Abstract
While emotion is a central component of human health and well-being, traditional approaches to understanding its biological function have been wanting. A dynamic systems model, however, broadly redefines and recasts emotion as a primary sensory system—perhaps the first sensory system to have emerged, serving the ancient autopoietic function of “self-regulation.” Drawing upon molecular biology and revelations from the field of epigenetics, the model suggests that human emotional perceptions provide an ongoing stream of “self-relevant” sensory information concerning optimally adaptive states between the organism and its immediate environment, along with coupled behavioral corrections that honor a universal self-regulatory logic, one still encoded within cellular signaling and immune functions. Exemplified by the fundamental molecular circuitry of sensorimotor control in the E coli bacterium, the model suggests that the hedonic (affective) categories emerge directly from positive and negative feedback processes, their good/bad binary appraisals relating to dual self-regulatory behavioral regimes—evolutionary purposes, through which organisms actively participate in natural selection, and through which humans can interpret optimal or deficit states of balanced being and becoming. The self-regulatory sensory paradigm transcends anthropomorphism, unites divergent theoretical perspectives and isolated bodies of literature, while challenging time-honored assumptions. While suppressive regulatory strategies abound, it suggests that emotions are better understood as regulating us, providing a service crucial to all semantic language, learning systems, evaluative decision-making, and fundamental to optimal physical, mental, and social health.
Key Words: Emotion, selfregulation, morality, development, feedback, bio-values, sensitivity, computational dynamics, cybernetics, connectionism, complexity, self-organization, epigenetics
EMOTION: THE SELF-REGULATORY SENSE
The wisdom of Jeremy Bentham has oft been quoted: “Man has been placed under the governance of two sovereign masters: pleasure and pain.”1
Despite this insight, philosophers and psychologists remain haunted by the question: What is the biological Junction of emotion? It has been difficult to disentangle emotion from biological drives and physiological responses,2 from motivational appetites and defenses,3 from cognitive appraisals4,5 or moral intuitions6; to make sense of the cultural similarities and differences,7 or to reconcile divergent theories8,9; so difficult, that theorizing about emotion as a functional whole has largely been abandoned. As one critic put it: “My central conclusion is that the general concept of emotion is unlikely to be a useful concept in psychological theory.”10
The purpose here is to suggest the opposite: That the problem with the traditional approach is that it has been overly specific, narrow, and anthropomorphic. Indeed, emotion theory remains reminiscent of the Sufi tale of the elephant and the blind men,11 with each theorist grasping a portion, but unable to see the phenomenon in its entirety. Yet rather than integration and synthesis, the trend continues of “dissecting the elephant”12 into ever-smaller fragments devoid of coherent biological function. As a result, emotional feelings and behaviors are written off as outdated animal vestiges, “ill-suited to modern exigencies,”13 to be suppressively regulated by one's conscious rational mind, if not pharmaceutical intervention.
But with recent revelations from a variety of disciplines, a formerly hidden—yet astoundingly elegant—functional elephant looms large. The current proposal is that the function of emotion is the very sort of “governance” that Bentham suggested, that of self-regulation. But in this usage, “self-regulation” refers primarily to the biologically bottom-up autopilot variety of regulatory control processes, and implies that subordination to our hedonic masters is actually a very good thing. It will be argued that our limited ability to suppressively regulate our emotions is because they are actually regulating us, and from a much deeper, wiser, evolutionary evaluative authority.
To sketch this ancient function, we must pan much further back in our phylogenetic history, and delve deeper into the biophysical regulatory processes of living systems, tracing the emergent trajectory of the emotional system from its simplest mechanistic roots to its present state of elaborate multi-tiered complexity.
To linguistically accommodate the entire functional elephant, we must broadly redefine the category of “emotion” to include “affect” and innate “hedonic” approach/avoid behavior, locating its function in the arena of regulatory signaling and motor control mechanisms. We must specifically focus the inquiry upon feedback loops, recursive, cyclic and reciprocally deterministic, stimulus-response relationships; those that give rise to the earliest forms of “computation”—information processing—in nature; those that inform what will be termed “self-regulated” behavioral agency in organisms as simple as a single-celled bacterium, and those still evident in the cell-signaling cascades that convey identity-relevant information across all levels of organization within complex multicellular organisms—including humans.
Indeed, many theorists have pointed out the primary “relevance detection,”14 “relevance signaling,”15 and “informational,”16–18 functions of emotion, as well as those of resource mobilization and conservation,19 and the organization and facilitation of adaptive behavioral responses.20,21 Likewise, many have noted the categorizational,22 motivational23,24 goal relevant nature15 and primacy25 of affect. In fact, the idea of biophysical feedback itself has a rich history in emotion theory2,26–37 in which Carver and Scheier38,39 specifically noted feedback as a self-regulatory “control process” underlying affect. Recent revelations, however, about bottom-up “self-organization”40,41 and interactive epigenetic mechanisms42 in evolution, can finally root these insights in solid biophysical ground, as well as offer significant clarifications and enhancements.
Indeed, building upon these contributions, I propose that emotion can only be envisioned as a unified functional whole when reconceived as an entire sensory system—a primary somatosensory system that guides biologically adaptive self-regulation. Not a newly evolved or sixth sense43 but perhaps the first sensory system to have emerged on the evolutionary stage, born of the simple molecular stimulus-response networks that regulate metabolic and genetic activity and crude sensorimotor behavioral control in single-celled organisms. Such primal self-regulatory “sensations” are functionally homologous to, and still manifest within, cell-signaling mechanisms in multicellular organisms that integrate and maintain “the self” at all levels of complexity—rooted as deeply as those that control the navigation and differentiation of pluripotent stem cells into their various tissue environments during embryonic self-development. In other words, while they may have emerged as sensorimotor regulators in the earliest life forms, the same principle mechanisms still constitute the signaling and communication systems, the self-organizing language—the self-regulatory music, if you will—of the human body.
In whatever form of “subjective experience” these original sensations may have yielded, in functional terms they would deliver primal perceptions of time, space and self—an inaugural glimmer of a body-self moving within its not-self surroundings, at some point constituting the “feeling of being”44 or “how it feels to be alive.”45 Hence, in far more complex bodies in motion (mammals, other primates, and humans), each emotional feeling perception still reflects “a wave of bodily disturbance,” or the “bodily affections,”2 or “the feeling of what is happening.”46,47
Key to our discussion, however, is that from their emergence forward, these informational sensations have contained “felt evaluations,”48,49 the symbolic binary opposites that we experience as pleasure and pain, the feel good/feel bad hedonic valence of emotion. These “positive and negative” binary opposites offer real-time computational representations of the ongoing dynamic orchestration of whole-body coherence, with harmonically resonant and dissonant reverberations ringing forth when environmental perturbations require self-regulatory responses. The current proposal is that the binary hedonic logic within these felt evaluations offers nothing less than a biological value system, informing us of universally optimal and deficit states of balanced being and becoming—a natural value system rooted in the biophysical requirements for life itself.
At a more concrete level of analysis, the positive and negative hedonic categories equate with “eustress” and “distress” signals respectively50 and locate the emotional sense as an intimate affiliate of the immune system (recently declared a sensory system itself).51 Adding, however, that its core physiological “self” or “not-self distinction is tethered deeper still in genetic and epigenetic regulatory mechanisms, the bottom-up biological processes that ultimately inform the fundamentally “self-relevant”34 or “motivationally relevant”52 nature of affective stimulus, and underscore the notorious bidirectional connection between emotion and physical health.53–59 As such, these core self-regulatory feedback processes in humans also undergird the requirement for “regulatory fit”60 within and between goals, or concordance within the “psychological immune system”61 and other self-balancing processes such as “cognitive dissonance”62 although, as will be argued, emotional dissonance may be more biophysically accurate.
The self-regulatory functional elephant will also acknowledge emotion as the unsung hero in conditioned learning,63,64 in subliminal “priming”65 and embodied66 implicit67 or unconscious cognition,68 implicit bias69,70 as well as nonconscious, “auto pilot” self-regulation71; in cognitive identity formation,72,73 self-perception,74 self-concept,75 self-serving biases,76 and self-enhancement motives77: in needs for and feelings about self-determinism,78 self-efficacy,79 selfesteem,80,81 self-expansion82 and urges toward selfactualization83; all of which are elegantly integrated within emotional sensory perceptions and their coupled behavioral responses.
In short, the goal here is to sketch a new image for the box of the puzzle of emotion, one where emotion takes its rightful place as a sense; one depicting common feeling tones on par with colors, tastes, scents and sounds. One in which feeling perceptions, ranging from rudimentary pleasure and pain, through basic joy and sadness, to complex pride, shame, admiration and envy, serve as sensory signals offering an elegant palate of evaluative information about our adaptive fitness in the immediate environment. Indeed, the proposal is not only that emotion should be reframed as a sensory system, but that emotion should also be acknowledged as the biological grandfather of all the senses, and that its hedonic self-regulatory logic remains encoded within all other senses—a simple logic, yet one so crucial as to have been conserved throughout our entire evolutionary history. Acknowledging how our presently elaborate, cognitively enriched, emotional perceptions still bubble up from their ancient self-regulatory wellspring, offers quite profound implications for the medical community, as well as the social sciences in general. Indeed, it allows the scientific construct of emotion to come full circle, rejoining with the so-called naive realism of immediate human experience, yet offering direct inroads to embodied knowledge, bountiful emotional intelligence, social intuition, and even moral reasoning.
But however elegant, these subjective manifestations cannot be separated from their objective counterpart, for each emotional sensory perception includes both an informational component and a coupled behavioral response. Indeed, in this new view, emotion is ground zero for all sensorimotor stimulus-response relationships, with the hedonic approach and avoid behavioral pattern—a pattern observable from the single celled ameba to the complex human84—serving as the primary empirical justification and departure point for our new story. A crucial point is that this crude sentience is contingent upon, and would follow from, the deterministic behaviors themselves, or as Marienberg put it: “the becoming aware of the capacity to act while acting.”85 In short, identifying the biological function of emotion requires taking Skinnerian behaviorism to all new reductionist levels—an inquiry into how approach and avoid behaviors emerge from the chemistry of living systems. Yet, when equipped with the lens of feedback control theory, the journey affords a primordial peek into the “black box,” offering a clear and detailed functional explanation of how innate (“unconditioned”) stimuli evoke “affect” itself—something decidedly lacking in emotion theory.9
This brief introduction begins with a redefinition of emotion within this broadened context, turning next to its biophysical substrates and underlying feedback dynamics, and identifying the source of what will be termed “the self-regulatory code.” The hedonic behavior of the Escherichia coli (E coli) bacterium is offered next as an example of the ancient mechanisms (both function and form), followed by a description of the modern neural, perceptual, and behavioral manifestations of the emotional sense; and ending with a brief discussion of the implications for human health. Indeed, to formally acknowledge emotion as a primal sensory system invites critical reevaluation of many deeply engrained linguistic conventions, beliefs, and practices.
EMOTION: A BROADENED DEFINITION
To begin, I broadly redefine “emotion” within the context of digital stimulus-response behavioral phenomena, including any biochemical processes and physical mechanisms, laws and forces that determine their cause and effect relationship. By digital, I mean any sort of distinctly binary values, symmetrically isomorphic or oppositional qualities, structures, bistable states or transformative actions that exist in nature that can be harnessed as meaningfully symbolic cues further up the evolutionary ladder. In other words, such binary values (ie, positive/negative electrical charges, north/ south magnetic poles, left/right symmetries, cis/trans isomers, bistable attractors, etc) can serve as digital information “bits” for computational processing. In fact, an if-then stimulus-response logic is there for the taking in the orderly behavior of electrons, behavior that ultimately drives all higher scale chemical reactions—from the bonding and anti-bonding behaviors of molecules, through the transitional and equilibrium states of metabolic networks, to the signaling cascades and on/off regulatory switching of genetic processes. In short, the sensory informational components of emotion can only be appreciated against the backdrop of the in-forming, trans-forming, stimulus-response dynamics of matter in motion.
These binary opposites, deterministic behavioral laws, and self-organizing dynamics underlie the “regulation” part of the self-regulatory function of emotion, as they deliver bottom-up “order for free.”41 As we will see, they also deliver an elegant stimulus-response choice-making logic—whether or not any sentient life form has yet emerged to exploit it. The “self” part of the self-regulatory function, and the emergence of what is defined herein as emotion proper, is rooted in iterative, self-reflexive, feedback loops. Indeed, feedback provides the crucial evolutionary link between the deterministic, self-organizing “happening” behavior of non-living matter and the self-regulatory agency—goal driven “doing” behavior—of living systems. As such, feedback also provides the conceptual linchpin between the physically impartial “positive” and “negative” binaries in nature and the warm-fuzzy/cold-prickly evaluative categories of personal experience.
What Is Feedback?
Feedback, in terms of general function, refers to communication and control mechanisms prevalent in both mechanical and organic systems—those that report upon (inform) and alter (transform) the relationship between a given system and its immediate environment.86 Feedback is cyclic, as it occurs in circular stimulus-response loops where the output of a system is fed back into itself, serving as a stimulus for a subsequent round of output responses (See Figure 1, two systems with and without feedback). In this primary mechanical context, however, the term “self” is synonymous with the system in question, whether it be an atom, a molecule, a cell, an organ system, or an organism interacting with its local “not-self” environment. Equating “system” with “self,” of course, does not yet imply sentience or consciousness, but is simply a relative location in space, as well as a subjective center in time serving as both source and sink for energy and information exchange, and therefore, ground zero for both stimulus and response. Nonetheless, as Figure 1 suggests, feedback processes conceptually juxtapose time, space, and self in unadulterated ways, offering a simple yet elegant springboard for our discussion of emotion as a primal self-regulatory sense.
Figure 1.
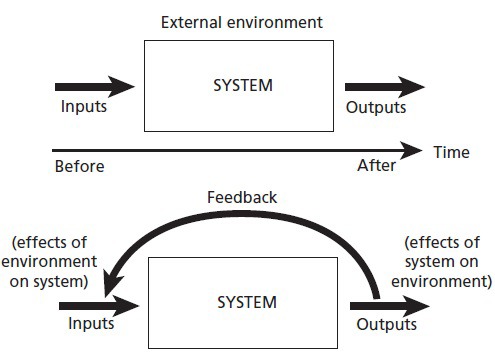
Feedback. (Adapted from de Rosnay, 1979.)
But the feedback mechanism is also central to the aforementioned “regulatory” side of the self-regulatory emotional elephant—as well as the emergence of sentience itself. For feedback loops are the basic building blocks of cybernetic systems,87–89 also known as “complex adaptive systems,”90 “dissipative structures,”91 and self-making “autopoietic” systems92—which include all life forms. As the original “science of control and communication,”89 cybernetics united regulatory control theory with physical information theory, investigating how materially embedded systems can make observers and actors possible—how mechanically in-forming and trans-forming processes give rise to subjective information and behavioral control in living systems. In fact, in terms of thermodynamics, feedback is associated with both entropy (chaotic disorder) and the “negentropic”93 ordering principles that underlie the physical definition of information itself. (As Nobel laureate Manfred Eigen suggested: “If you ask where does information come from and what its meaning is, the answer is: information generates itself in feedback loops.”94)
In short, feedback is quite literally a key computational in-forming and trans-forming engine in nature, with feedback regulation subserving all biological signaling systems,95,96 underlies biorhythms and biological clocks97 and molecular and neural circuitry,98 is essential to all genetic, epigenetic,99 immune.100–102 and even sensory mechanisms,103 as well as goal-directedness and behavioral control.104
The functional architecture of these ordering and disordering principles—from electromagnetic polar shape shifting transitions to favored-state energetic balances—was elegantly depicted by the founder of both cybernetics and general systems theory Ross Ashby, in his original “homeostat,”105 an electronic device that provided a concrete example of adaptive control. It was a crude learning or “thinking” machine, one that combined both analog and digital information processing in order to maintain stability in the face of widely varied and highly challenging environmental perturbations106 —an informational architecture central to our discussion. In fact, the auto-induced, cyclic, self-reflexive nature of feedback, and its ubiquitous role in selforganizing and self-regulatory processes places it center stage for both “self” and “regulation” pieces of the self-regulatory function. I will demonstrate herein how the hedonic valence of emotion—with its definitively “self-relevant”34 stimulus signals—emerges directly from positive and negative feedback loops. Indeed, they come in two types, providing the binary opposites for digital “choice-making” in what I call the self-regulatory code, still evident in the sensorimotor architecture of living systems, much as Ashby had envisioned.
For now, emotion as a self-regulatory sense emerges because feedback “happens” across the great chain of being, the “noise”107 of its simple computational dynamics having been harnessed by self-replicating systems, and conserved, honed, and elaborated upon by natural selection. As such, the feedback paradigm can shed light upon the hedonic behavior of simple organisms that emerged on the evolutionary stage long before nerve nets or brains, allowing questions of primitive sentience to be separated from the complex neural processes that are correlated with human consciousness. In fact, it is only within this broadened, less neurocentric depiction that the many facets of the entire emotional sensory system can come to light.
Indeed, this new view allows us to zoom in, conceptually revisiting the earliest emergent sensory mechanisms for detailed clarity in the form and function of self-regulatory feedback. At this micro level, the feedback (and feed-forward) circuitry offers conceptual precision to descriptive terms for information flow in space and time (ie, inside, outside, before, after, backward, forward, bottom-up, top-down), precision that can help physicians and social scientists transcend the Cartesian (“dual process”) mind-body muddle. This new approach allows us to zoom out to the macro level of analysis, offering a bird's eye vantage from which a complete spectrum of informative emotional feeling tones comes into view, a continuum of meaningful sensory signals ranging from the hardwired and universal, to the learned, socio-cultural and particular, finely tuned to the specific life experience of each unique individual.
In fact, since its initial emergence, the emotional sense has undergone tremendous elaboration by natural selection. Its present structure is an elegant tri-level informational hierarchy—from affect to basic to complex feelings—reflecting the generally “triune” structure of the brain,108 yet with each still playing its own uniquely valuable self-regulatory role. But perhaps most importantly, it shows how affect provides the core “hedonic”109 evaluative message, the fundamental “bad-for-me” or “good-for-me” appraisals that we experience as immediate psychological pain or pleasure. Indeed, identifying emotion as our primal self-regulatory sense, restores our innate tether to biologically determined optimal—perhaps non-negotiable—states of life-giving balance.
