts. A–L: control MDCK, MDCK with scrambled shRNA, and MDCK with MMP1 shRNA (KD) cells were grown in type I collagen for 10 days. Whereas both control (A–D) and scrambled shRNA (E–H) cells are able to form hollow cysts, KD of MMP1 disrupts cyst formation in 3D-grown MDCK cells (I–L). Note that the polarity of cell clusters is reversed in MMP1-KD (I–L) cells compared with control (A–D) or scrambled (E–H) MDCK cells. For example, in MMP1-KD cells, cortical actin (I) and GP-135 (J) are localized on the basolateral side of the cell clusters (arrowheads in I and J) compared with control and scrambled shRNA cysts, where cortical actin (A and E) and GP-135 (B and F) are localized to the inner (apical) surface of the cysts. E-Cadherin is localized to the basolateral membrane in control and scrambled shRNA cells (C and G, respectively), whereas it fails to localize clearly to the cell membrane in MMP1-KD cells (K). Additionally, caspase-3 is increased in MMP1-KD cells (L) compared with control and scrambled shRNA cells (D and H, respectively). Scale bars = 25 μm.
In addition to being unable to form lumen-filled cysts and having inverted cell polarity, we observed increased apoptosis in MMP1-KD MDCK cells, as visualized by increased staining for cleaved (active) caspase-3 (Fig. 5L) compared with control and scrambled shRNA cells (Figs. 5, D and H).
Depletion of MMP1 leads to disruptions in tubule formation in 3D-cultured MDCK cells.
Although MMP1-KD cells, somewhat surprisingly, were able to extend cell chains outside of the initial cell clusters after 24 h (Fig. 6), they were unable to form lumen-filled tubules after 72 h of HGF treatment (Fig. 7).
Fig. 6.
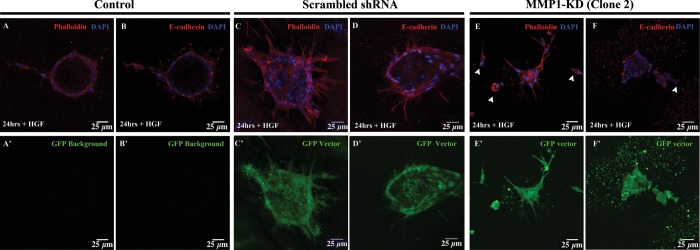
MMP1-KD cells migrate out of cell clusters. After 24 h of HGF treatment, control MDCK cell cysts have chains emerging from the cysts (A and B). MDCK cells with scrambled shRNA also show chains emerging from the cysts (C and D). At this time, MMP1-KD cells are able to migrate out of the cell clusters; however, not all cells are found in chains. Instead, many cells seem to migrate out of the clusters (E and F, arrowheads). Scale bars = 25 μm.
Fig. 7.
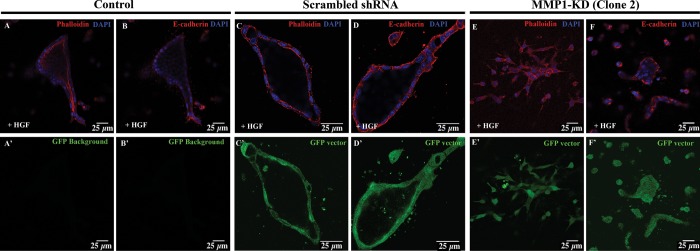
MMP1 KD disrupts tubule formation in 3D MDCK cells. 3D-cultured control (A and B), scrambled shRNA (C and D), and MMP1-KD (E and F) MDCK cells stained for phalloidin (red; A, C, and E) and E-cadherin (red; B, D, and F) are shown at 72 h post-treatment with HGF. At this time, control and scrambled shRNA cells are able to form polarized tubules with lumens (A–D). Control cells do not express GFP, whereas scrambled shRNA and MMP1-KD cells can be readily visualized using GFP coexpression as a marker (C′–F′). MMP1-KD cells fail to form polarized, lumen-filled tubules at 72 h after HGF treatment (E and F). Note that the distribution of actin is concentrated along the cell junctions in the MMP1-KD cells (E), in contrast with the enriched cortical actin expression, visible in the control cells (A). Scale bars = 25 μm.