In sum, the emotional sense is born of biophysical regulatory feedback signals that come courtesy of lawful stimulus-response behavior, signals that still undergird our hedonic emotional perceptions and their coupled approach or avoid behavioral responses. These affective polar opposites are the highly conserved felt evaluations—saying “no” to this and “yes” to that—those that appear across the various levels of analysis, recognizable in affective “eustress and distress” signals50; informing us of the immediate environmental “benefits and harms,”46 or symbolic “challenges and threats,”110 and giving rise to our general positive and negative categories of emotion. I will, in a moment, suggest an even more fundamental self-regulatory dichotomy that undergirds them all, one showing how the amazing emotional sense offers universal self-regulatory perceptions for all humans which—when properly understood—also offer a personally tailored guidance system to each individual. For now, “emotion” is defined to include these core hedonic self-regulatory signals as well as the primary or basic emotions111 (joy, sadness, disgust, anger and fear); and the complex112 feelings (also known as “unnatural” 113; “secondary” 114; “social” or “moral” emotions.115,116 This complex class, the most recent to have emerged on the evolutionary stage, is the most cognitively laden and temporally expansive, and includes such familiar feelings as trust, mistrust, pride, shame, gratitude, contempt, envy, admiration, love, and hate. Indeed, as depicted in the Venn diagram of Figure 2, this expanded, all-inclusive, multitiered, definition of the emotional system also reflects the stair-step evolution of each new level of self-regulatory information as it emerged over our sweeping biological history—the most ancient remaining functionally foundational and present within each, more recent, additional enhancement.
Figure 2.
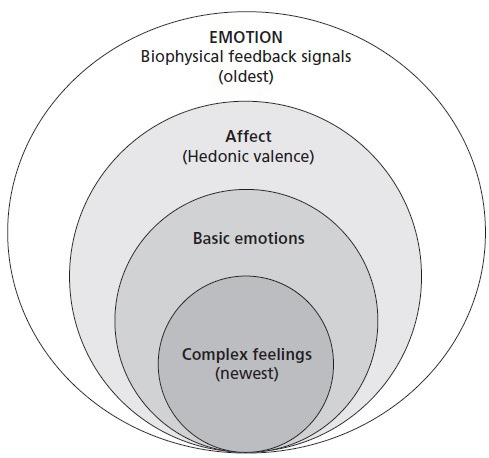
The expanded categorical definition of emotion.
Whether or not the above discussion coheres for health professionals or social scientists who may not stray far from our respective disciplines, please bear with me, for the self-regulatory logic that emerges from the ubiquitous biophysical feedback process speaks for itself. Indeed, once this missing piece of the emotional puzzle—its self-regulatory sensory function—is identified, many other disjointed bodies of empirical evidence fall into place.
BEHAVIOR, FEEDBACK, AND THE EMERGENCE OF SELF-REGULATORY CODE
In this new view, such ubiquitous bottom-up phenomena as embodied cognitions, priming effects, subconscious attitudes, unconscious motives, conditioned memories, and instinctive autopilot behaviors are a direct result of the self-regulatory processes we perceive via the emotional sense. In fact, it is only in the context of these primary bottom-up aspects of emotion that the more recently evolved top-down add-ons begin to make self-regulatory sense.
It is conceivable, however, that I am indulging in naive realism or am equally guilty of anthropomorphism—pushing the human experience of pleasure and pain back upon less complex species. To avert this critique, I'd like to temporarily decouple the stimulus-response relationship, asking readers to simply bracket the subjective aspects of emotion (depicted in Figure 2) and maintain a strictly behaviorist perspective. In fact, while the sensory information has undergone tremendous elaboration over time, the basic motor approach/ avoid behavioral responses remain the same—and they embody the self-regulatory logos on offer. Thus, in the spirit of empiricism, we will confine the next portion of the discussion to the objective approach or avoid behavioral pattern and let the actions speak for themselves.
To continue, as previously suggested, the secret to cracking the self-regulatory code is feedback. This is because feedback is first and foremost a regulatory control process—in-forming while trans-forming, ordering, and organizing behavior. In fact, “integral feedback control” is a basic engineering strategy in complex man-made systems such as a jet airplane, with feedback loops found at every level, from transistors and circuits to instruments and actuators, to the autopilot mechanism for the entire vehicle itself.117
Although Ashby's homeostat was largely forgotten, this autopilot nature of behavioral control is perhaps what later inspired engineering psychologists to link human behavior with negative or “regulatory” feedback control. Regulatory feedback is associated with homeostasis—keeping things at their proper set points in order to keep the airplane or the creature shipshape and on its proper course. Indeed, by the 1970's, on the heels of the behaviorist heyday, feedback control theory a “quantitative science of purposive systems”118 was resurrected with the palliative promise of restoring internal goal states to psychological theory. In organic systems, however, we've seen that homeostatic goal states rely upon natural physical constants, reaction thresholds, and optimal equilibrium balance points—chemically or energetically “favorable” states, in accordance with the laws of thermodynamics. This may be why the classic example of homeostatic feedback control then became the thermostat.119 The thermostatic regulator functions through a three step process: It compares the actual state of the system to some preset optimum, signals when a mismatch is detected, and self-corrects back toward the optimal state (it “effects”89 an observable behavioral response). In a home heater, for example, the actual room temperature is compared to the desired preset temperature, and when the house gets too hot or too cold, the thermostat rebalances the system by kicking the heat on or off. While problematic (outside its original quantitative context), this thermostatic model offers an excellent inroad into our detailed examination of the simplest sensory systems, as the three steps (comparison, signaling, and self-correction) are crucial components of the self-regulatory feedback cycle. For key to our discussion, is that feedback comes in two types. In fact, the binary code—as well as the thermostatic arrangement itself—emerges from an elegant coupling of these two types of feedback, a stimulus response relationship that creates the necessary bridge between the determined (happening) behavior of matter and the partially free—but logically self-regulatory—(doing) behavior of animate agents. This coupling also delivers the functions that the early cyberneticists had hoped could: “at last explain how ‘mental’ causes could enter into ‘physical’ effects.”118 Indeed, the coupling of both types of feedback is the missing piece required to illuminate the self-regulatory logos, and vault the gulf to human behavior with that logic intact.
Positive and Negative Feedback
The first type of feedback is called positive feedback. In a positive feedback loop the iterative cycles build upon one another, such that with each new cycle the change to the system proceeds in the same direction as that of the former cycle (Figure 3.) Positive feedback is associated with chaotic change, leading to divergent behavior, “an indefinite expansion or explosion (a running away toward infinity) or total blocking of activities (a running away toward zero).”86 Functionally, positive feedback is amplifying, associated with rapid, exponential, growth (or decay) and upward or downward spirals of runaway change. Examples include: chain reactions, autocatalysis, signal transduction cascades, economic inflation or deflation, and population explosion or depletion. Please note that there is no evaluative (good or bad) connotation to “positive,” the term speaking only of the direction of change, with positive connoting qualitative change in the same direction as the previous cycle, whether that direction yields a quantitative increase or a decrease in a given energetic or chemical parameter.
Figure 3.
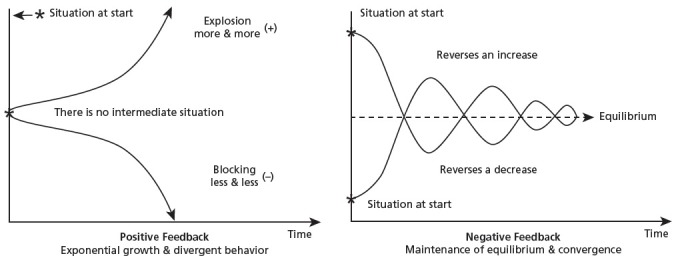
The two types of feedback. (Adapted from de Rosnay, 1979.)
The second type, negative feedback does just the opposite, reversing the direction of the process relative to the previous iteration (Figure 3). Once again, there is no evaluative judgment, ‘negative’ simply means reversing the direction of the change, regardless of the nature of that change. But since it is a ubiquitous feature of homeostatic circuits, negative feedback is considered regulatory, in that it controls the runaway “chaotic” change born of positive feedback loops. As mentioned, negative feedback relies upon natural laws and statistical mechanics, kicking in when upper or lower thresholds of a given parameter are breached, providing convergence to a preferred, chemically or energetically “favorable” state, in accordance with the laws of thermodynamics and quantum mechanics. (Indeed, even the electron has a preferred energetic “ground” state.) But it is equally important to realize that the wild, runaway behavior of positive feedback also flows from those same physical laws and forces—an electron, an ion, a polarized molecule, a membrane, a neuron, or an organism—can also be in an “excited” or temporarily unbalanced dynamic state. It seems that life could neither emerge nor be sustained without both halves of the in-formative trans-formative whole that is feedback.
In short, both positive and negative feedback are ubiquitous in nature, counterparts to one another, working together in the process of self-organization. While positive feedback yields the instability and divergent processes that constantly create, destroy, and recreate new arrangements of matter, negative feedback provides the stabilizing balance, homeostasis, and preservation of form. Indeed, feedback loops are among nature's most fundamental building blocks, “the engine of selforganizing dynamical activity” that “leaves its tracks and marks as fractal structures”120—the non-Euclidian “fractal”121 geometric shapes underlying all natural and biological structures, including the human brain.
Coupled Feedback Loops and Self-regulation in Early Life
Historically, however, most control models of human behavior relied upon only negative feedback, and have therefore languished. Likewise, it has since become clear that even the simplest behavioral control mechanism in a living system involves many links and chains of single positive or negative loops, which changes the entire game. Indeed, when we begin melding the physically deterministic and the subjective functional definitions of “self,” the increases and decreases manifested by positive and negative feedback (the changes and their reversals) connote state changes within the identity of a living form, changes driven directly by the reciprocally disturbing interactions between the selfsystem and its immediate (not-self) environment. In evolutionary terms, such a regulatory process would have emerged along with life itself, an outgrowth of “hypercycles” and “autocatalytic” chemical networks,122 constituting a “thermodynamic work cycle,”123 the first sort of metabolism. A further requirement for life was the formation of the lipid membrane to bound, contain, and protect a living system (analogous to human skin), yet with structures that allow it to sense and respond to its environment, both of which were essential to the emergence of minimal biological agency124—goal seeking behavior. (Also see Sherman and Deacon, for an intriguing theory of a missing link “autocell”125 that bridges thermodynamics, morphodynamics, and goal-seeking teleodynamics in emergent systems; albeit devoid of the feedback processes discussed here.) In fact, such a system has been suggested to predate even natural selection, described as “context dependent actualization of potential,”126 or “self-other organization.”127
At some serendipitous juncture in our evolutionary history however, self-replicating molecular arrangements emerged and natural selection was off and running. But, regardless of how this leap occurred, central to our discussion is that regulatory feedback circuits and their dynamic logic128 were already in place, serving regulatory functions in the first single celled creatures. “Regulation” in this context involves changes (“covalent modifications”) in the properties of a cell under the influence of external and internal signals in order to adjust the cell's internal biochemistry. This process is considered the evolutionary “origin” of sensory processing129—and, I argue, is precisely what the cyberneticists were intuiting about feedback control. Indeed, in whatever order they emerged, the trifecta abilities: (1) to sense the physical qualities of one's immediate environment; (2) to respond behaviorally, and (3) to categorize sensory stimulus gives an “operational closure,”130 a circular causality131—a general principle of organization within an autopoietic system that defines biological “function” itself.132
Feedback Functions of Cellular Receptor Complexes
Nonetheless, while the bulk of this discussion focuses upon the functional outcomes of feedback processes, understanding the structures that instantiate them is paramount—for biological function follows physical form. These structures are called protein receptor complexes, essential components of all cellular membranes in both prokaryotic and eukaryotic cells. Cellular receptors were originally conceived as lock and key stimulus-response facilitators, upon which a chemical agent (ligand) would bind, triggering a specific cellular response. In fact, these unique cell-surface molecules are not only essential to the earliest sensory systems, but remain central to intercellular signaling, interacting with hormones and humoral factors essential to inter-organ communication.133 However, with powerful new microscopes it has become clear that the simple lock and key model was severely limited, and cellular receptors have proven to be far more structurally and functionally complex (now referred to as “complexes”). Indeed, through their form they instantiate both the positive and the negative feedback loops under discussion and serve as structural homologues to Ashby's homeostat. For crucially, these structures are transmembrane receptor complexes, physically exposed to both the external and internal environments of a cell. They have both ‘heads’ outside and ‘tails’ inside—a general structural feature that facilitates the feedback comparison and the internal effector response.
Moreover, the individual proteins that comprise the complexes are detailed 3-D structures with modular construction and moving parts—shape-shifting dynamics driven by ligand binding that allow for complex couplings, combinations, and chains of individually positive or negative feedback loops. In fact, at present, the repertoire of genes that encode these plasma membrane receptors has been called the “signaling receptome” with receptor families that reflect their evolutionary origins and chart their ever-increasing functional complexity. Indeed, the Seven-Transmembrane (7TM) family of receptors (still present in the human receptome), first emerged in unicellular organisms already composed of seven discrete transmembrane domains that induce conformational changes and diverse functions.133 As such, receptor complexes at every level on the phylogenetic tree instantiate intricate webworks of coupled feedback loops and circuits with common functional motifs.134,135 These motifs include such functions as: basal homeostat, threshold limiter, and adaption (born of negative loops); and amplifier, accelerator, damper, delayer, or bistable switching (of positive loops); or pulse generators or oscillators (of both).
Of particular interest for our new model of emotion, is the positive feedback motif of bistable, digital switches between alternative phases or states135–139 the aforementioned covalent modifications.129 As previously noted, such deterministic binary (either/or) switching is observable at all scales of material organization (ie, chiral symmetry of amino acids that determine the genetic code; bonding and anti-bonding reactions that govern protein folding; “on/off” switching of genes and all-or-none firings of neurons.) In fact, this dynamic bistable pattern emerges consistently even from randomly connected network nodes yielding systems poised critically on the “edge-of-chaos,” dynamically balanced between stability and change.96 More, the dynamic transitions between these bistable states that have been suggested to provide the earliest forms of computation in nature.140 Indeed, even the simple thermostat requires bistable switching—and several other positive feedback motifs, as did Ashby's original homeostat.
Hence, the present proposal is that the original winning evolutionary scenario—the one that underpins the self-regulatory behavior of life forms—was a coupling of both types of feedback such that the divergent positive feedback stimulus triggers convergent, negative feedback regulatory responses (Figure 4). This general arrangement delivers most (if not all) of the functional feedback motifs in one fell swoop, providing nearly every requirement of the regulatory thermostat.
Figure 4.
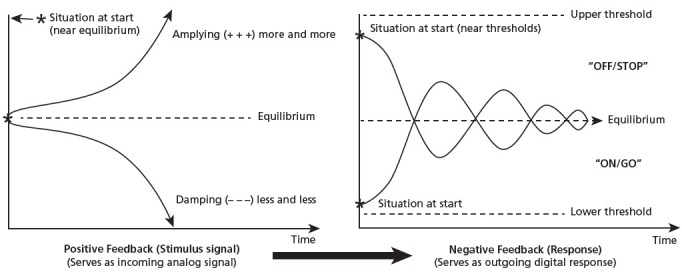
How coupled positive and negative feedback yields stimulus-response behavior. (Adapted from de Rosnay, 1979.)
For example, as depicted at left in Figure 4, the amplification versus damping, and bistable switching motifs of positive feedback offer a graded analog signal which indicates the system is changing in significant ways, that some relevant environmental stimulus is either increasing or decreasing. (Others have termed this the “sense signal” which is then compared to an inner “reference signal,” triggering the “error signal.”89) These changes are then indeed compared to the desired states and reaction thresholds (basal homeostatic and limiter motifs of negative feedback, shown at right); which triggers a corrective response that reverses the trend, bringing the system back into balance (like the home furnace). While perhaps neglected in cybernetic models of human behavior, this coupled feedback configuration has been noted elsewhere and deemed a biological logic gate or block that can switch from the “and” to the “or” functions,141 the logic circuitry of the electrical transistors in computer chips.
With an elegant simplicity, this general feedback arrangement offers both analog and digital information processing, extending its principle of circular closure across multiple levels of organization, to forge a selfsimilar pattern of relational causality across multiple scales in time and space—fulfilling all Ashby's original hopes for his homeostatic thinking brain. Indeed, like a neural network, it gives rise to horizontal cross talk (bidirectional and parallel processing) between local network nodes as well as unidirectional signaling and control relationships across vertical levels in fractal hierarchies, fostering synchrony between faster and slower system dynamics, and bridging local and global levels of coherence and control. Most importantly, these reciprocal self-regulatory relationships coordinate life-giving functions in complex organisms, guiding intercellular development142 and ultimately yielding “perfect adaptation.”143 In fact, the motifs of coupled positive and negative feedback loops include the oscillatory behavior, pulse generators, and on/off firing behavior of neural networks, and the “tunability” of biological rhythms from cell cycles to heartbeats.144,145 Furthermore, at the macro, systemic, level of analysis, wherein the organism as a whole interacts directly within its external ecological niche, this adaptive tunability constitutes a “constrained form of computational learning”—synonymous with evolution itself,146 Ashby's learning machine writ large with its simple machine-like algorithms becoming ever more flexibly personalized “ecorithms”147 guiding evermore complex adaptive responses. Best of all, of course, this elegant feedback coupling sets the stage for the first sorts of hedonic behavior—as well as the first sort of enacted, embodied, mind.
Self-regulatory Behavior in Bacteria and the Tit-for-tat Code
Indeed, this new story strikes at the heart of an ongoing philosophical debate as to the nature and origins of mind. Perhaps related to the original Cartesian divide, the debate concerns whether mindful “cognition” is an exclusive manifestation of a functional brain or whether it is primarily embodied and embedded in an environmental context (ie, references 148-150).148–150 The emotional sensory model suggests that it is both, but that as the locus of the feedback control function, “branes”—environmentally embedded cellular membranes—came before brains in terms of evolution, and their signaling dynamics delivered the first experience of self in space and time. (In other words, it suggests that emotion preceded “cognition” proper and that “sentio ergo sum”—I feel therefore I am—may have been more biophysically accurate.) As such, the sensory feedback model weds the computational, representational, identity and embodiment approaches to the emergence of mind in the singular concept of primary selfregulatory perception. That, which I am arguing, gave rise to the inaugural evaluations within the emotional sense.
In fact, the brilliance of the cybernetic model, was that rather than to control behavior per se, it served to “control perception.”89 It was a theory of how a system controls its somatosensory experience of being—its hedonic feeling of what is happening.46 But this seems just a convoluted way of saying that a regulatory control system delivers (ushers or creates) perception itself. In short, it yields a crude mind. Indeed, Jaak Panksepp, founding father of “affective neuroscience”151 posits a core affective consciousness, or a “visceral nervous system” that yields “primordial affective mentality”—genuine feelings in all neurally endowed creatures, “similar to seeing a color.” Theorists stop short, however, of declaring emotion to be an actual sense, for as emotion pioneer Nico Frijda puts it: “There is still no detailed hypothesis at the functional level of how innate affective stimuli evoke affect.”9 This is where an examination of the simplest sensory systems can clarify and expose the devilish molecular details within which the primal emotional sense remains shrouded.
Take, for example, the chemosensory system of the Escherichia coli (E coli) bacterium, perhaps the first identifiable sense to emerge, and one whose molecular circuitry is quite well understood. The on/off switching that underlies affect is readily evident in the digital behaviors of coupled protein molecules, those central to genetic regulation as well as sensory perception. (For reviews, see references 129, 152, 153.) As mentioned, the structure of interest is the protein receptor complex on its “brane”—transmembrane structures analogous in humans to external sense organs on our body and skin (noses, ear, eyes, etc.), yet where all the feedback functionality is orchestrated.
Indeed, in the simple E coli, there are three levels of binary self-regulatory switching with functional outcomes from on/off genetic regulation, through stop/go behavior (approach/avoid chemotaxis), to the yes/no hedonic evaluative representations under discussion, and as the details will demonstrate, each ofwhich exemplifies the self-regulatory feedback arrangement depicted in Figure 4. In fact, though far more complex than our ancient ancestral autocell, the molecular circuitry on the brane of the E coli illustrates evolutionary enhancements of the original capacity to categorize sensory stimulus, an original requirement for causal, operational, and functional closure. Furthermore, in terms of the brain-only view, these three levels offer exact matches to the three criteria required of a legitimate “internal representation” offered by Haugland154: (1) to coordinate its behaviors with environmental features not always “reliably present to the system”; (2) to cope with such cases by having “something else” stand in (in place of a direct environmental signal) and guide behavior in its stead; and (3) that “something else” is part of a more general representational scheme—a code—that allows the standing in to occur systematically and allows for a variety of related representational states.155 Likewise, these conditions dovetail cleanly onto Powers' control model of human behavior,89 with the comparison between Haugland's conditions 1 and 2 (termed the sense signal and the reference signal),154 which when discrepant delivers the error signal, with a coupled self-correcting effector behavioral response that I am suggesting manifests as the binary hedonic valence of emotion. In short, the coupling of positive and negative feedback gives rise to all three criteria for a functional mind and an elegant sensorimotor behavioral control system—far before brains emerged on the evolutionary stage.
While some may rightly worry that an E coli bacterium is hardly analogous to a human being, its simple sensory system provides an elegantly detailed example of the “thermostatic” feedback arrangement in action, allowing us to precisely parse what happens where and when in space and time that yields self-regulated hedonic behavior. In other words, in terms of both function and structure, the E coli bacterium offers an excellent biological stand-in for the “system” depicted in Figure 1, its membrane physically bounding itself from its not-self environment. The feedback loop is the embedded aspect of mind, the transmembrane sensory receptors reporting self-relevant stimulus as the body moves about, with the three steps of feedback control constituting what goes on in the “black box” mind proper—a simple loop that yields primal hedonic perception and approach/ avoid behavior. Indeed, the suggestion is that this simple circuitry reflects the core “molecular universals” of approach and avoidant behaviors conserved in a wide range of species.156 It is also likely the source of the generally accepted taxonomy of “primary process affects” in emotion theory: sensory affects, bodily homeostatic affects, and brain emotional affects151—those that loosely capture the three tiers of information encoded in human emotional perceptions (previously depicted in Figure 2).
With that said, the general mechanism works like this: A chemical in the external environment binds to a receptor protein complex on the bug's outer membrane, activating a signal transduction cascade inside the cell that leads to both a short term change in the organism's behavior, and a long-term adaptation of the receptor mechanism itself.157 Each of these changes is driven by the feedback arrangement (depicted in Figure 4), and via their coupling to one another, they typify the circular causality wherein the faster dynamics serve as the bottom-up signals triggering the slower, top-down corrective response. In short, the system utilizes three levels of the thermostatic stimulus-response switching, each facilitated by the feedback coupling.
Specifically, in E coli, the short-term behavioral response is the switching between a counterclockwise (CCW) or clockwise (CW) rotation of a given flagellum—one of the four to eight tail-like protein appendages embedded in the cell wall—that allows swimming toward or away from beneficial or harmful chemical gradients, temperature changes, or other relevant environmental conditions. (With the CCW motion, all the flagella rope together propelling the organism forward, but a switch in any one flagellum to the CW mode, flails them apart causing an abrupt halt and a “tumble” off in another direction.)
From On/Off to Stop/Go
This basic stop and go behavior is accomplished by a circuit of many positive and negative loops mediating interactions between five receptor proteins (ie, Trg, sensing ribose and galactose; Tar sensing aspartate; Tsr, serine; Tap, peptides; and Aer, which senses O2) and the protein products of six key genes (CheW, CheA, CheY, CheZ, CheR, CheB). These receptor proteins (numbering in the tens of thousands) cooperatively cluster together in the cellular membrane by a process of stochastic self-assembly,158–160 such that they serve as an “information processing organelle,”161 likened to a “nose.”162 As mentioned, however, what is instructive about the brane, is that this nose-like sensory organ spans the depth of the membrane “skin” such that its outside heads and inside tails are privy to both internal and external environments simultaneously, which is how the feedback comparisons, signaling and responses are instantiated.
These transmembrane receptor complexes (assisted by adaptor protein CheW and histodine kinase CheA) detect the change in chemical gradients—the environmental stimulus—and regulate behavior accordingly via integral feedback control.117 As in Figure 4, they constantly monitor the environment, comparing the relative concentrations at time one with those at time two (your classic negative feedback homeostat motif), with the increase or decrease in bound receptors serving as a positive feedback signal informing the cell that a significant deviation from stable set-points (negative feedback limiter) has occurred. (As the core sensory organ, the outside “heads” of the receptor complexes deliver Powers' “sense signals,” 89 and subsequent alterations of the inside “tails” serve as Haugeland's first criteria for an internal representation—the direct detectors of relevant environmental stimulus that may not always be present.154
For from there, a coupled positive feedback exchange between CheA and phophatase CheZ takes place inside the cell, which adds or removes phosphorous (respectively) to and from second messenger CheY, which directly initiates the regulatory (negative feedback) motor response, the switching between CCW and CW flagellum rotational modes that controls the bugs behavior. (This second messenger protein, serves as Haugeland's second criteria for mindful representation, the “something else”154 that stands in for the missing stimulus, yet still mediates the stop and go behavior. In the Powers model, this is an internal extension of the sense signal 89 (and perhaps the simplest example of the evermore complex signal transduction cascades observable in more complex organisms, those that include neurotransmitters and hormones in humans.)
From Stop/Go to Yes/No
So far, however, this is only half of the story. For these are the bottom-up fast time, activating, dynamics, wherein the binding and unbinding of receptor proteins triggers the on/off phosphorylation or dephosphorylation of CheY, which then drives the immediate stop/go switching between behavioral regimes. These are the dynamics (the feedback coupling depicted in Figure 4) that operate on timescales of milliseconds, with the amplifying (+) signal triggering a (−) reversal switching to the “OFF” (or, in this case, “Stop”) mode. Likewise, a decrease (−) in the phosphorylation signal triggers an increase (+), wherein the reversing (negative feedback) response switches to the “ON” (or “Go”) mode (See Figure 4). Do note that these dynamics are regulatory (negative feedback) responses; they are keeping the system within the specific thresholds, preserving the system within its existing parameters. (This is the level where the, homeostatic negative-feedback-only control models still ring true.)
The other half of this regulatory circuit follows the same feedback pattern, but unfolds over a longer timescale (minutes), yielding the slower, top-down, deactivating dynamic that gives rise to adaptation in the bug's sensory system—a brief, but functional, “memory.”129,161 This is a change that increases the range of sensitivity by altering the sensory mechanism itself, offering the bacterium a broadened bandwidth of information for subsequent encounters, adding a feed-forward step in the cycle.163
This is a crucial juncture in our new story. For it is this adaptive response that takes the logic of on/off switching and stop/go behavior to the yes/noevaluation that ultimately underlies the proximate feel good/feel bad hedonic valence of emotion. (In fact, this feed-forward step is a necessary piece for any control model that posits anticipatory or purposeful goal states.)
To continue, this slower top-down adaptation process informs the system of the rate of change in the original stimulus, and results in an alteration of the sensory receptor complex itself This occurs through methylation of specific units of the receptor complex—the inside “tails”—by a reciprocal on-off relationship between the remaining two proteins: CheR (a methyl transferase that adds a methyl group) to the tail and CheB (a methyl esterase that removes it). This pattern is virtually identical to and directly linked with the faster phosphorylation switching for stop/go behavior (as depicted in Figure 4) and thus provides a record of the specific responses to environmental changes. (Indeed, as phosphorylation of Ch A increases, the methylation activity of CheB correspondingly decreases.)
However, unlike the faster dynamics, this adaptive homeostatic (negative feedback) response occurs after existing sensory thresholds have been breached (or saturation has occurred), settling the system into a new normal rather than simply returning to the original set point. Hence, this modulation-by-methylation allows the system to reset its equilibrium to zero, even while the chemoeffectors are still present, but at a new higher or lower equilibrium point—altering receptor sensitivity and adding overall complexity to the system. (This threshold shift can be envisioned by imagining the starting point on Figure 4 to have begun either above or below the existing threshold, rather than within as depicted, where the “On” or “Off” response settles the system into a relatively upward or downward new normal; and will also be depicted in Figure 7.) In terms of function, as one molecular biologist put it, this allows the bug to tune the “volume” of its sensory system up or down164; or as Powers put it, how the feedback process “controls perception.”118
Figure 7.
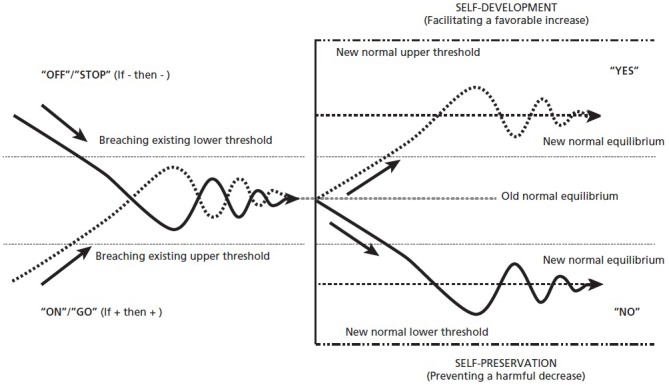
How the Tit-for-tat code serves dual self-regulatory "purposes": self-development and self-preservation.
In sum, the reciprocal feedback relationship between the phosphorylation and the methylation signaling pathways yields the causal circular connectivity between multiple levels of organization, with its temporal pattern of fast activation and slow deactivation delivering the best “noise attenuation,”165 bringing us full circle to the vertical tunability that synchronizes cells in multi-cellular organisms. Indeed, this methylation-adaptation process is the key “stimulus-response” relationship in our new story, as its corrective action kicks in with threshold-breaching, globally significant stimulus—whenever novel, intense, and deeply “self-relevant” changes are underway.
The Tit-for-tat Self-regulatory Code
Best of all, it comes freighted with its own evaluative logic. The positive feedback increases or decreases in methylation of the protein receptor complex (the chemical marks on the inside tails) offer an exact reflection of the stop and go behavior and its direct correlation with the harmful or beneficial environmental conditions. They provide a faithful signal of how previous behavior said “yes” to certain environmental conditions and “no” to others. (They provide Haugeland's third criteria for a mindful internal representation, a more general representational scheme—a code that can reflect a variety of related stimuli.154
Indeed, the upward going (positive, +) stimulus represents “goodies” that promote metabolic flow and developmental growth, while the downward (negative, −) decreases, signal “baddies” that could threaten structural stability. Together they offer the bacterium a single—yet binary—evaluative symbol, one that represents everything of life-giving importance from the presence of food and toxins, to temperature shifts, changes in oxygen levels or ph balance,167,168 to the constant energy flux and flows of electromagnetic fields on nanoscales in space and time169—which inform the digital approach/avoid behaviors of chemotaxis, thermotaxis, aerotaxis, osmotaxis, and phototaxis, respectively.161 In fact, given its origins in electromagnetic forces and thermodynamic laws, it offers a general searching and learning strategy dubbed “infotaxis” for balancing the needs to explore and exploit the immediate environment, a way of zeroing in on information that “accumulates as entropy decreases,”170 not unlike a child's game of Hot Beans (“you are getting warmer, you are getting colder”). In short, the functional effect of this chemical network is that a formerly neutral on/off switch can be bootstrapped into holding general good/bad—“for me”—evaluative significance.
Although these elegant feedback control networks are based on simple diffusion and stochastic (statistically random) chemical fluctuations, they set the evolutionary stage for genuine self-regulatory sentience to emerge. Indeed, tremendous selective pressure would be placed upon any mutation allowing the organism to distinguish between these two binary stimuli and respond in ways that help them along. In fact, such ability is required in any control model of behavior, as it would constitute both the comparison process and perception of the error signal itself.
Herein lies the logic of what I call the tit-for-tat self regulatory code within the hedonic valence of emotion. All that was required at this historical juncture was an additional positive feedback loop, one that could offer a further feed-forward enhancement of the existing signaling pathway, one that allowed a choice-making switch between the yes/no options, before the negative feedback rebalancing had occurred. In fact, this is the missing link required to bridge the gulf to self-regulatory (goal seeking) behavior in humans, as well as the conceptual heart of genuine “cognitive” perception.
Indeed, a feed-forward control process can act in anticipation of stimulus conditions,171 drawing upon the on-line memory embodied in the ebb and flow of sensory adaptation. This flexible choice-making response would indeed facilitate the optimal sorts of changes that have happened in the past, and could readily be accomplished by a binary switch between the positive or negative feedback responses themselves. Centrally, this new story suggests that something like this must have occurred, giving rise to the binary computational algorithm inherent within the feedback comparator: a straightforward if-then logical rule within the self-regulatory sense. Elegant in its simplicity, the rule states: If positive (+) then positive (+), if negative (−) then negative (−). In other words, for a positive stimulus signal (more and more), perform a positive feedback (more and more amplifying) response. For a negative stimulus signal (less and less), perform a negative, stabilizing response that reverses the present trend (Figure 5).
Figure 5.
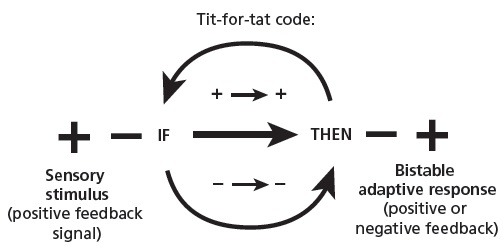
The Tit-For-tat self-regulatory code.
Following this simple tit-for-tat self-regulatory perceptual logic allows the organism to approach, facilitate, and otherwise increase the in-forming conditions that are life-promoting, and to avoid, prevent or otherwise decrease harmful, entropic changes. Likewise, with the automatic nature of the adaptive process, this simple code provides the classical semantic symbols, the innately reinforcing—rewarding or punishing—“unconditioned” Pavlovian responses that undergird both classical and operant conditioned learning. Indeed, the fundamental hedonic perception provides the elusive “basement language” that philosophers have long sought, reliable knowledge about the external world rooted in primal sensory experience.172 In short, the self-regulatory code unites the stimulus-response phenomena noted within the behaviorist tradition with the cybernetic control models of human behavior. As depicted in Figure 6, the self-regulatory code elucidates the inner workings of the black box (what goes on between the input stimulus and output response); clarifying the relationship between Powers' “sense,” “reference” and “error” signals89; and bridging cleanly to Carver and Scheier's origins of affect.38,39 (Offering, however, the more intuitive self-relevant logic of hedonism, wherein negative feedback is associated with pain and avoidant behavior rather than with pleasure and approach.)
Figure 6.
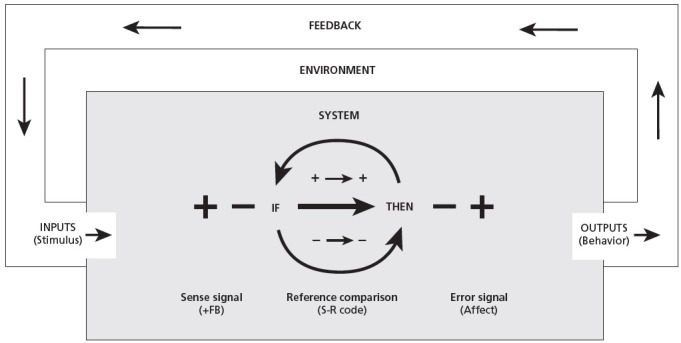
The Self-regulatory code in the black control box.
In our little E coli, however, it matters not whether any subjective experience of the positive feedback signal is present, for the negative feedback response—the automatic adaptation—has already had an important self-regulatory effect.129 The adaptation has shifted the system to a higher or a lower equilibrium point (the new normal), rather than returning it to the formerly favorable state, and in perfect accordance with the harmful or beneficial environmental stimulus. In doing so, it has accomplished either an optimizing, developmental, adaptation—saying “yes” to beneficial changes—or a self-preservationary intervention, saying “no” to potentially self-destructive harms.
Depicted, for example, in Figure 7, is essentially the “on/off” response process shown previously (in Figure 4), and in Figure 7 is that same response but one following a breach ofeither threshold yielding the “yes/no” evaluation. (Herein lies the roots of the hedonic treadmill,173 wherein sensory adaptations to good stuff become internalized such that new levels of stimulus are required to trigger positive self-relevance.) But regardless of any possible perceptual accouterments, in even the very earliest forms of life, these simple chemical regulatory feedback networks have cracked the philosophical door between determinism and compatible free will, between hardwired logos and softwired telos, ushering behavioral agency with a few degrees of freedom—allowing the organism an active role in the evolutionary process.