DISCUSSION
This study demonstrates the importance of using 3D-grown cells to investigate complex morphological processes, such as cystogenesis and tubulogenesis. By comparing gene-expression profiles from 2D- and 3D-grown MDCK cells, treated with HGF, we were able to determine that changes in gene expression in response to HGF are very different in 2D- vs. 3D-cultured cells (Fig. 1A). We were able to identify 2,539 genes that are upregulated in response to HGF treatment. 42% (1,064) of those genes are upregulated only in 3D. (Fig. 1B) and would not be and were not identified in microarray studies, including ours (16), using only 2D filter-grown cells. The same trend was observed for genes downregulated by HGF treatment; out of a total 3,019 genes downregulated upon HGF treatment, 45% (1,353) were downregulated exclusively in 3D-culture conditions. It should be noted that scatter factor and HGFs were originally identified separately and were named for the different physiological effects. It was only later that these two factors were shown to be one and the same. Consistent with our results, when HGF acts on its c-met tyrosine kinase receptor, genes involved in mitogenesis, motogenesis, and morphogenesis are activated (2).
Interestingly, many of the genes most highly downregulated in 3D + HGF but not in 2D + HGF, are chemokines (e.g., IL-8, CCL-7, CCL-2, CXCL-10). Given that macrophage infiltration (often mediated by chemokines) has been implicated recently in abnormal cyst formation in ADPKD (17) and that an increase in levels of CCL-2 has been correlated with acute kidney injury (31), our results point to biologically significant targets, previously not identified using 2D culture. Supporting this idea is the fact that the gene most highly downregulated, exclusively in 3D, in response to HGF, was IL-8 (#1 in Table 1), which was recently shown to participate in the formation of cysts by MDCK cells in 3D culture, with HGF stimulating tubulogenesis through the suppression of IL-8 (45).
Additionally, we identified glypican-3 (GPC-3) among the genes that are most highly upregulated in 3D + HGF but not in 2D + HGF (#6 in Table 2). GPC-3 is a glycoprotein, previously identified in the regulation of branching morphogenesis (13). GPC-3 knockout mice have kidney cysts and severe defects in a number of other tubule-based organs, such as the lungs (8). Together, these results suggest that the present approach allows for the identification of genes highly relevant for cystogenesis and tubulogenesis in vivo.
Our previous work, using a MAPK subtraction pathway microarray analysis of 2D-cultured MDCK cells, allowed us to identify 2,105 HGF-responsive, MAPK-dependent genes (16). Consistent with that previous work, in this study, we identified a total of 2,841 MAPK-dependent genes (Fig. 3), out of which 840 (30% of total) are novel MAPK-dependent genes that respond only in 3D culture (Fig. 3B).
We previously looked at inhibitors of MMP activity during cystogenesis and tubulogenesis in 3D culture of MDCK cells (32) and had shown that tissue inhibitor of metalloproteinase 1 (TIMP1), TIMP2, and TIMP3 were all necessary to prevent the redifferentiation stage of tubulogenesis (in which actin extensions and chains of cells form tubules). The fact that all three TIMPs were necessary to prevent tubulogenesis (broad-spectrum inhibitors of MMPs, such as BB-94, GM6001, and AG3340, also inhibited tubulogenesis) suggested that there were more MMPs involved than just MMP13, which we had identified in that study (as well as in our previous 2D microarray studies) (1, 16) as being necessary for tubulogenesis. This was confirmed in our identification of MMP1 as a tubulogene in this paper. Interestingly, MMP1 was the MAPK-dependent gene most highly upregulated in 3D + HGF culture (Table 2). Consistent with our hypothesis about the importance of 3D-culture systems, we did not identify MMP1 as a tubulogene in our previous 2D subtraction pathway microarray analysis (16) nor did we here in the 2D analysis (Fig. 2).