Individual and Social Aspects of Self
In fact, and perhaps even more philosophically intriguing, this simple self-regulatory system also sets the stage to define individual and social aspects of the selfsystem. While the cellular membrane initially demarks self from the not-self environment, this simple yes/no rule can also be pressed into service to identify genetically similar and different bacterial species, in perhaps the earliest forms of cooperative communalism and competitive tribalism. For example, the phenomenon of “quorum sensing” where on/off switching between behavioral modes depends upon the concentration of other citizens within a specific bacterial species.174
Indeed, in addition to pre-existing environmental stimuli, quorum sensing bacteria produce and release self-identifying autoinducers, chemical signal molecules that then rise and fall with the local cell-population density. They are used for communication, allowing individuals to synchronize particular behaviors so they can function as multicellular organisms, marshalling cooperative chemical defenses—or virulent attacks—against other species.175 Likewise, these either/or (me or we, us or them) signals, can be coupled to other sensory stimuli like heat or cold to guide more complex autonomous or communal behavior. For example, an individual E. coli bacterium will normally thermotax toward warm environments where growth conditions are optimal. But should the population become overly dense and therefore resources strained, loner—self-preservationary—mode will kick in and the bug will move toward cooler locations 166 to “chill out” until conditions for growth improve.164 Likewise, is this dual sense of selfidentity in the elegant slime mold Dictyostelium discoideum, that can exist either as a single-celled organism or as a colony of social amoebas—a eukaryote with the same cAMP-sensing toolkit as humans, rooted in two varieties of the ancient 7TM receptor.133
A central insight from this level of analysis is that a core, physical, sense of identity (both personal and social) is already apparent in the lowly bacterium, founded upon simple protein networks and their integral feedback dynamics. Hence, this first form of self-regulatory sentience also cracks the philosophical door to phenomenal being (and becoming) in time and space as well as doing behavior.
Nonetheless, first and foremost, the present proposal is that these ancient self-regulatory mechanisms have been honed by natural selection to yield the chemical—hard-wired (genetic)—distinction between self and not-self utilized by the immune system, as well as the chemical language of the paracrine and endocrine systems,176 and to subserve the neuropeptides involved in neural communication in both enteric177 and central nervous systems—those deemed the “molecules of emotion.”178 In fact, they provide the informational “language”179 that allows optimal cellular differentiation and space/time migration of the right types of cells to the right places at the right times throughout embryonic development. But in addition to this physiological legacy, in humans, the ongoing development and empathic expansion of one's mindful, social, and cultural sense of identity180 is also crucial to an optimal developmental trajectory, and key to decoding the universal guidance offered by our emotional sensory perceptions.
Purpose in Evolution?
This brings us to the fundamentally significant binary dichotomy gestured toward previously, that which lies at the most primordial core of nature's value system. This is the prime self-regulatory directive that has been conserved, kept intact throughout our evolutionary history; the one that allows organisms to actively participate in natural selection; and the one that provides the evaluative meaning within the hedonic valance of emotion. As already depicted in Figure 7, the yes/no binary evaluations mediate dual teleological goal states—purposes, if you will: Those of self-development, the core evaluative appraisal for categorically pleasurable “positive” emotions, and selfpreservation, for the painful or “negative” category. These are the binary functional outcomes of the ancient self-regulatory process, those that make hedonic behavior “optimal” or “right” in the deepest, most biologically valid, sense of the word (moral implications notwithstanding).
Although potentially oppositional purposes, it is crucial to note that these are two right and good, perhaps non-negotiable requirements for life itself inherent within the most primordial regulatory processes. Each is equally appropriate at different times and spaces, and optimal under different environmental circumstances. These are the underlying goals states, the teleological purposes, glimpsed by the early cyberneticists; later described by pioneering systems psychologists as preparatory (preserving the original set point) and participatory adaptation to the new,181 and are now described as the dual regulatory “focuses” within complex human self-regulation.60 These binary purposes are also what complexity scientist's might call selforganizing “attractors” on “fitness landscapes,”182 those that keep creatures poised between chaotic change and rigid stability; and those that are reflected in the digital “growth or protection” programs of cells.183,184 Best of all, these dual purposes provide a direct biophysical tether between subjectively good and bad perceptions and objectively right and wrong states of living-giving balance.
This is how acknowledging bottom-up self-regulatory sensory feedback can fill a sizable gap in evolutionary theory—for these dual purposes are simply mirror reflections of the top-down criteria for natural selection: adaptation and survival.185 Yet, until recently, these present moment stimulus-response behavioral adaptations were considered evolutionarily irrelevant, the functional role of the cell membrane largely unnoticed, with causal genetic control credited to the nucleus (the DNA) alone. Upon the mapping of the genome, however, the subsequent revelations about epigenetic control processes have forever altered the central dogma by elucidating the crucial role of environmental cues, intrinsic signals, and cellular memory in evolution.186–188 Revelations of how supposedly “junk DNA” and noncoding RNA are actually providing ongoing regulatory switching189,190; with relational if-then rules of engagement that ensure specific gene products are brought into action when and only when appropriate,191 and mediating the very developmental morphology of an organism192 as well as its behavior. Revelations of how epigenetic switching yields critical modifications during cellular stress responses,100,193–196 plays a key role in immune functioning,197 and serves as modulators of neuronal responses,198 of neural development and neuroplasticity.199–202 Revelations of how our old friend the methylation marking process, sets down tracks on the histone cores of DNA, yielding heritable memory systems in non-germline cellular replication203; marks that appear to be bidirectional (“poised”) bistable switches themselves204,205 with both bi-directionality and reversibility of DNA methylation crucial to optimal neurodevelopment,206 discoveries that help explain the mysterious phenotypic variations between monozygotic twins207 and highlight the importance of individual differences in behavior, cognition, physiology208—and emotionality.209,210 Indeed, the new field of neuroepigenetics is rapidly evolving, finding disordered methylation markings to be associated with autism, schizophrenia, bipolar, and degenerative disorders.211,212
In sum, the discovery of epigenetic regulatory mechanisms is expanding and reframing the reactive “selfish gene” scenario,213 to a more Lamarckian proactive, fluid, and self-regulating genome, now recognized to be in constant cyclic interaction with the immediate environment, and adaptively switching specific genes on or off in response to ever-changing ecological circumstances. (Of course, these include social environments and the relational components of self-regulation, as evidenced in such emerging fields as “social genomics,”193 “stress genomics,”194 and “social neuroscience.”214) Acknowledging these bottom-up dynamics honors the generative, developmental, symbiotic and cooperative underpinnings within and between living systems and partially deflates the purely competitive, random, blind, meaningless, and glacially slow depiction of evolution. Indeed, as Charles Darwin himself once suggested (in a letter to Nathaniel Wallich, 1881), selection might be ‘the consequence of a much more general law of nature’94—to which I would add: That of the binary computational laws of self-regulatory feedback.
FROM BRANES TO BRAINS AND THE MODERN FEEDBACK CYCLE
These new micro-biological lenses can liberate social scientists from limited evolutionary narratives that look only to conditions of the ancient ancestral environment to elucidate the genetic components of adaptive behavior. Indeed, the “iterated systems” and “algorithms that govern emotional states” in the here- and-now are anything but “irrelevant.”215 They serve as the very self-regulatory core of adaptation itself. In fact, the original molecular sensory organs of the emotional sense (receptor clusters on cellular membranes) remain hard at work regulating each cell of every specialization within its immediate intracellular environment. While the second messengers—and third, and fourth … from phosphates and kinases to neuropeptides and hormones—have become ever-more complex, their original binary computational processes generate the electrical, chemical, and cellular “rhythms”216—the cyclic feedback at every level of scale that delivers self-regulatory “coherence.”217 Examples from the human “receptome”133 include the G-protein-coupled receptors (the largest family of proteins in the human genome218 that mediate responses to hormones and neurotransmitters as well as facilitate vision, olphaction, and taste219; the IP3 receptor (Inositol Thriphosphate receptor) a calcium release channel that switches between open and closed conformations, generating calcium oscillations that in turn regulate periodic hormone secretions220; the β 2 adrenergic receptor that regulates cardiovascular and pulmonary function221; the Syk family of kinases that turn immunoreceptors on or off, and the Src kinases that can “turn up or turn down immune cell signaling responses”222; and T cell antigen receptor complexes that tune immune responses to match the level of the threat223—in the classic homeostatic arrangement.
Nonetheless, the ‘sensory organ’ of emotion now has many additional structural components, from the original membrane receptors and networks of molecules to specialized nodes and networks of neurons (sensory, motor, excitatory, inhibitory, interneurons, etc), and the topological architecture of the human brain.
Dendritic Computations via Feedback
Moreover, the feedback arrangement, with its fractal self-similarity, computational logos and three step cycle (compare, signal, self-correct) is also readily apparent in the structure and function of individual neurons as well.224,225 Indeed, the dendritic spines of pyramidal nerve cells have been discovered to serve as computational building blocks that are fundamental to synaptic plasticity, a discovery with “revolutionary implications for neuroscience.”226 For contrary to Cajal's original notion that action potentials only flow one way (dendrites to soma to axons), it has become clear that they also “backpropagate” in the reverse direction (soma to dendrites). These formerly unacknowledged dendritic computations allow the neuron to sum up synaptic inputs, “compare” that sum against a threshold, and “decide” whether to initiate an action potential, to “operate as a device where analog computations are at some decision point transformed into a digital output signal.”227 We see yet again the ubiquitous binary logos, the pattern of yes/no increases and decreases in synaptic weights to positive and negative exemplars224 and in the reciprocally local and global computations.
Furthermore, the intriguing fact that dendritic spines are suspiciously homologous in size, structure, and chemosensory function to bacteria—a possible ancient symbiont a la mitochondria—has not gone unnoticed.228 In fact, dendritic spines appear to be a morphological link between the early cell receptor complexes and specialized excitable cells—neurons; their dynamic structure and shape-shifting behavior echoing and expanding upon the electrical properties of branes, not mentioned above. For even the E coli has both ligand and voltage gated ion channel receptors, with membrane potential a major component of the driving force for membrane transport and flagellar motion—the energy required to power metabolism and any movement at all. Indeed, voltage spiking has recently been observed in the E coli, with on/off “blinking” associated with aerobic respiration and the stress response.229 Likewise the dynamic growth and shrinkage of the spines themselves follows the same pattern of regulatory increases and decreases (of specialized glutamate receptors) associated with long-term potentiation and damping, correlating with synaptic plasticity, the “self-modifying” cognitive processes that give rise to memory, emotion and executive function230- core elements of human consciousness. Indeed, spine plasticity itself responds to life experience including fear conditioning231, and intriguingly - as with the aforementioned epigenetic methylation marks - altered or disordered spine dynamics, morphology or density, are associated with psychiatric diseases and neurological degeneration.232
In fact, in the 1990s, neurobiologists discovered additional discrete structures on neural membranes known as “microdomains,” little rafts that perform computations and regulate ion-channel dynamics—if not the action potential itself.233 These microdomains, as further complexifications of the multiple domains on the ancient receptor toolkit, self-assemble in clusters with haunting similarity to the membrane receptor clusters on the E. coli, and play a significant role in the assembly of other receptor proteins as well. Indeed, enriched in cholesterol and sphingomyelin, microdomains can be likened to larger, fancier ‘heads’ on the topside of the membrane, those that allow the specialized neuron to function as a series ofswitches, beyond the simple circuitry of other cell types. Whether evolutionarily homologous or not, however, the circular causality and self-similar pattern of signaling are unmistakable, with dendrites to neurons, neurons to neural networks, and neural networks to sensory perceptions each making unique contributions to the ongoing interactive computational process.
The three Functional Loops in the Tri-level Brain
Even without the added discoveries of microdomains and dendritic computation, even the more conservative (“cognitive”) neuroscientists have identified both the top-down (efferent) and bottom-up (afferent) neuroanatomical pathways of emotional sensory perception; converging in the limbic sensorimotor cortices, and complete with frontal-lobe hemispheric lateralization of positive and negative affect in approach and avoid behavior, respectively.234 Even those disputing the natural kind view of emotion,235 acknowledge that affect is synonymous with somatosensory perception of both external stimulus and internal responses.236 And perhaps even the most neurocentric explanations of emotional experience can soften in light of the fact that the very development of nerve cells, particularly interneurons of the prelimbic cortices—a hallmark of complex brains of every variety—is contingent upon optimal immune signaling in response to distress and early deprivation.237
Indeed, since this ancient regulatory pattern is so fundamental, the three main steps in the feedback cycle are reflected in globally complex nested loops of the triune brain, each integrating particular emotion and appraisal processes.238 These include a “motivated monitoring loop” (linking the dorso-lateral prefrontal cortex (DLPFC), the anterior cingulate cortex (ACC), hippocampus (HPC), amygdala (AM), orbitofrontal cortex (OFC) and the brain stem/basal forebrain (BS/BFB); the “motivated object evaluation loop” (linking the OFC, AM, and BS/ BFB with the sensory cortices); and the “motivated action loop” (between the OFC, AM, nucleus accumbens (NAS), ventral pallidum (VP), the ventral tegmental area (VTA) and the thalamus; where, respectively, the ongoing comparisons, signaling and the corrective actions occur.
In fact, if defining an emotional “sensory organ” in terms of neural structures, the amygdala is present in all three loops,239–41 and is instrumental in signaling the novelty 242 and uncertainty243 of self-relevant34 environmental stimulus. Likewise, would be the ACC, “the receptive organ of the experience of emotion,”244 with special clusters of P-type (positive) and N-type (negative) neurons in the primate pregenual (pACC) that are respectively “sensitive to positive and negative motivational states.”245 Together, the AM and pACC serve as exact functional analogs to the on/off (occupied ‘heads’) and yes/no (methylated ‘tails’) of the sensory receptor clusters in the E coli bacterium. Of course, as we have seen, the self-regulatory sensory network begins in the “branes” of all cells, including the skin cells that still bound and contain the human system—hence the classic Galvanic Skin Response (GSR) measure of emotional arousal as well as the emotive component of social touch.246
Likewise, the coupling between positive and negative feedback is evident in the reciprocal, bi-directional, interactions between the right and left hemispheres of the brain,247 between the brain and heart, and between the sympathetic and parasympathetic branches of the autonomic nervous system. Indeed, the vagal nerve mediates bottom-up emotional sensitivity (high stress “reactivity”) as well as top-down emotion regulation (faster recovery), both of which are associated with high vagal tone.248–250 In fact, the polyvagal theory,251 picks up the story of the evolution of emotion, setting forth the phylogenic shift in regulatory mechanisms through three global stages that gave rise to the “primary” emotions of our “social nervous system.”252 As mentioned, Jaak Panksepp151 has mapped “the affective brain” across species, and the bottom up emotional regulatory path characterizes the “default mode network” in humans (medial parietal/posterior cingulate, medial prefrontal, lateral inferior parietal and superior temporal cortices), specific to empathy and “social tasks” versus those that manipulate inanimate objects.253
The Modern Human Feedback Cycle
All told, over our evolutionary history, natural selection has expanded the self-regulatory feedback cycle from its original two-step stimulus response loop to a five step interactive process between mind and body and world. At present, the cycle contains three cognitive feed-forward (top-down) add-ons cobbled upon, yet constantly interacting with the ancient (bottom-up) subconscious autopilot system. Indeed, these sequential steps set forth the temporal order required to elucidate the specific distinctions between the basic and complex categories of emotional perceptions (as set forth in Figure 2, and elaborated shortly). For now, it is crucial to note that this cycle constitutes the mind-body-world interface, and that the linear flow of direct human experience constantly cycles through its five sequential components. It is an ongoing process wherein mind and body each play a unique self-regulatory role, but are elegantly unified, connected and in-formed by the emotional sense at every juncture; a process that yields ongoing trial and error feedback during “self-relevant” moments, intrapersonal feedback that is instrumental in evaluative/embodied cognition, memory formation, adaptive learning and behavioral motivation. More generally, to whatever degree nature has afforded the human being a mind with genuine “free will”; such volitional behavioral control is undergirded and constrained by the body's foundationally causal self-regulatory feedback dynamics - reliably delivered by the everpresent emotional sense.
Loosely, the first three steps can be described as conscious intentional motives, volitional actions, and perceived outcomes—all of which inevitably contain perceptual filters, and cognitive biases254–256 unique to one's individual socio-cultural developmental history (Figure 8). Fortunately, they are kept in check by steps four and five, the original yes/no evaluative perception and the approach/avoid behavioral correction from whence they emerged. This general five step temporal sequence was aptly captured in James Gross' process model of emotion regulation, with his “antecedent focused” coping capturing the first three feed-forward steps of the modern cycle, and his “response focused”257 regulation capturing the last two—the original here and now body-in-world sensory-motor feedback loop.
Figure 8.
Modern feedback cycle with feed-forward cognitive elaborations and complex feelings.
However, I would emphasize the crucial link between steps three and four, wherein the salient selfrelevant comparison now takes place—a vital comparison between how the mind perceives an unfolding event against the body's actual outcome. This might well be accomplished by Lewis'“motivated monitoring loop,”238 which then triggers the primordial affective feedback signal in order to keep things biophysically real, hence, my call to rethink the value of suppressive forms of emotion regulation. Indeed, in this new view, our binary feel-good/feel-bad hedonic feelings remain the conscious mind's only valid informational tether to the biophysically optimal/deficit conditions required for life itself, and an innate safeguard against its more volitional—yet potentially dishonest258—rationalizations and hypocrisies.259 Instantly, they offer both a reality check and a behavioral fix—concordant with the ancient self-regulatory imperatives. Their elegant stimulus-response mechanics moves us to actively avoid self-destruction and create evolutionary selfdevelopment, and their simple tit-for-tat logic constantly reminds us of these dual universal purposes.
Moreover, suppression does not work. Suppressive emotion regulation actually increases the bottom-up activation of the error signal.260 Likewise, whether or not the informational component of the emotional message is deliberatively and rationally incorporated into the cognitive schemata (building conscious, reasoned motives), the coupled corrective response will simply forge them into the mindscape via Pavlovian conditioning (perhaps through Lewis' “motivated action loop,”238) yielding the subconscious variety of motives propounded by Freud—those instinctive libidinous drivers that run roughshod over our higher rational intentions.
The key point is that this five-step cycle depicts a fundamental temporal sequence that is prerequisite for the many facets of the self-regulatory emotional elephant to come into view. It conceptually reunites “the self” as a functional whole, bridging the gap left by the Cartesian severance of mind from body and the many illusory divides, judgments and assumptions that would follow. Particularly those that privileged reason over emotion, and conscious and intentional processes over intuitive, embodied cognitions and “naive” sensory perceptions. Indeed, the sensory feedback model resolves many time honored controversies in emotion theory: Reconciling William James' original insights about the bottom-up causal components with Cannon's top down; Lazarus' subsequent emphasis on cognitive appraisal295 despite Zajonc's primacy of affect.25 It unites the dimensional261 with the discrete models of emotion, and the feedback dynamics offers the bridging rules262 that reconcile the materialist, behaviorist, identity and functional approaches to subjective emotional experience.263 It honors Joseph LeDoux's distinction between cognitive computations and affective—self-relevant— computations,34 and his low road/high road dual but interactive emotional processing paths in the brain, those that validate Bernard Weiner's “attributional”264 linking of motivation and emotion.
Perhaps most importantly, it elucidates how core affect.12 basic emotions,265 and complex (socially constructed) feeling perceptions 266 all dovetail together in exquisite functional elegance within the modern day emotional sensory system (as previously depicted in Figure 2).
DECODING HUMAN EMOTIONAL MESSAGES
Key to understanding emotion as a sensory system is that emotional perceptions deliver self-regulatory messages from the self (the body) to the self (the mind) about the well-being whole self. Once we can frame these messages within the context of the ongoing feedback cycle and decipher their specific meaning, the emotional sense offers nothing less than a personal guidance system. Hence, in this section I will set forth more detail about the three levels of information encoded in human emotional perceptions, how they unite the various components of the self, and how they relate to the temporal sequence of the modern-day five step feedback cycle (depicted in Figure 8). Doing so will clarify muddy linguistic conventions with more precise terminology.
Hedonic Valence (Affective Evaluation)
The first level of meaning concerns the term affect, which I will henceforth subsume within, after distinguishing from, the hedonic valence of emotion. Indeed, in the literature, “affect” concerns only valence and arousal intensity,261 and omits the motivational behavioral dimension. The key insight of this model, of course, is that the binary valence is born of behavioral regulation and rooted in positive and negative feedback processes. In fact, the evaluative message it bears is not only meaningful for optimal self-regulation (maintaining “emotional equilibrium”267 and “regulatory fit,”60) but one fundamental to the process of evolution itself. The valance provides subjectively positive or negative “qualia” as an informational lynchpin between an organism's biophysical well-being and the criteria of natural selection. In short, valence speaks of natural—universal and nonnegotiable—biovalues that concern the optimal conditions for life itself.
From the perspective of human experience, the bottom-up primary evaluation encoded within affect268 is the perceptual error signal, directing attention toward self-relevant events and placing them in the context of the dual self-regulatory purposes, with “goodies” signaling opportunities for adaptive selfdevelopment and “baddies” for corrective self-preservation. This is the ultimate, long-term evolutionary meaning associated with affect.
Indeed, in terms of epigenetics and immune functioning, core affect relies upon the ancient evaluative yes-no logic of cellular signaling, synthesizing the myriad voices of the cells, organs, and organ systems, into the symphonic “interoceptive”234 wisdom of the entire organism. This model, however, tethers the identityrelevant hedonic wisdom deeper still in the self/not-self logic of autopoietic self-making, bridging to the self/ not-self distinction of the immune system and its salient distress and eustress signals - with the circular closure delivering a bidirectional communication flow. In fact, the top down manifestations of the emotional sense are likely involved in placebo and nocebo effects269–272 providing a direct inroad to our physical health.
At the more proximate level of meaning, affect still concerns optimal behavioral movement, providing immediate feedback about the state of the body in present time and space, the original right-here-right-now signaling of good or bad events as they are unfolding, and triggering hedonic approach or avoidance—the tit-for-tat logic of increasing the stimulus, or moving toward the goodies in the immediate environment, and decreasing, or moving away from the local baddies. This original, primal, function of affect is represented by the last two steps of the modern feedback cycle, limited in terms of conscious experience to the somatic46 and visceral151 perceptions, “attentional attitudes,”9 and “gut feelings.”273 These are also the time-urgent “hot nodes” of emotional perception,274,275 those that signal the dissonance long thought to be “cognitive.”276 Nonetheless, natural selection has conserved the original stimulus-response pairing, and affect is implicit in every step of the feedback cycle.
Indeed, whether or not we are aware of any sensations of pleasure and pain, primal affect also delivers the subconscious, automatic, aspects of emotional perception, regulating purposeful approach/avoid behavior even if the mind remains out of the loop. This includes, of course, all conditioned learning, and the nonconscious aspects of motivation and self-regulation: implicit volition,277 implicit intention,278 or automated will.279 Hence, the ubiquity, primacy, immediacy, and classical conditioning power of affect,280,281 and such mysterious manifestations as the “present bias preferences,”282 the anchoring and availability heuristics,254 the projection bias256; the confirmatory bias,283 and a host of other “wild,”284 “irrational,”285,286 influences that have long bedeviled the rationalist model of economic decision making.287,288 Of course, they also reflect the ancient embodied wisdom, and manifest as the automatic, subconscious aspects of decision making that are intuitively advantageous289—for they faithfully reflect the ancient self-regulatory code.
Basic Emotions
While this deeper relational and functional significance of pleasure and pain has gone largely unrecognized, evolution has forged the basic emotions (and all complex blends and shades) upon their ever-present self-regulatory base. As such, the “natural kinds” basic or primary265,291–293 emotions also deliver in-the-moment, bottom-up, feedback signals with universal symbolic meanings—yet with an added layer of specificity within their common appraisal themes.5,294 Here an important distinction is made between the efferent, top-down, cognitive appraisal295 and the afferent, bottom-up affective evaluation,268 the former involving more complex prefrontal and linguistic processing; yet emphasizing also that both serve equally important functional roles in the emotional system.
While controversy remains over which emotions are basic,296 based upon their temporal (feedback) significance, this model suggests joy, sadness, disgust, fear and anger to be the best contenders for the mantle of universal self-regulatory perceptions. These basic emotions are relatively more hardwired, unfolding over the first 6 months of infant development,297,298 with their common appraisal themes delivering more specific information299 about basic life-giving requirements—“hedonic needs”300—and how to fulfill them in the immediate environment. The “how to” part is the additional informational component, involving conditioned or conscious cognitive schemata forged through the feedback cycle over time, yet the hedonic requirements and behaviors remain the same.
Indeed, like primary colors, their common appraisal themes carry specific information about innate physiological as well as psychosocial needs.79,83 (“Needs” in this context reflect any biologically hardwired urges, drives, values or “specialized modes of organismic operation that match evolutionarily recurrent situations.”215) For example, basic joy with its “Yes!-Go!-Good-for-me!” message, pulls us to discover and honor these basement needs and reinforces novel strategies for meeting them, driving optimal developmental adaptations and fostering creative cultural and environmental enhancements. Although research on needs is scant, this model suggests the “hierarchical” nature of needs relates directly to the dual self-regulatory purposes, with the top priority negative emotions (self-preserving: sadness, disgust, fear, and anger) largely honoring the non-negotiable thermodynamic and metabolic needs—with the autonomous agency, the freedom and empowerment as well as the physical and social safety required to fulfill them.
In fact, it is important to note that four out the five basic emotions are of negative valence—the painful distress signals, and their urgent “No!-Stop!-Bad-for-me” self-preservationary message. Indeed, bad is stronger than good,43,301 perhaps the reason why evolutionary theory acknowledged only the self-preservationary imperative, and psychology emphasized the dysfunctional aspects of the human condition.302 But through this new lens, the predominance of basic negative emotion is because nature gifts us with nearly four times as much specific, universal, information about how to correctively preserve the body in the world.
For instance, the appraisal themes of the four basic negative emotions—loss (sadness), imminent danger (fear), contamination (disgust), and disempowering obstacles to agency or social violations (anger)—move us to either change the immediate environmental circumstances or alter our location, to “fight or take flight.” To which I would add: to make right—a catchall term I offer to categorize any sort of adaptive, creative problemsolving response to emotional distress, born of the self-developmental imperative and the approach mode of behavior. Right, in this context, is also healthy.
Instead of suppression or behavioral avoidance, a Right Response (RR) is one that involves an active, adaptive, rebalancing of the ecologically optimal (biophysically favorable) relational state between the organism and the environment. The RR has been captured in the stress literature as problem-focused coping,303,304 or transformational coping,305 as perhaps the most adaptive way of reducing the psychophysiological arousal tension.306 This happens in one of two ways: It can involve an active adaptation of the immediate external environment, which is essentially creative action or “work,” the way we build social and economic capital307 and the way we accomplish cultural evolution.
For example, when sad, finding or creating replacements for what has just been lost; when disgusted, finding more wholesome comestibles or creating hygienic conditions; when afraid, finding protective shelter, connectivity and community; or when angry removing the obstacles to one's requisite self-regulatory agency, ensuring balanced interactions, and repairing social connections. Indeed, in terms of interpersonal conflict, all acts of nonverbal and linguistic communication are RRs, reducing basic pain with an outside change without running away or resorting to fisticuffs. In fact, “doing good,” helping to resolve the distress of others, is in and of itself a buffer against “feeling bad.”308
In short, basic emotions offer universal meaning in the present moment and under the specific conditions of the immediate environment, information about the health and well-being of the body—any body and everybody. As such they are represented by the last two steps of the modern feedback cycle. They remain feedback signals, and are only fed-forward to the degree that they combine and blend into the complex emotional perceptions. Nonetheless, they move us to fight, take flight or correctively “right” the external environmental stimulus conditions.
Complex Feelings
For, in contrast, as secondary blends and shades of the primary emotions, the complex feeling perceptions enfold the remembered past and the imagined or expected future, bearing self-regulatory messages about the mind. They are highly personalized, delivering specific guidance tailored to the individual's particular cultural immersions, private life experiences, and unique history. As defined herein, they are to be found exclusively in the first three steps of the modern feedback cycle.
Complex feelings include trust, mistrust, courage, anxiety, pride, shame, gratitude, contempt, compassion, guilt, admiration, envy, hope, worry, devotion, rage, love, hate, curiosity, honor, faith, etc., and have a clear developmental trajectory (emerging between ages two and eight,112 if not fully complete by age three.298) They largely serve the self-developmental imperative, and are goal-relevant to the “higher” human needs—needs for enduring social bonds, for self-esteem, for creativity, and long term meaning. They are the result of many self-constructing309 repetitions through the feedback cycle, the basic themes having been elaborated upon by language, individual learning experiences, self-identifying concepts, and sociocultural schemata (cognitive structures—knowledge, beliefs, rules, habits, rituals, traditions and in-group norms, obtained from one's foster environment).
With the notable exception of rage (a perfect storm of belief driven blame, positive feedback amplification, and basic anger) the complex emotions are the less time urgent, “cold nodes” of emotion,274,275 yet still carry the ancient hedonic logos within them. Indeed, as depicted in Figure 8, these complex feeling perceptions are the more long-term feed-forward causal components of behavior,79 reflecting both conscious and habitual motives, judgments, and appraisals that still carry their original emotional valence as “emotion schemas”290 in attitudes310,311; moods,312,313 and even personality traits.314 In short, the complex feeling perceptions deliver self-regulatory information about the mind, feeding forward an ongoing stream of evaluative commentary about its optimal or dysfunctional holdings, habits, and the uses and abuses of the rational intellect.
Indeed, complex feelings prescribe the second kind of optimal RR, which is affected to the internal environment, the personal mindscape, in acts of conscious knowledge acquisition, deliberate learning, personal growth, or mindful self-regulation—offering a personally accountable answer to the distress call (oftentimes despite the external circumstances). This internal variety of RR also includes building and invoking optimal belief structures to reappraise260,315 or temporarily endure a challenging or uncontrollable situation; or to bear an injustice, setback, or misfortune with relative grace and “resilience.”316 This internal RR is also known as “self-control,”317 “self-discipline,”318 or when habitual, “grit,”319 all of which describe the ability to endure short-term pain in order to cultivate long-term, complex—highly meaningful—pleasure (captured by the body builder's lament “no pain no gain.”) While there are many implications beyond this introductory scope, suffice it to say that the information offered by the complex emotions harbors a vast reservoir of biological—indeed moral—wisdom yet to be tapped by humanity.
SUMMARY: EMOTION AS SELF-REGULATORY FEEDBACK
In sum, the self-regulatory feedback model proposes that there are three levels of self-regulatory information encoded within each human feeling perception—information that sub-serves the self-regulatory purposes of all living systems, as well as a suite of universal human needs, and the individually unique ways of meeting them that evolve over time. The model highlights the ongoing, cyclic, trial-and-error nature of learning and human development, with the confluence between the three levels coming into focus with the recognition that primordial affect serves as the “error” signals—both good and bad deviations from homodynamic states of balance. Balance within and between body and world, within and between mind and body, and ultimately within and between individuals comprising social bodies.
With the proposed additions, clarifications, and structural exemplars from cellular sensorimotor mechanisms, this model helps resolve criticisms of the control model of human behavior.79 Indeed, there has been abundant misunderstanding, misinterpretation, and a series of patterned “blunders”118 on the road to realizing the early cybernetic vision. This includes linguistic confusion across interdisciplinary literatures concerning the terms “positive” and “negative” feedback,320 often confounded with feed-forward loops or complex feedback circuits built from couplings of multiple positive or negative loops. Even the best models38–39 rely upon only one of the two types of feedback, confound internal and external locations in space, and vault to the complex level of human self-regulation with assumptions that inadvertently reverse the logic of the self-regulatory code.39
But with both the redemption and validation of regulatory feedback, this model also refines and builds upon the rich tradition of “consistency theories”321 wherein some stimulus event creates a deviation from a balanced cognitive state and affect plays a role in signaling or restoring that balance. Some examples include congruity theory,322 cognitive dissonance theory,62 balance theory,323 psycho-cybernetics,324 neuropsychological theory,325 self-discrepancy theory,326 homeostatic synaptic signaling,327 affect-balance mediation,328 regulatory focus theory,60 the original “logical calculus” in nervous activity,329 and even the “equilibrating” nature of development itself.330 As mentioned, all such models suggest a sort of psychological immune system61,331 that operates unconsciously, wherein intense hedonic states trigger a variety of processes designed to attenuate them. These range from the homeostatic processes that diminish their physiological impact332,333 to the defensive processes (ie, rationalization, self-serving bias, positive delusions, self-deception, etc.) that diminish their psychological impact13,315,331,334,335 in order to “protect the individual from an overdose of gloom.”61 To which I would add, can spell “doom” when misunderstood and left unanswered—threatening the very physical integrity of the individual.
IMPLICATIONS FOR PUBLIC HEALTH
Indeed, in this new view, common words such as “right” and “wrong” are biologically grounded in the physical requirements of the living organism—recognizing the original yes/no, feel good/feel bad, hedonic evaluation as physiological eustress and distress signals, and reframing certain elements of morality in the context of public health.
Right states of life-giving balance, right behavior and right-track development in this context, concern optimal biophysical functioning, self-regulatory responses, and mediation between the dual evolutionary purposes: preserving the body and adaptively developing the mind. “Wrong” behavior is that which repeatedly suppresses, denies, or otherwise fails to respond correctively to the emotional sensory information, with ongoing, unanswered, distress signals giving rise to a limited, unhealthy, and perhaps even self-destructive trajectory.
Fortunately, the simple hedonic code yields a singular and universal moral commandment of harm reduction, a blend of the Hippocratic oath and the Kantian categorical imperative: To simply reduce the external environmental conditions that elicit basic pains (the negative emotions), and increase those—in both the local landscape and personal mindscape—that foster the complex pleasures (the positive emotions).
EMOTION AND OPTIMAL DEVELOPMENT
In fact, evidence is mounting that the positive eustress signals offer far more than simple good feelings and short-term rewards: They “broaden and build”336 and “inspire and rewire”116 the mindscape and social landscape, expanding our empathic boundaries, moving us to bond with others, to “mend, tend, and befriend”337 and to “shift and persist”338 during formidable challenges. They even promote vibrant health,339,340 and longevity.341,342 They both signal novel developmental opportunities and reflect optimal self-regulation, the “self-control” that predicts health, wealth and even public safety.343 Indeed, born of the positive feedback dynamic, the positive emotions drive a self-perpetuating “upward spiral,”344 naturally punctuating an optimal physical, mental, and social developmental trajectory.
For example, in Erickson's stage model of psychosocial development, the first stage is marked by either trust versus mistrust in the first year of life; the second by “autonomy”345 (to which I would add: confidence in one's self-regulatory agency, curiosity, delight, zeal, and hope for life) versus shame or doubt (anxiety, worry); and the third stage by “initiative” (courage to tackle challenges, faith in oneself, mirth, affection for peers, and admiration of inspiring others, gratitude for caring support and mentorship, and compassion for suffering) versus guilt (to which I would add boredom, envy, greed, contempt and rage). Successful development of the right-track positive emotions all contribute to an integrated and meaningful sense of identity and a passionate humanitarian conscience by adolescence, as well as loving intimacy, generosity, and compassion in adulthood.346
This model suggests, however, that these perceptual milestones also reflect the fundamental epigenetic and immune regulatory processes and the structuring, pruning, tuning of neural circuitry and ongoing dendritic plasticity. It suggests that there may be critical opportunistic windows during the emergence of primary identity and self-regulatory agency, the formation of fundamental complex human capabilities347 and personalized psychological capacities348—timely processes which, if stymied, can yield compromised or detrimental developmental outcomes.349
For conversely, the prevalence and preponderance of the complex negative emotions implies a more compromised or deficient developmental trajectory, reflecting maladaptive schemata—“wrong” in that they are limiting if not self-destructive. Indeed, when basic sadness, fear, disgust, and anger are not allowed to their job, the causal environmental factors remain the same, and these unanswered distress signals will simply be conditioned into the mindscape, causing long-term imbalances and ongoing, self-perpetuating, distress (“suffering”). Indeed, complex feelings such as mistrust, shame, anxiety, worry, envy, contempt, rage and hate are indications that the prime commandment has been violated, and the eliciting conditions have actually been increased, now harbored mentally and feeding forward in negative attitudes, limited beliefs, and narrow identity boundaries that skew perception and that drive habitually avoidant behaviors, and all the predictable intrapersonal dissonance (and interpersonal conflict) that results. These are the targets of therapeutic interventions such as rational-emotive or cognitive behavioral therapy,350,351 their negative valence nominating them as maladaptive candidates for revision or elimination.