We, therefore, decided to study further the role of MMP1 as a novel tubulogene. To do this, we generated stable shRNA-mediated MMP1-KD MDCK cells (Fig. 4) to confirm that MMP1 is required for tubulogenesis. We found that MMP1-KD cells are unable to form cysts when grown in collagen, and whereas the polarity of 2D-cultured cells is normal, the polarity of 3D-cultured cells is inverted (Figs. 4C and 5, respectively). Additionally, although MMP1-KD cells were able to migrate out of the cell clusters and form single-cell chains upon treatment with HGF (Fig. 6), we found no evidence of lumen formation in the elongated cell chains and tubules (Figs. 6 and 7). MMP1-KD cells also had increased levels of apoptosis (this was noticed during cell passaging and confirmed via a cleaved caspase-3 stain; Fig. 5). These data, together, suggest that the MMP1-KD cells have defects in lumen formation due to disruptions in cell polarization in 3D culture. We hypothesize that this leads to apoptosis, as the cells are unable to orient and tubulate correctly (tubulogenesis begins on the basolateral surface). MMP1a knockout mice were generated (12); however, there was no developmental phenotype, most likely due to redundancy from MMP1b (no double-knockout MMP1a/1b mice have been generated).
In summary, this study provides strong evidence that use of 3D- instead of 2D-cultured cells is essential for the characterization of signaling pathways that regulate complicated morphogenic programs, such as cystogenesis and tubulogenesis. In the present work, we identified 2,417 3D-specific candidate tubulogenes, out of which 840 are dependent on MAPK signaling, thus furthering the hypothesis that MAPK signaling plays an important (but likely not exclusive) role in the regulation of tubulogenesis. We also show evidence that MMP1 is a previously unidentified, MAPK-dependent tubulogene, required for cystogenesis and tubulogenesis in 3D-cultured MDCK cells.
GRANTS
Support for this work was provided, in part, by grants from the VA Merit Award (I01BX000820 to J. H. Lipschutz), National Institute of Diabetes and Digestive and Kidney Diseases (DK069909 and DK047757 to J. H. Lipschutz and DK093625 to L. Huang), NephHope Foundation (fellowship to M. F. Chacon-Heszele), Satellite Healthcare (Norman S. Coplon Extramural Research Grant to J. H. Lipschutz), and University of Pennsylvania Translational Medicine Institute (Pilot Grant to J. H. Lipschutz).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.F.C-H. and J.H.L. conception and design of research; M.F.C-H., X.Z., N.E.H., and S.M. performed experiments; M.F.C-H., S.Y.C., L.H., J.W.T., K.M.P., and J.H.L. analyzed data; M.F.C-H., S.Y.C., L.H., J.W.T., K.M.P., and J.H.L. interpreted results of experiments; M.F.C-H. and J.H.L. prepared figures; M.F.C-H. and J.H.L. drafted manuscript; J.H.L. edited and revised manuscript; M.F.C-H. and J.H.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The University of Pennsylvania Biomedical Imaging Core Facility of the Cancer Center is acknowledged for providing imaging services.
REFERENCES
- 1.Balkovetz DF, Gerrard ER, Li S, Johnson D, Lee J, Tobias JW, Rogers KK, Snyder RW, Lipschutz JH. Gene expression alterations during HGF-induced dedifferentiation in 2013 of a renal tubular epithelial cell line (MDCK) using a novel canine DNA microarray. Am J Physiol Renal Physiol 286: F702–F710, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Balkovetz DF, Lipschutz JH. Hepatocyte growth factor and the kidney: it is not just for the liver. Int Rev Cytol 186: 225–260, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Balkovetz DF, Pollack AL, Mostov KE. Hepatocyte growth factor alters the polarity of Madin-Darby canine kidney cell monolayers. J Biol Chem 272: 3471–3477, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Balkovetz DF, Sambandam V. Dynamics of E-cadherin and γ-catenin complexes during dedifferentiation of polarized MDCK cells. Kidney Int 56: 910–921, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Bao Q, Hughes RC. Galectin-3 and polarized growth within collagen gels of wild-type and ricin-resistant MDCK renal epithelial cells. Glycobiology 9: 489–495, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Bao Q, Hughes RC. Galectin-3 expression and effects on cyst enlargement and tubulogenesis in kidney epithelial MDCK cells cultured in three-dimensional matrices in vitro. J Cell Sci 108: 2791–2800, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Boccaccio C, ò M, Tamagnone L, Bardelli A, Michieli P, Battistini C, Comoglio PM. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature 391: 285–288, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Cano-Gauci DF, Song HH, Yang H, McKerlie C, Choo B, Shi W, Pullano R, Piscione TD, Grisaru S, Soon S, Sedlackova L, Tanswell AK, Mak TW, Yeger H, Lockwood GA, Rosenblum ND, Filmus J. Glypican-3-deficient mice exhibit developmental overgrowth and some of the abnormalities typical of Simpson-Golabi-Behmel syndrome. J Cell Biol 146: 255–264, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Crepaldi T, Gautreau A, Comoglio PM, Louvard D, Arpin M. Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cells. J Cell Biol 138: 423–434, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Fanjul-Fernández M, Folgueras AR, Fueyo A, Balbín M, Suárez MF, Fernández-García MS, Shapiro SD, Freije JM, López-Otín C. Matrix metalloproteinase MMP-1a is dispensable for normal growth and fertility in mice and promotes lung cancer progression by modulating inflammatory responses. J Biol Chem 288: 14647–14656, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grisaru S, Cano-Gauci D, Tee J, Filmus J, Rosenblum ND. Glypican-3 modulates BMP- and FGF-mediated effects during renal branching morphogenesis. Dev Biol 231: 31–46, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Gumbiner BM. Epithelial morphogenesis. Cell 69: 385–387, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Hellman NE, Greco AJ, Rogers KK, Kanchagar C, Balkovetz DF, Lipschutz JH. Activated extracellular signal-regulated kinases are necessary and sufficient to initiate tubulogenesis in renal tubular MDCK strain I cell cysts. Am J Physiol Renal Physiol 289: F777–F785, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Hellman NE, Spector J, Robinson J, Zuo X, Saunier S, Antignac C, Tobias JW, Lipschutz JH. Matrix metalloproteinase 13 (MMP13) and tissue inhibitor of matrix metalloproteinase 1 (TIMP1), regulated by the MAPK pathway, are both necessary for Madin-Darby canine kidney tubulogenesis. J Biol Chem 283: 4272–4282, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Karihaloo A, Koraishy F, Huen SC, Lee Y, Merrick D, Caplan MJ, Somlo S, Cantley LG. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 22: 1809–1814, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leighton J, Brada Z, Estes LW, Justh G. Secretory activity and oncogenicity of a cell line (MDCK) derived from canine kidney. Science 163: 472–473, 1969 [DOI] [PubMed] [Google Scholar]
- 19.Leighton J, Mark R, Justh G. Patterns of three-dimensional growth in vitro in collagen-coated cellulose sponge: carcinomas and embryonic tissues. Cancer Res 28: 286–296, 1968 [PubMed] [Google Scholar]
- 20.Lindstedt R, Apodaca G, Barondes SH, Mostov KE, Leffler H. Apical secretion of a cytosolic protein by Madin-Darby canine kidney cells. Evidence for polarized release of an endogenous lectin by a nonclassical secretory pathway. J Biol Chem 268: 11750–11757, 1993 [PubMed] [Google Scholar]
- 21.Lipschutz JH, Guo W, O'Brien LE, Nguyen YH, Novick P, Mostov KE. Exocyst is involved in cystogenesis and tubulogenesis and acts by modulating synthesis and delivery of basolateral plasma membrane and secretory proteins. Mol Biol Cell 11: 4259–4275, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipschutz JH, Kissil JL. Expression of beta-catenin and gamma-catenin in epithelial tumor cell lines and characterization of a unique cell line. Cancer Lett 126: 33–41, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Lipschutz JH, Lingappa VR, Mostov KE. The exocyst affects protein synthesis by acting on the translocation machinery of the endoplasmic reticulum. J Biol Chem 278: 20954–20960, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Lipschutz JH, O'Brien LE, Altschuler Y, Avrahami D, Nguyen Y, Tang K, Mostov KE. Analysis of membrane traffic in polarized epithelial cells. Curr Protoc Cell Biol Chapter 15: Unit 15.5, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Marrs JA, Napolitano EW, Murphy-Erdosh C, Mays RW, Reichardt LF, Nelson WJ. Distinguishing roles of the membrane-cytoskeleton and cadherin mediated cell-cell adhesion in generating different Na+,K(+)-ATPase distributions in polarized epithelia. J Cell Biol 123: 149–164, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McAteer JA, Dougherty GS, Gardner KD, Jr, Evan AP. Polarized epithelial cysts in vitro: a review of cell and explant culture systems that exhibit epithelial cyst formation. Scanning Microsc 2: 1739–1763, 1988 [PubMed] [Google Scholar]