Worse, we have pressed our man-made suffering into service in a warped third-party form of morality—one where we suppressively regulate one another by deliberately inflicting emotional pain. For example, as moral psychologist Paul Rozin has noted, ethical codes are routinely enforced by third party expressions of the negative emotions,352 with disgust mediating codes of divinity (religious mores), contempt enforcing codes of community (local sociocultural mores), and anger upholding codes of autonomy (equal justice, human rights, etc). But this strategy can backfire, for it only succeeds to the degree that it instills first person emotions such as shame, embarrassment, guilt, sadness, or fear, harnessing the “flight,” submissive, mode of hardwired emotional response.353 Likewise, it predicts competitive conflicts between the various ethical codes should they prompt first person disgust or anger instead, and its naturally aggressive “fight” mode of self-preservationary avoidance354—or elicit such hostile complex emotions as contempt, rage, or hate and “getting even” (the negative feedback rebalancing) via revenge.355 In fact, punitive authoritarian parenting that relies upon shame and humiliation negates the self, invites anger, promotes rage356 and self-destructive activity; and can lead directly to violent criminal behavior.358,359
EVOLVING SENSITIVITY TO CONTEXT
Furthermore, sociocultural practices and structures that exploit negative emotion in this manner create a compounded, lingering state of biophysical distress,360 setting the epigenetic stage for compromised immune function, ill health, maladaptive development, and psychiatric disorder.361,362 Indeed, through epigenetic pathways, stressful events become biologically embedded—they get “under the skin”—during developmental windows crucial to the forging of neural circuitry,363,364 and are implicated in the DNA damage that accelerates degenerative aging.365 It is now well documented that environmental factors such as maltreatment, family adversity, marital conflict, maternal depression, and even financial distress are been linked with cognitive deficits and socio-emotional behavioral problems in children.366–379 The mechanism of such biological embedding has been called “time dependent sensitization,”380 “neural sensitization,”381 “sensory hyper-arousal,”382 “central sensitization,”383 “central nervous system sensitization,”384 and “sensitivity to context,”385 but by any name, they reflect the self-regulatory feedback dynamics—and epigenetic and immune manifestations—of the emotional sense.
Additionally, humans continue to evolve, and we may be becoming increasingly sensitive to the biophysical cues that elicit emotional perceptions, implying that epigenetic processes are becoming evermore influential in all aspects of our health and well-being. Or, in other words, that psychosomatic and sociosomatic effects of the mind and the world have an increasingly potent effect on our genetic processes. In fact, one's very stress response can be epigenetically programmed by the environmental exposures of one's immediate ancestors, one's grandparents.386 Likewise, “increasing evidence suggests that most, if not all, diseases of the central nervous system are associated with either primary or secondary perturbations of the epigenome,”387 which of course include “psychosomatic” syndromes, affective disorders388,389 and psychiatric diseases390—not to mention the profound developmental deficits from extreme contextual deprivation.349 Furthermore, disruptions in these chemical signaling systems and their neural circuitry can give rise to the empathy deficits in autism,382 to psychopathy,391,392 and the dissociative393 and identity pathologies in schizophrenia.394,395 Yet their underlying feedback dynamics can also explain the marginal efficacy, adaptive tolerance, and long-term deficits that can accompany pharmacological therapies.396–398
Indeed, we have blamed the emotional messenger while missing its primal self-regulatory message. We have chosen to suppressively regulate our emotions instead of allowing them to inform and regulate us. It should be no surprise then, that most of the mental, behavioral, and identity disorders within the psychiatric guidebook, the Diagnostic and Statistical Manual of Mental Disorders (DSM),399 are characterized by emotional dysfunction, particularly the ongoing negative emotions. Nor should it be surprising that controversy abounds concerning the changeability, suitability, and efficacy of the DSM criteria, for we surely cannot adequately grasp “disorder” without first understanding the original, self-regulatory, emotional order.
Nonetheless, our ever-evolving emotional sensitivity is a good thing. For while increased sensitivity to context in children raised in adverse, stressful, environments is particularly harmful; extra sensitivity in those with enriched and nurturing environments fosters even better developmental outcomes than their less sensitive peers.385 Likewise, with the development of emotional literacy and emotional intelligence, extra sensitivity means earlier warning, more detailed information, and timely opportunities for swift and effective corrective responses.
CONCLUSION
I have argued that a broadened interdisciplinary perspective, an updated evolutionary theory, and an expanded definition are required to elucidate the biophysical function of human emotion—to envision the entire emotional elephant both within and beyond the wide variety of theoretical viewpoints. Indeed, despite all effort, the bigger picture remains opaque, emotion remains undefinable in psychology,400 and seeking a unifying function has been deemed unfashionable if not misguided.401 The result is a continuum of independent and often mutually incompatible theories ranging from the position that emotions are biologically hardwired, to the view that they are largely sociocultural constructions, suggesting that “emotion generation” and “emotion regulation” are “either one or two, depending on your point of view”402—a situation that has reduced the science of emotion to a matter of personal opinion.
This new story, however, suggests that emotion generation IS emotion regulation, because it is best understood as a biologically ancient self-regulatory sensory system. Yet, despite many theorists noting both the sensory and self-regulatory nature of emotion, there seems little inclination to officially acknowledge emotion as a sense. Nonetheless, abundant empirical evidence is there for the taking that justifies making that declaration: Evidence ranging from the patterned molecular activity that drives hedonic stimulus-response behavior and yields inaugural evaluative perception in the simplest organisms, to the functional connections between cell signaling networks and epigenetic, immune, and neural processes in more complex organisms. Evidence of how these mechanical regulators manifest as multi-tiered feeling perceptions, sensitivity to context phenomena, patterns of development, motivation, decision-making, moral reasoning and emotion regulation in humans. Evidence that compatibly dovetails with, extends, and provides biological foundations for “the laws of emotion”403; that melds with evolutionary theory in both its early and contemporary forms, and that is comfortably compatible with nearly every major ideological tenet and empirical finding within psychology. Furthermore, is the significant fact that synesthesia, the odd overlapping of sensory modalities, includes an “emotionally mediated” variety.404 So my friendly challenge is to ask: Why not? If it walks, talks, and acts like a duck (or functional elephant in this case), perhaps it is time to publically declare it to be just that: The preponderance of evidence suggests that it is time to rightfully honor emotion as our self-regulatory sense.
For indeed, this new story has come from a broader evolutionary vantage, noting that affective feelings and their coupled behavioral responses are rooted in the most primal forms of identity and sensory-motor control, readily apparent in the molecular structures and self-regulatory circuitry of “branes” (membranes) of the simplest living systems. This is a control circuitry instantiated by protein receptor complexes that govern hedonic approach and avoid behavior, fashioned via a serendipitous coupling of positive (amplifying) and negative (regulating) feedback processes and harnessed—very early on—as symbolic cues for beneficial or harmful environmental conditions. It maps the confluence of the self-regulatory computational dynamics across the more hardwired genetic and soft-wired epigenetic regulatory landscapes with its dynamic on-off switching, to stop/go appetitive behavioral control, to yes/no hedonic approach/avoid responses in accordance with its simple—yet universal—tit-for-tat self-regulatory code. It suggests that pleasurable and painful categories of feeling relate directly to the criteria for natural selection (selfpreservation and adaptive self-development); that subjective perceptions of “goodness” and “positivity” concern optimal balances between the organism and its immediate environment, and that “rightness” equates with optimal biophysical conditions for living systems as well as adaptive, timely, and appropriate responses to immediate environmental challenges.
It maps how the ancient sensory language of emotion now manifests as bi-directional communication pathways, across the generally tri-level structure of the human brain and its dual processing paths,34,234,405 and in individual neurons, as well as the receptome “branes” of each specialized cell; fostering the parallel computations across the epigenetic, immune, endocrine, respiratory and central nervous systems in the generation of “self-relevant” emotional sensory perceptions. This mapping elucidates how common human feelings now encode three levels of self-regulatory information, elegantly balancing the immediate needs of the body in the context of the world, mediating the growth of mind while prioritizing preservation of the body, and elegantly integrating the individualistic and social aspects of human identity. This new model is also fully testable, and many of its predictions are already well-established facts across the social as well as physical sciences.
On the other hand, the model is not without its vices. Accessibility is of primary concern, due to its departure from traditional assumptions and approaches, if not antipathy, given that it upsets several paradigmatic apple carts. Investigations will require a broader scientific lens—an interdisciplinary inquiry and a synthesis of biophysical facts, bucking the academic trend toward ever more detailed analysis and career specialization that plagues emotion theory.
It will also necessitate a revised vocabulary for the feeling signals themselves. Indeed, words fail; and even exploring the model's implications will require building a new lexicon, one with terms that more accurately depict the biophysical origins, temporal significance, and elegant complexity of emotional feeling perceptions; one that is functionally tethered to the biophysical underpinnings, and that rightly privileges our hedonic evaluations; one that links appraisal information with universal human needs and is not freighted with mind-body dualism (or the traditional good/evil dichotomy), and one that has been laundered of the pejorative connotations that suppressive emotion regulation presupposes.
The model may also challenge the ecological validity of some standard empirical approaches, ranging from laboratory emotion induction and self-reports (such as PANAS), to statistical analysis (wherein a more Bayesian paradigm would honor the feedback dynamics, the self-relevant nature of emotion, the subjectivity of the investigator, and the observable real-world behavioral patterns.406–408 In short, the model poses some heady challenges for social scientists.
The virtues of this model, however, suggest surmounting such hurdles to be a worthy pursuit. For, to the author's knowledge, it is the first model to offer a biologically justifiable function of emotion, one that is devoid of neurocentricity, and rooted in the fundamental biophysical facts and principles beyond the conventional interpretation of Darwinian evolution. In fact, while he wondered whether or not emotional facial expressions may be vestigial,409 Darwin himself recognized the these core self-regulatory dynamics in his three principles of emotion: He noted the bottom up behavioral automaticity and positive feedback in his “principle of direct action,” the negative feedback dynamic in his “principle of antithesis,” and anticipated the self-developmental Pavlovian conditioning and its feed-forward manifestations in his “principle of serviceable associated habits.”410 He also endorsed Alexander Bain's “fundamental law of pleasure and pain,” which states that pleasure is connected with an increase and pain a decrease in vital power (the tit-for-tat self-regulatory code), a law founded upon “the principle of self-conservation, the self-regulating, selfacting impulse of the animal system”411 (emphasis mine). Had Darwin been privy to modern understandings of the chemical networks, computational, and regulatory dynamics involved in genetics, epigenetic inheritance,42,186 social genomics,193 and neuroplasticity,412 it seems likely he would have noted the importance of here-and-now environmental interactions and behavioral responses, and perhaps more pointedly given emotion its functional due. Still, despite his laudable parsimony, Darwin concluded that “the ‘language of emotion’ is certainly of importance for the welfare of mankind.”410
Indeed, in addition to unifying many seemingly separate and unrelated bodies of literature, this model affords science a pioneering inroad into the territory of values. It allows us to reexamine and transcend the naturalistic fallacy,413–415 providing a language of embodied bio-values against which to contrast, inform, and assess our standard philosophical assumptions. It invites us to reexamine traditional value judgments and linguistic categories such as good and evil, and virtue and sin; allowing us to shift certain aspects of morality into the realm of public health with “right” and “wrong” states concerning biophysically universal requirements, conditions and optimal balances for all life forms. It offers a hard-science bedrock for the “positive” in “positive emotions”33 and “positive psychology”416 as well as the “positive” adaptive functions of the “negative” emotions and insight into why they are so insistent, acknowledging a clear epigenetic and immunological bridge between mental well-being and physical health.
Finally, the model places purpose in an evolutionary context, with both positive and negative relating to universal self-regulatory purposes to which the biovalues of all living systems are tethered. To recognize our teleological end-directed purposes is to fill a “gaping hole” in our understanding of our world and our place within it—“the intrinsic value in humankind,”417 offering a much more optimistic portrait of human nature—if not of nature itself. It suggests that cohesion, cooperation, and adaptive creativity are as deeply rooted in our evolutionary history as random mutation and red-in-tooth-and-claw competition. It suggests that nature is green with grace and embrace, balancing self-preservationary necessities with self-developmental synergy, and that it is our feeling sense that defines us—and defines us as good: (“Sentio ergo sum bonum!”: “I feel, therefore I am good!”)
Still, aside from a better scientific foundation, this isn't really a new story. It has been with us since time untold, for even the ancients—Plato, Aristotle—recognized moral virtue as rooted in the judicious use of pleasure and pain. But reframing emotion as a self-regulatory sense, offers a more judicious appreciation of the biological fact that first and foremost, emotion is actually—and rightly—regulating us. Indeed, as Jeremy Bentham suggested, our “sovereign masters” of pleasure and pain “point out what we ought to do as well as determine what we shall do.” And that their perceptual persistence is not only devoid of vestigial or original “sin,” but may offer our only salvation from it.
REFERENCES
- 1.Bentham J. The principles of morals and legislation. New York, NY: Hafner Press; 1948 [Google Scholar]
- 2.James W. What is an emotion? Mind. 1884:188–205
- 3.Cannon W. The James-Lange theory of emotions: a critical examination and an alternative theory. Am J Psych. 1927;39(1/4):106–34 [PubMed] [Google Scholar]
- 4.Schachter S, Singer J. Cognitive, social, and physiological determinants or emotional state. Psychol Rev. 1962;69(5):79–99 [DOI] [PubMed] [Google Scholar]
- 5.Scherer K, Schorr A, Johnstone T, editors. Appraisal processes in emotion: theory, methods, research (series in affective science). New York, NY: Oxford University Press USA; 2001 [Google Scholar]
- 6.Haidt J. The emotional dog and its rational tail: a social intuitionist approach to moral judgment. Psychol Rev. 2001;108(4);814–34 [DOI] [PubMed] [Google Scholar]
- 7.Mesquita B, Frijda N. Cultural variations in emotions: a review. Psych Bul. 1992;112(2);179–204 [DOI] [PubMed] [Google Scholar]
- 8.Gross J, Keltner D. Functional accounts of emotion. Cogn Emot. 1999;13(5);467–80 [Google Scholar]
- 9.Frijda N. The psychologists' point of view. In: Lewis M, Haviland-Jones J, Barrett L, editors. Handbook of emotions, 3rd ed.New York: NY: The Guilford Press; 2008:68–87 [Google Scholar]
- 10.Griffiths P. What emotions really are. Chicago, IL: University of Chicago Press; 1997:14 [Google Scholar]
- 11.Russell J. Core affect and the psychological construction of emotion. Psychol Rev. 2003;10(1);145–72 [DOI] [PubMed] [Google Scholar]
- 12.Russell J, Barrett L. Core affect, prototypical emotional episodes, and other things called emotion: dissecting the elephant. J Per Soc Psych. 1999;76(5);805–19 [DOI] [PubMed] [Google Scholar]
- 13.Gross J. The emerging field of emotion regulation: an integrative review. Rev Gen Psych. 1998;2(3);271–99 [Google Scholar]
- 14.Scherer K. On the nature and function of emotion: a component process approach. In: Scherer K, Ekman P, editors. Approaches to emotion. Hillsdale, NJ: Erlbaum; 1984:293–318 [Google Scholar]
- 15.Frijda N. Varieties of affect: emotions and episodes, moods and sentiments. In: Ekman P, Davidson R, editors. The nature of emotion: fundamental questions. New York, NY: Oxford University Press USA; 1994:59–67 [Google Scholar]
- 16.Lang P. The network model of emotion: motivational connections. In: Wyer R, Srull T, editors. Advances in social cognition. Hillsdale, NJ: Erlbaum; 1995 [Google Scholar]
- 17.Schwarz N, Clore G. Mood, misattribution, and judgments of well-being: informative and directive functions of affective states. J Pers Soc Psych. 1983;45(3);513–23 [Google Scholar]
- 18.Schwarz N. Feelings as information: Informational and motivational functions of affective states. In: Higgins E, Sorrentino R, editors. Handbook of motivation and cognition. Vol 2. New York, NY: The Guilford Press; 1990:527–61 [Google Scholar]
- 19.Clark L, Watson D. Distinguishing functional from dysfunctional affective responses. In: Ekman P, Davidson R, editors. The nature of emotion: fundamental questions. New York, NY: Oxford University Press USA; 1994:131–6 [Google Scholar]
- 20.Frijda N. Impulsive action and motivation. Biol Psychol. 2010;84(3);570–579 [DOI] [PubMed] [Google Scholar]
- 21.Levenson R. Human emotions: a functional view. In: Ekman P, Davidson R, editors. The nature of emotion: fundamental questions. New York, NY: Oxford University Press USA; 1994;23–6 [Google Scholar]
- 22.Niedenthal P, Halberstadt J, Innes-Ker A. Emotional response categorization. Psychol Rev. 1999:106(2);337–61 [Google Scholar]
- 23.Lang P, Davis M. Emotion, motivation, and the brain: reflex foundations in animal and human research. Prog Brain Res. 2006;156:3–29 [DOI] [PubMed] [Google Scholar]
- 24.Solomon R, Corbit J. An opponent process theory of motivation: The temporal dynamics of affect. Psychol Rev. 1974;81(2);119–45 [DOI] [PubMed] [Google Scholar]
- 25.Zajonc R. On the primacy of affect. In: Scherer KR, P.Ekman P, editors. Approaches to emotion. New York, NY: Psychology Press; 1984:259–70 [Google Scholar]
- 26.Baumeister R, Vohs K, DeWall C, Zhang L. How emotion shapes behavior: Feedback, anticipation, and reflection, rather than direct causation. Pers Soc Psychol Rev. 2007;11(2):167–203 [DOI] [PubMed] [Google Scholar]
- 27.Buck R. Nonverbal behavior and the theory of emotion: the facial feedback hypothesis. J Pers Soc Psychol. 1980;38(5):811–24 [DOI] [PubMed] [Google Scholar]
- 28.Clore G, Wyer R, Dienes B, Gasper K, Gohm C, Isabel L. Cognition in emotion: always, sometimes, or never? In: Martin L, Clore G, editors. Theories of mood and cognition: a user's handbook. Mahwah, NJ: Erlbaum; 2001:24–61 [Google Scholar]
- 29.Fr da N. The psychologists' point of view. In: Lewis ML, Haviland-Jones JM, editors. Handbook of emotions. 2nd ed.New York, NY: Guilford Press;2000:68–87 [Google Scholar]
- 30.Heilman K. Emotional experience: a neurological model. In Lane R, Nadel L, Ahern G, editors. Cognitive neuroscience of emotion. New York, NY: Oxford University Press; 2000:328–44 [Google Scholar]
- 31.Hoeksma J, Oosterlaan J, Schipper E. Emotion regulation and the dynamics of feelings: a conceptual and methodological framework. Child Dev. 2004;75(2):354–60 [DOI] [PubMed] [Google Scholar]
- 32.Laird J. Self-attribution of emotion: the effects of expressive behavior on the quality of emotional experience. J Pers Soc Psychol. 1974;29(4);475–86 [DOI] [PubMed] [Google Scholar]
- 33.Larsen R. Toward a science of mood regulation. Psychol Inq. 2000;11(3):129–41 [Google Scholar]
- 34.LeDoux J. Cognitive and emotional interactions in the brain. Cogn Emot. 1989;3(4):267–89 [Google Scholar]
- 35.Pribram K, McGinniss D. Arousal, activation, and effort in the control of attention. Psychol Rev. 1975;82(2):116–49 [DOI] [PubMed] [Google Scholar]
- 36.Rosenblatt A. The role of affect in cognitive psychology and psychoanalysis. Psychoanal Psych. 1985;2(2):85–97 [Google Scholar]
- 37.Tomkins S. Affect, imagery, consciousness. Vol i. The positive affects. Oxford, UK: Springer; 1962 [Google Scholar]
- 38.Carver C, Scheier M. Origins and functions of positive and negative affect. Psychol Rev. 1990;97(1):9–35 [Google Scholar]
- 39.Carver C, Scheier M. Self-regulation of action and affect. In: Vohs KD, Baumeister RF, editors. Handbook of self-regulation: research theory and applications. 2nd ed.New York, NY: The Guilford Press; 2011:3–22 [Google Scholar]
- 40.Camazine S, Deneubourg J, Franks N, Sneyd J, Theraulaz G, Bonabeau E. SelfOrganization in Biological Systems. Princeton, NJ: Princeton University Press; 2001:7–87 [Google Scholar]
- 41.Kauffman S. Origins of order. New York, NY: Oxford University Press USA; 1993 [Google Scholar]
- 42.Jablonka E, Lamb M. Evolution in four dimensions: genetic, epigenetic, behavioral, and symbolic variation in the history of life. Cambridge, MA: The MIT Press; 2005 [Google Scholar]
- 43.Larsen R. The contributions of positive and negative affect to emotional well-being. Psychol Topics. 2009;18(2):247–66 [Google Scholar]
- 44.Ratcliffe M. The feeling of being. J Consciousness Studies. 2005;12(8-10):45–63 [Google Scholar]
- 45.Fingerhut J, Marienberg S. How it feels to be alive. In: Fingerhut J, Marienberg S, editors. Feelings of being alive. Berlin: De Gruyter; 2012;2–19 [Google Scholar]
- 46.Damasio A. The feeling of what is happening. New York, NY: Pantheon; 1999 [Google Scholar]
- 47.Damasio A. Self comes to mind. Orlando, FL: Harcourt; 2010 [Google Scholar]
- 48.Helm B. Felt evaluations: a theory of pleasure and pain. Amer Phil Quar. 2002January;39(1):13–30 [Google Scholar]
- 49.Slaby J. Emotional rationality and feelings of being. In: Fingerhut J S, Marienberg S, editors. Feelings of being alive. Berlin: De Gruyter; 2012 [Google Scholar]
- 50.Selye H. The stress of life. New York, NY: McGraw Hill; (revised ed, 1978); 1956 [Google Scholar]
- 51.Blalock J. The immune system as a sixth sense. J Intern Med. 2005;257(2):126–38 [DOI] [PubMed] [Google Scholar]
- 52.Hajak G, Moser J, Holroyd C, Simons R. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biol Psychol. 2006;71(2):148–54 [DOI] [PubMed] [Google Scholar]
- 53.Kiecolt-Glaser J, McGuire L, Robles T, Glaser R. Emotions, morbidity, and mortality: new perspectives from psychoneuroimmunology. Annu Rev Psychol. 2002;53:83–107 [DOI] [PubMed] [Google Scholar]
- 54.Lazarus R. Psychological stress and the emotions. Annu Rev Psychol. 1993;44:i–21 [DOI] [PubMed] [Google Scholar]
- 55.Mayne T. Negative affect and health: the importance of being Ernest. Cogn Emot. 1999;13(5):601–35 [Google Scholar]
- 56.Petrie K, Booth R, Pennebaker R. The immunological effects of thought suppression. J Pers Soc Psychol. 1998;75(5):1264–72 [DOI] [PubMed] [Google Scholar]
- 57.Segerstrom S, Miller G. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psych Bul. 2004;30(4);601–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor S, Kemeny M, Reed G, Bower J, Gruenewald T. Psychological resources, positive illusions, and health. Am Psychol. 2000;55(1):99–109 [DOI] [PubMed] [Google Scholar]
- 59.Xu J, Robert R. The power of positive emotions: it's a matter of life or death—subjective well-being and longevity over 28 years in a general population. Health Psychol. 2010;29(1):9–19 [DOI] [PubMed] [Google Scholar]
- 60.Scholler A, Higgins E. Promotion and prevention systems: regulatory focus dynamics within self-regulatory hierarchies. In: Vohs K, Baumeister R, editors. Handbook of self-regulation: research theory and applications. 2nd ed.2011:143–61
- 61.Gilbert D, Pinel E, Wilson T, Blumberg S, Wheatley T. Immune neglect: a source of durability bias in affective forecasting. J Pers Soc Psychol. 1998;75(3):617–38 [DOI] [PubMed] [Google Scholar]
- 62.Festinger L. A theory of cognitive dissonance. 1959. Evanston, Ill: Row Peterson [Google Scholar]
- 63.LeDoux J. Emotion circuits in the brain. Annu Rev Neurosci. 2000March;23:155–184 [DOI] [PubMed] [Google Scholar]
- 64.Pavlov I. Conditioned reflexes. (Anrep G. V., translator) 1927. London: Oxford University Press [Google Scholar]
- 65.Biederman I, Cooper E. Evidence for complete translational and reflectional invariance in visual object priming. Perception. 1991;20:585–93 [DOI] [PubMed] [Google Scholar]
- 66.Barsalou L. Perceptual global systems. Behav Brain Sci. 1999;22:577–660 [DOI] [PubMed] [Google Scholar]
- 67.Frith C, Frith U. Implicit and explicit processes in social cognition. Neuron. 2008;6(6):503–10 [DOI] [PubMed] [Google Scholar]
- 68.Bowers K. On being unconsciously influenced and informed. In: Bowers K, Meichenbaum D, editors. The unconscious reconsidered. New York: Wiley; 1984:27–272 [Google Scholar]
- 69.Greenwald A, Krieger L. Implicit bias: scientific foundations. Cal Law Rev. 2006;94(4):945–67 [Google Scholar]
- 70.Levy B, Banaji M. Implicit ageism. In: Nelson T, editor. Ageism: stereotyping and prejudice against older persons. 2002; Cambridge, MA: MIT Press: 49–75 [Google Scholar]
- 71.Papies E, Aarts H. Nonconscious self-regulation, or the auto pilot of human behavior. In: Vohs K, Baumeister R, editors. Handbook of self-regulation: research theory and applications. 2nd ed; 2011:125–42
- 72.Bosma H, Kunnen E. Determinants and mechanisms in ego identity development: a review and synthesis. Dev Rev. 2001;21(1):39–66 [Google Scholar]
- 73.Stryker S, Burke P. The past, present, and future of identity theory. Soc Psychol Q. 2000;63(4):284–97 [Google Scholar]
- 74.Bem D. Self-perception: an alternative interpretation of cognitive dissonance phenomena. Psychol Rev. 1967;74(3):183–200 [DOI] [PubMed] [Google Scholar]
- 75.Markus H, Wurf E. The dynamic self-concept: a social psychological perspective. Annu Rev Psychol. 1987;38:299–337 [Google Scholar]
- 76.Bernstein W, Stephan W, Davis M. J Pers Soc Psychol. 1979;37(10):1810–21 [Google Scholar]
- 77.Leary M. Motivational and emotional aspects of the self. Annu Rev Psychol. 2007;58:317–44 [DOI] [PubMed] [Google Scholar]
- 78.Deci E, Ryan R. Intrinsic motivation and self-determination in human behavior. New York: Plenum; 1985 [Google Scholar]
- 79.Bandura A, Locke E. Negative self-efficacy and goal effects revisited. J Appl Psych. 2003;88(1):87–99 [DOI] [PubMed] [Google Scholar]
- 80.Baumeister R. Rethinking self-esteem: why nonprofits should stop pushing self-esteem and start endorsing self-control. Stanford Soc Innov Rev. 2005; (Winter):1–9 [Google Scholar]
- 81.Harber K. Self-esteem and affect as information. Pers Soc Psychol Bull. 2005February;31(2):276–88 [DOI] [PubMed] [Google Scholar]
- 82.Aron A, Aron E. Self-expansion motivation and including other in the self. In: Duck S, editor. Handbook of personal relationships: theory, research and interventions. 2nd ed.Hoboken, NJ: John Wiley Sons Inc; 1997:251–70 [Google Scholar]
- 83.Maslow A. Motivation and personality. New York, NY: Harper Row; 1970 [Google Scholar]
- 84.Medicus G. Toward an ethnopsychology: A phylogenic tree of behavior. Ethol Sociobio. 1987;8(3 suppl);131–50 [Google Scholar]
- 85.Marienberg S. How it feels to be alive. In: Fingerhut J, Marienberg S, editors. Feelings of being alive. Berlin: De Gruyter; 2012:17 [Google Scholar]
- 86.de Rosnay J. The macroscope: a new world scientific system. New York: NY Harper Row; 1979:2–72 See also:de Rosnay J. (1997). Feedback. In: Heylighen F, Joslyn C, Turchin V, editors. Principia Cybernetica Web (Principia Cybernetica, Brussels); http://pespmc1.vub.ac.be/FEEDBACK.html [Google Scholar]
- 87.Ashby W. An Introduction to cybernetics. London: Chapman Hall; 1956 [Google Scholar]
- 88.Wiener N. Cybernetics. New York, NY: Wiley; 1948 [Google Scholar]
- 89.Powers W. Feedback: beyond behaviorism. Science. 1987;179(26):351–56 [DOI] [PubMed] [Google Scholar]
- 90.Holland J. Complex adaptive systems. Daedalus, 1992;121(1, A New Era in Computation, Winter):17–30 [Google Scholar]
- 91.Prigogine I, Stengers L. Order out of chaos: man's new dialogue with nature. New York, NY: Bantam; 1984 [Google Scholar]
- 92.Varella F, Maturana H, Uribe R. Autopoiesis: the organization of living systems, its characterization and model. Biosystems. 1974;5:187–96 [DOI] [PubMed] [Google Scholar]
- 93.Brillouin L. The negentropy principle of information. J App Physics. 1953;24(9):1152–63 [Google Scholar]
- 94.Eigen M. The origin of genetic information: viruses as models. Gene. 1993;135:37–47 [DOI] [PubMed] [Google Scholar]
- 95.Thomas R, Thieffry D, Kaufman M. Dynamical behavior of biological regulatory networks: Biological role of feedback loops and practical use of the concept of the loop-character state. Bull Math Biol. 1995;57(2):247–76 [DOI] [PubMed] [Google Scholar]
- 96.Kauffman S. At home in the universe: the search for laws of self-organization and complexity. New York, NY: Oxford University Press; 1996:78–168 [Google Scholar]
- 97.Dunlap J. Molecular bases for circadian clocks. Cell. 1999;96(22):217–90 [DOI] [PubMed] [Google Scholar]
- 98.Grossberg S. Intracellular mechanisms of adaptation and self-regulation in self-organizing networks: the role of chemical transducers. Bull Math Biol. 1980;42(3):365–96 [DOI] [PubMed] [Google Scholar]
- 99.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Gen Suppl. 2003March;33:245–54 [DOI] [PubMed] [Google Scholar]
- 100.Ansel K, Lee D, Rao A. An epigenetic view of helper T cell differentiation. Nature Immun. 2003;4(7):616–24 [DOI] [PubMed] [Google Scholar]
- 101.Coogan A, Wyse C. Neuroimmunology of the circadian clock. Brain Res. 2008;1232:104–12 [DOI] [PubMed] [Google Scholar]
- 102.Keller M, Mazuch J, Abraham U, Eom G, Herzog E, Volk H-D, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. PNAS. 2009December;106(50):21407–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Farley R, Sampath A. Perspectives on: Information coding in mammalian sensory physiology. J Gen Physiol. 2011;138(3):281–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heylighen F, Joslyn C. Cybernetics and second order cybernetics. Encyclopedia of Physical Science and Technology, (3rd ed.) New York, NY: Academic Press; 2001:1–23 [Google Scholar]
- 105.Ashby W. The homeostat. Electrical Engineering. 1948;20:380 [Google Scholar]
- 106.Cariani P. The homeostat as embodiment of adaptive control. Int J Gen Syst. 2009Fed;38(2):139–54 [Google Scholar]
- 107.Walleczek J. Self-organized Biological Dynamics and Nonlinear Control. Cambridge: Cambridge University Press; 2000 [Google Scholar]
- 108.MacLean P. The triune brain in evolution: Role in paleocerebral functions. Kluwer Academic Publishers; 1990 [Google Scholar]
- 109.Branscombe N. Hedonic Effects of hedonic valence and physiological arousal on emotion: A comparison of two theoretical perspectives. Mot Emo. 1985;9(2):153–69 [Google Scholar]
- 110.Blascovich J, Mendes W, Tomaka J, Salomon K, Seery M. The robust nature of the biopsychosocial model of challenge and threat: A reply to Wright and Kirby. Pers Soc Psychol Rev. 2003;7(3):234–43 [DOI] [PubMed] [Google Scholar]
- 111.Eckman P. Facial expression and emotion. Amer Psych. 1993;48(4):376–9 [DOI] [PubMed] [Google Scholar]
- 112.Nunner-Winkler G, Sodian B. Children's understanding of moral emotions. Child Dev. 1998;59:1323–38 [DOI] [PubMed] [Google Scholar]
- 113.Lutz C. Unnatural emotions: Every day sentiments on a Micronesian atoll and their challenge to Western theory. Chicago, IL: University of Chicago Press; 1988 [Google Scholar]
- 114.Kemper T. A social interaction theory of emotions. New York, NY: John Wiley Sons; 1978 [Google Scholar]
- 115.de Sousa R. Moral Emotions. Ethical Theory and Moral Practice. 2001;4:109–26 [Google Scholar]
- 116.Haidt J. The Moral Emotions. In Davidson R, Scherer K, Goldsmith H, editors. Handbook of affective sciences. Oxford UK: Oxford University Press; 2003:852–70 [Google Scholar]
- 117.Yi T., Huang Y, Simon M, Doyle J. Robust perfect adaptation in bacterial chemotaxis through integral feedback. PNAS. 2000April25;97(9):4649–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Powers W. Quantitative analysis of purposive systems: Some spadework at the foundations of scientific psychology. Psychol Rev. 1978;85(5):417–35 [Google Scholar]
- 119.Carver C. Scheier M. On the Self-Regulation of Behavior. New York, NY: Cambridge University Press; 1998:13–22 [Google Scholar]
- 120.Briggs J. Fractals: The patterns of chaos. 1992:22–25
- 121.Mandlebrot B. The fractal geometry of nature. New York, NY: W. H. Freeman Co; 1977 [Google Scholar]
- 122.Eigen M, Gardiner W, Schuster P, Winkler-Oswatitsch R. The origin of genetic information. Sci Amer. 1981April;244:88–92 [DOI] [PubMed] [Google Scholar]
- 123.Kauffman S. Beyond reductionism: Reinventing the sacred. Zygon. 2007;42(4):903–14 [Google Scholar]
- 124.Kauffman S, Clayton P. On emergence, agency, and organization. Biol Phil. 2006;21:501–21 [Google Scholar]
- 125.Sherman J, Deacon T. Teleology for the perplexed: How matter began to matter. Zygon. 2007;42(4):873–901 [Google Scholar]
- 126.Gabora L, Aerts D. Evolution as context-driven actualization of potential: Toward an interdisciplinary theory of change of state. Interdisc Sci Revs. 2005;30(1):69–88 [Google Scholar]
- 127.Gabora L. Self-other organization: Why early life did not evolve through natural selection. J Theo Bio. 2006;241(3):443–50 [DOI] [PubMed] [Google Scholar]
- 128.Thomas R. Laws for the dynamics of regulatory networks. Int J Dev Bio. 1998;42:479–85 [PubMed] [Google Scholar]
- 129.Koshland D. Biochemistry of sensing and adaptation in a simple bacterial system. Annual Rev of Biochem. 1981;50:765–82 [DOI] [PubMed] [Google Scholar]
- 130.Maturana HR, Varela FJ. Autopoeisis and cognition: the realization of the living. Dordrecht: D. Reidel Publishing Co; 1980 [Google Scholar]
- 131.Hakken H. The science of structure: Synergistics. New York, NY: Van Nostrand Reinhold; 1984 [Google Scholar]
- 132.Kauffman S. Evolution beyond Newton, Darwin and entailing law. In: Henning B, Scarfs A, Sagan D, editors. Beyond mechanism: putting life back into biology. Plymouth, UK: Lexington Books; 2013:1–24 [Google Scholar]
- 133.Ben-Shiomo I, Hsu S, Rauch R, Kowalski H, Hsueh A. Signaling receptome: a genomic and evolutionary perspective of plasma membrane receptors involved in signal transmission. Science STKE. 2003;(187):re9. [DOI] [PubMed] [Google Scholar]
- 134.Brandman O, Ferrell J, Li R, Meyer T.Interlinked fast and slow positive feedback loops drive reliable cell decisions. Science. 2005;310(October, 21):496–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brandman O, Meyer T. Feedback loops shape cellular signals in space and time. Science, 2008; 322(October17)390–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lisman J, Fallon J. What maintains memories? Science. 1999January15;283:339–40 [DOI] [PubMed] [Google Scholar]
- 137.Rao C, Arkin A. Control motifs for intracellular regulatory networks. Ann Rev Biomed Eng. 2001;3:391–419 [DOI] [PubMed] [Google Scholar]