- 28.McAteer JA, Dougherty GS, Gardner KD, Jr, Evan AP. Scanning electron microscopy of kidney cells in culture: surface features of polarized epithelia. Scan Electron Microsc 1135–1150, 1986 [PubMed] [Google Scholar]
- 29.McAteer JA, Evan AP, Gardner KD. Morphogenetic clonal growth of kidney epithelial cell line MDCK. Anat Rec 217: 229–239, 1987 [DOI] [PubMed] [Google Scholar]
- 30.Montesano R, Schaller G, Orci L. Induction of epithelial tubular morphogenesis in vitro by fibroblast-derived soluble factors. Cell 66: 697–711, 1991 [DOI] [PubMed] [Google Scholar]
- 31.Nishihara K, Masuda S, Shinke H, Ozawa A, Ichimura T, Yonezawa A, Nakagawa S, Inui K, Bonventre JV, Matsubara K. Urinary chemokine (C-C motif) ligand 2 (monocyte chemotactic protein-1) as a tubular injury marker for early detection of cisplatin-induced nephrotoxicity. Biochem Pharmacol 85: 570–582, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Brien LE, Tang K, Kats ES, Schutz-Geschwender A, Lipschutz JH, Mostov KE. ERK and MMPs sequentially regulate distinct stages of epithelial tubule development. Dev Cell 7: 21–32, 2004 [DOI] [PubMed] [Google Scholar]
- 33.O'Brien LE, Zegers MM, Mostov KE. Building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol 3: 531–537, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Ojakian GK, Romain RE, Herz RE. A distal nephron glycoprotein that has different cell surface distributions on MDCK cell sublines. Am J Physiol Cell Physiol 253: C433–C443, 1987 [DOI] [PubMed] [Google Scholar]
- 35.Pollack AL, Runyan RB, Mostov KE. Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev Biol 204: 64–79, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Rogers KK, Jou TS, Guo W, Lipschutz JH. The Rho family of small GTPases is involved in epithelial cystogenesis and tubulogenesis. Kidney Int 63: 1632–1644, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Sakurai H, Barros EJ, Tsukamoto T, Barasch J, Nigam SK. An in vitro tubulogenesis system using cell lines derived from the embryonic kidney shows dependence on multiple soluble growth factors. Proc Natl Acad Sci USA 94: 6279–6284, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakurai H, Tsukamoto T, Kjelsberg CA, Cantley LG, Nigam SK. EGF receptor ligands are a large fraction of in vitro branching morphogens secreted by embryonic kidney. Am J Physiol Renal Physiol 273: F463–F472, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Santos OF, Nigam SK. HGF-induced tubulogenesis and branching of epithelial cells is modulated by extracellular matrix and TGF-β. Dev Biol 160: 293–302, 1993 [DOI] [PubMed] [Google Scholar]
- 40.Sato S, Burdett I, Hughes RC. Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: a pathway independent of the endoplasmic reticulum-Golgi complex. Exp Cell Res 207: 8–18, 1993 [DOI] [PubMed] [Google Scholar]
- 41.Saxen L. Organogenesis of the Kidney. Cambridge, UK: Cambridge University Press, 1987 [Google Scholar]
- 42.Simons K, Fuller SD. Cell surface polarity in epithelia. Annu Rev Cell Biol 1: 243–288, 1985 [DOI] [PubMed] [Google Scholar]
- 43.Smyth BJ, Snyder RW, Balkovetz DF, Lipschutz JH. Recent advances in the cell biology of polycystic kidney disease. Int Rev Cytol 231: 51–89, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Vainio S, Müller U. Inductive tissue interactions, cell signaling, and the control of kidney organogenesis. Cell 90: 975–978, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Wells EK, Yarborough O, Lifton RP, Cantley LG, Caplan MJ. Epithelial morphogenesis of MDCK cells in three-dimensional collagen culture is modulated by interleukin-8. Am J Physiol Cell Physiol 304: C966–C975, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zegers MM, O'Brien LE, Yu W, Datta A, Mostov KE. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol 13: 169–176, 2003 [DOI] [PubMed] [Google Scholar]