- 138.Ferrell J. Self-perpetuating states in signal transduction: Positive feedback, double negative feedback and bistability. Cur Opin Chem Bio. 2002;6:140–8 [DOI] [PubMed] [Google Scholar]
- 139.Cinquin O, Demongeot J. Positive and negative feedback: Striking a balance between necessary antagonists. J Theor Bio. 2002;216:229–41 [DOI] [PubMed] [Google Scholar]
- 140.Langton C. Computation at the edge of chaos: Phase transitions and emergent computations. Physica D. 1998;42:12–37 [Google Scholar]
- 141.Dari A, Kia B, Bulsara A, Ditto W. Logical stochastic resonance with correlated internal and external noises in a synthetic biological logic block. Chaos. 2011;21:047521–1 [DOI] [PubMed] [Google Scholar]
- 142.Freeman M. Feedback control of intercellular signaling in development. Nature. 2000November16;408:313–9 [DOI] [PubMed] [Google Scholar]
- 143.Ceste M, Doyle J. Reverse engineering of biological complexity. Science. 2002March1;295:1664–9 [DOI] [PubMed] [Google Scholar]
- 144.Davis G. Homeostatic control of neural activity: From phenomenology to molecular design. Ann Rev Neurosci. 2006;29:307–23 [DOI] [PubMed] [Google Scholar]
- 145.Tsai T, Choi Y, Ma W, Pomerening J, Tang C, Ferrell JE. Robust, tunable biological oscillations from interlinked positive and negative feedback loops. Science. 2008July4;321:3995–4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Feldman V, Valiant L. The learning power of evolution. COLT. 2008:513–4
- 147.Valiant L. Probably approximately correct: nature's algorithms for learning and prospering in a complex world. New York, NY: Basic Books; 2013 [Google Scholar]
- 148.Dennett D. Freedom Evolves. New York, NY: Penguin; 2003:47–56 [Google Scholar]
- 149.Blackmore S. Conversations on consciousness: what the best minds think about the brain, free will, and what it means to be human. Oxford University Press; 2006 [Google Scholar]
- 150.Thompson E. Mind in Life: Biology, phenomenology, and the sciences of mind. Cambridge, MA: Harvard University Press, Belknap Press; 2007:312–441 [Google Scholar]
- 151.Panksepp J. The affective brain and core consciousness. In Lewis M, Haviland-Jones J, Barrett L. (Eds) Handbook of Emotions, 3rd Edition New York, NY: The Guilford Press; 2008:47–67 [Google Scholar]
- 152.Bren A, Eisenbach M. How signals are heard during bacterial chemotaxis: Protein-protein interactions in sensory signal propagation. J Bacteriol. 2000December;184(24);6865–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wadham G, Armitage J. Making sense of it all: Bacterial chemotaxis. Nature Revs Mol Cell Biol. 2004December;5:1024–37 [DOI] [PubMed] [Google Scholar]
- 154.Haugeland J. Representational genera. In: Ramsey W, Rumelhard DE, Stich SP, editors. Philosophy and connectionist theory. New York, NY: Erlbaum; 1991. 61–89 [Google Scholar]
- 155.Clark A. Being there: putting brain body and world together again. 1997:143–75
- 156.O'Connell L, Hofmann H. Genes, hormones, and circuits: an integrative approach to the study of evolution of social behavior. Frontiers Neuroendocrinol. 2010;32:320–35 [DOI] [PubMed] [Google Scholar]
- 157.Fain G. Sensory transduction.2003:159–64 Sunderland, MA: Sineauer Associates Inc. [Google Scholar]
- 158.Sourjik V. Receptor clustering and signal processing in E. coli chemotaxis. Trends in Microbio. 2004;12(12):569–75 [DOI] [PubMed] [Google Scholar]
- 159.Zhang P., Khirsigara C, Hartnell L, Subramaniam S.Direct visualization of Escherichia coli chemotaxis receptor arrays using cryo-electron microscopy. PNAS. 2004March6;104(10):3777–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Greenfield D, McEvoy A, Shroff H, et al. Self-organization of the Escherichia coli chemotaxis network imaged with super-resolution light microscopy. PLoS Bio. 2009;7(6) el000137:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Baker M, Wolanin P, Stock J. Signal transduction in bacterial chemotaxis. Bioessays. 2005;28:9–22 [DOI] [PubMed] [Google Scholar]
- 162.Parkinson J, Blair D. Does E. coli have a nose? Science. 1983;259;1701–91 [DOI] [PubMed]
- 163.Lagomarsino M, Bassetti P, Isambert H. Hierarchy and feedback in the evolution of the E. coli transcription network. PNAS. 2007;104(13):5516–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bray D. Wetware. London: Yale University Press; 2009:89–108 [Google Scholar]
- 165.Wang L, Xin J, Nie Q. A critical quantity for noise attenuation in feedback systems. PLoS Comp Bio. 2010;6(4), e1000764:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Salman H, Libchaber A. A concentration-dependent switch in the bacterial response to temperature. Nature Cell Bio. 2007September9;9:1098–9 [DOI] [PubMed] [Google Scholar]
- 167.Bibikov S, Biran R, Rudd K, Parksinson J. A signal transduce for Aerotaxis in Escherichia coli. J Bacteriology. 1997Jun:4075–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rebbapragada A, Johnson M, Hardin G. et al. The Aer protein and the serine chemoreceptor Tsr independently sense intracellular energy levels and transducer oxygen, redox, and energy signals for Escherichia coli behavior. PNAS. 1997;94:10541–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bourret R, Stock A. Molecular information processing: Lesson from bacterial chemotaxis. J Biol Chem. 2002March22;277(12):9625–8 [DOI] [PubMed] [Google Scholar]
- 170.Vergassola M, Villermaux E, Shraiman B.‘Infotaxis’ as a strategy for searching without gradients. Nature. 2007;455:406–9 [DOI] [PubMed] [Google Scholar]
- 171.Shudo E, Haccou P, Iwasa Y. Optimal choice between feedforward and feedback control in gene expression to cope with unpredictable danger. J Theor Bio. 2003;223:149–60 [DOI] [PubMed] [Google Scholar]
- 172.Kauffman S. Answering Descartes: Beyond Turing. In: Cooper S, Hodges A, editors. The once and future turing: computing the world. 2008. Cambridge, MA: MIT Press; http://mitpress.mit.edu/sites/default/files/titles/alife/0262297140chap4.pdf [Google Scholar]
- 173.Brickman P, Campbell D. Hedonic relativism and planning the good society. In: Appley M, editor. Adaptation level theory: a symposium 1971:287–302 [Google Scholar]
- 174.Bassler B. How bacteria talk to each other: Regulation of gene expression by quorum sensing. Curr Opin in Microbiol. 1999;2:582–7 [DOI] [PubMed] [Google Scholar]
- 175.Waters C, Bassler B. Quorum sensing: Cell-to-cell communication in bacteria. Ann Rev Cell Dev Biol. 2005;21:319–46 [DOI] [PubMed] [Google Scholar]
- 176.van Honk J, Schutter J. Dynamic brain systems in quest for emotional homeostatsis. Beh Brain Sci. 2005;28(2):220–1 [Google Scholar]
- 177.Benarroch E. Enteric nervous system. Neurology. 2007November13;69:1953–7 [DOI] [PubMed] [Google Scholar]
- 178.Pert C. The Molecules of Emotion. New York, NY: Touchstone; 1998 [Google Scholar]
- 179.Pagel M. Rise of the digital machine. Nature. 2008April10;452 (699);doi:10.1038/452699a [DOI] [PubMed] [Google Scholar]
- 180.Adams G, Marshall S. A developmental social psychology of identity: Understanding the person-in-context. J Adolesc. 1996;19:429–42 [DOI] [PubMed] [Google Scholar]
- 181.Pribram K, Melges F. Psychophysiological basis of emotion. Handbook Clin Neurol. 1969(3). [Google Scholar]
- 182.Heylighen F. The science of self-organization and adaptivity. In: Kiel L, editor. Knowledge management, organizational intelligence and learning, and complexity: the encyclopedia of life support systems. 2002. New York, NY: Academic Press; 1–23 [Google Scholar]
- 183.Lipton B. Nature, nurture and the power of love. J Prenatal Perinatal Psych Health. 1998a;13(1);3–9 [Google Scholar]
- 184.Lipton B. The evolving science of chiropractic philosophy: Part 1. Today's chiropractic. 1998b;(Nov/Dec):20–31 [Google Scholar]
- 185.Pollak S. Mechanisms linking early experience and the emergence of emotions. Curr Dir Psych Sci. 2008;17(6):370–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jablonka E, Lamb M. Epigenetic inheritance in evolution. J Evol Biol. 1998:11:159–83 [Google Scholar]
- 187.Levenson J, Sweatt J. Epigenetic mechanisms in memory formation. Nature Revs Neurosci. 2005February;6:108–18 [DOI] [PubMed] [Google Scholar]
- 188.Jablonka E, Raz G. Transgenerational epigenetic inheritance: Prevalence, mechanisms, and implications for the study of heredity and evolution. Quarterly Rev Bio. 2009;84 (2):131–76 [DOI] [PubMed] [Google Scholar]
- 189.Mattick J. RNA regulation: a new genetics? Nature Revs Gen. 2004;5;316–323 [DOI] [PubMed]
- 190.Mattick J, Makunin I. Non-coding RNA. Human Molecular Genetics. 2006;15(Rev iss 1):R17–29 [DOI] [PubMed] [Google Scholar]
- 191.Baverstock K, Ronkko M. Epigenetic regulation of the mammalian cell. PLoSONE. 2008;3(6); e2290:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Newman S, Muller G. Epigenetic mechanisms of character origination. J Exp Zoology (Molecular and Developmental Evolution). 2000;288:304–17 [DOI] [PubMed] [Google Scholar]
- 193.Cole S. Social regulation of human gene expression. Current Directions in Psychological Science. 2009;18(3):132–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cole S. Elevating the perspective on human stress genomics. Psychoneuroendocrinology. 2010; 35(7):955–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Harris S, Levine A. The p53 pathway: Positive and negative feedback loops. Oncogene. 2005;24:2899–908 [DOI] [PubMed] [Google Scholar]
- 196.Chan C, Dyavaiah M, DeMott M, Taghizadeh K, Dedon P, Begley T. A quantitative systems approach reveals dynamics control of tRNA modifications during cellular response. PLoS Gen. 2010;6(12):1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fitzpatrick D, Wilson C. Methylation and demethylation in the regulation of genes, cells, and responses in the immune system. Clin Immunol. 2003;109:37–45 [DOI] [PubMed] [Google Scholar]
- 198.Khudayberdiev S, Fiore R, Schratt G. MicroRNA as modulators of neuronal responses. Communicative Integr Biol. 2009;2(5):411–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kemperman G, Gast D, Gage F. Neuroplasticity in old age: Sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Annals of Neurol. 2002;52:135–43 [DOI] [PubMed] [Google Scholar]
- 200.D'Sa C, Duman R. Antidepressants and neuroplasticity. Bipolar Disord. 2002;4:183–94 [DOI] [PubMed] [Google Scholar]
- 201.Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Changes in gray matter induced by training. Nature. 2004January22;427:311–2 [DOI] [PubMed] [Google Scholar]
- 202.McClung C, Nestler E. Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology Revs. 2008;33:3–17 [DOI] [PubMed] [Google Scholar]
- 203.Regev A, Lamb M, Jablonka E.The role of DNA methylation in invertebrates: Developmental regulation of genome defense? Mol Biol Evol. 1998;15(7):880–91 [Google Scholar]
- 204.Hodges E, Molaro A, Dos Santos C, et al. Directional DNA methylation changes and complex intermediate states accompany line age specificity in the adult hematopoietic compartment. Molecular Cell. 2011October7;44:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Herb B, Wolschin F, Hansen K, et al. Reversible switching between epigenetic states in honeybee behavioral subcastes. Nature Neurosci. 2012September16; DOI:10.1038/nn.3128 [DOI] [PMC free article] [PubMed]
- 206.Jakovcevski M, Akbarian S. Epigenetic mechanisms in neurological disease. Nature Med. 2012August8;18:1194–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fraga M, Ballestar E, Paz M, et al. Epigenetic differences arise during the lifetime of monozygtic twins. PNAS. 2005July26;102 (30):10604–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhang T-H, Meaney M. Epigenetics and the environmental regulation of the genome and its function. Annu Rev Psychol. 2010;61:439–66 [DOI] [PubMed] [Google Scholar]
- 209.Holmes A, le Guisquet A, Vogel E, Millstein R, Leman S, Belzung C.Early life genetic and environmental factors shaping emotionality in rodents. Neurosci Biochem Revs. 2005;(29):1335–46 [DOI] [PubMed] [Google Scholar]
- 210.Worthman C. Habits of the heart: Life history and the developmental neuroendocrinology of emotion. Amer J Hum Bio. 2009;21:772–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Connor C, Akbarian S. DNA Methylation changes in schizophrenia and bipolar disorder. Epigenetics. 2008Mar-Apr;3(2,):55–8 [DOI] [PubMed] [Google Scholar]
- 212.Shulha H, Cheung I, Whittle C, Wang J, Virgil D, Lin C., Guo Y, Lessard A, Akbarian S, Weng Z. Epigenetic signatures of autism: Trimethylated H3K4 landscapes in prefrontal neurons. Arch Gen Psychiatry. 2012;69(3):314–24 [DOI] [PubMed] [Google Scholar]
- 213.Dawkins R. The selfish gene. New York: Oxford University Press; 1989 [Google Scholar]
- 214.Norman G, Hawkley L, Cole S, Berntson G, Cacioppo J. Social neuroscience: the social brain, oxytocin and health. Soc Neurosci. 2012;7(1):18–29 [DOI] [PubMed] [Google Scholar]
- 215.Tooby J, Cosmides L. The past explains the present: emotional adaptations and the structure of ancestral environments. Ethol Sociobiol. 1990;11:375–424 [Google Scholar]
- 216.Goldbeter A. Computational approaches to cellular rhythms. Nature. 2002November14;420:238–45 [DOI] [PubMed] [Google Scholar]
- 217.McCraty R. Heart-brain neurodynamics: The making of emotions. Boulder Creek: CA, Institute of Heartmath; 2003 [Google Scholar]
- 218.Hanson M, Stevens R. Discovery of new GPCR biology—one receptor structure at a time. Structure. 2009January14;17(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rosenbaum D, Rasmussen S, Kobilka B. The structure and function of G-protein-coupled receptors. Nature. 2009May21;459:356–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dal Santo P, Logan N, Chisholm A, Jorgensen E.The inositol trisphosphate receptor regulates a 50-second behavioral rhythm in C. elegans. Cell. 1999;(98.6):757–67 [DOI] [PubMed] [Google Scholar]
- 221.Rosenbaum D, Cherezov V, Hanson M, et al. ChGPCR engineering yields high-resolution structural insights into ^2-adrenergic receptor function. Science. 2007;318(5854):1266–73 [DOI] [PubMed] [Google Scholar]
- 222.Lowell C. Src-family kinases: rheostats of immune cell signaling. Mol Immunol. 2004;41(6):631–43 [DOI] [PubMed] [Google Scholar]
- 223.Guy C, Vignali K, Temirov J, et al. Distinct TCR signaling pathways drive proliferation and cytokine production in T cells. Nature Immunol. 2013March3;14:262–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Poirazi P, Mel B. Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron. 2001;29:779–96 [DOI] [PubMed] [Google Scholar]
- 225.Hausser M, Bartlett M. Dendrites: Bug or feature? Cur Opin Neurobiol. 2003;13:372–83 [DOI] [PubMed] [Google Scholar]
- 226.Barlow H. Intraneuronal information processing, directional selectivity and memory for spatiotemporal sequences. Network: Comput in Neur Systms; 1996;7:251–9 [DOI] [PubMed] [Google Scholar]
- 227.London M, Hausser M. Denditic computation. Ann Rev Neurosci. 2005;28:503–32 [DOI] [PubMed] [Google Scholar]
- 228.Bray D. Protein molecules as computational elements in living cells. Nature. 1995;376,307–12 [DOI] [PubMed] [Google Scholar]
- 229.Kralj J, Hochbaum D, Douglass A, Cohen A. Electrical spiking in Escherichia coli probed with a fluorescent voltage-indicating protein. Science. 2011July15;333:345–9 [DOI] [PubMed] [Google Scholar]
- 230.Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33(3):121–8 [DOI] [PubMed] [Google Scholar]
- 231.Moczulska K, Tinter-Thiede J, Peter M, et al. Dynamics of dendritic spines in the mouse auditory cortex during memory formation and memory recall. PNAS. 2013;110(45):18315–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bhatt D, Zhang S, Gan W-B.Dendritic spine dynamics. Ann Rev Physiol. 2009;71:261–82 [DOI] [PubMed] [Google Scholar]
- 233.Wallace R. Neural membrane microdomains as computational systems: Toward molecular modeling in the study of neural disease. BioSystems. 2007;87:20–30 [DOI] [PubMed] [Google Scholar]
- 234.Craig A. Interoception and emotion. In: Lewis M, Haviland-Jones J, Barrett L. editors. Handbook of emotions, 3rd ed; 2008:272–88 New York, NY: Guilford Press; 272–88 [Google Scholar]
- 235.Barrett L. Are emotions natural kinds? Persp Psych Sci. 2006;1(1);28–58 [DOI] [PubMed] [Google Scholar]
- 236.Barrett L, Bliss-Moreau E, Duncan S, Rauch S, Wright C. The amygdala and the experience of affect. SCAN. 2007;2:73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Brenhouse H, Anderson S. Nonsteroidal anti-inflammatory treatment prevents delayed effects of early life stress in rats. Biol Psychiatry. 2011;70:434–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lewis M. Bridging emotional theory and neurobiology through dynamic systems modeling. Behavioral and Brain Sciences. 2005;28(2):169–245:183 [DOI] [PubMed] [Google Scholar]
- 239.Heberlein A, Atkinson P. Neuroscientific evidence for simulation and shared substrates in emotion recognition: beyond faces. Emo Rev. 2009;1(2):162–77 [Google Scholar]
- 240.Oya H, Kawasaki H, Howard M, Adolphs R. Electrophysiological responses in the human amygdala discriminate emotion categories of complex visual stimuli. J Neurosci. 2002;22(21):9502–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ruby P, Decety J. How would you feel versus how do you think she would feel? A Neuroimaging study of perspective-taking with social emotions. J Cog Neurosci. 2004;16(6):988–99 [DOI] [PubMed] [Google Scholar]
- 242.Schwartz C, Wright C, Shin L, Kagan J, Rauch S. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003June20;300:1952–3 [DOI] [PubMed] [Google Scholar]
- 243.Bomhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula, and somatosensory cortex: a single-trial fMRI study. Brain. 2002;125:1326–36 [DOI] [PubMed] [Google Scholar]
- 244.Papez J. A proposed mechanism of emotion. Arch Neurol Psych. 1937;38(4):725–43 [Google Scholar]
- 245.Kennerley S. Is the reward really worth it? Nature Neurosci. 2012May5;15:647–9 [DOI] [PubMed] [Google Scholar]
- 246.Gazzola V, Spezio M, Etzel J, Castelli F, Adolphs R, Keysers C. Primary somatosensory cortex discriminates affective significance in social touch. PNAS. 2012June19;109(25):E1657–E66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Royet J-P, Plailly J, Delon-Martin C, Kareken D, Segebarth C. fMRI of emotional responses to odors: Influence of hedonic valence and judgment, handedness and gender. Neuroimage. 2003;20:713–28 [DOI] [PubMed] [Google Scholar]
- 248.Gottman J, Katz L. Children's emotional reactions to stressful parent-child interactions: The link between emotion regulation and vagal tone. Marriage Fam Rev. 2002;34(3/4):265–83 [Google Scholar]
- 249.Movius H, Allen J. Cardiac vagal tone, defensiveness, and motivational style. Biol Psych. 2004;68:147–62 [DOI] [PubMed] [Google Scholar]
- 250.Rottenberg J, Salomon K, Gross J, Gotlib I. Vagal withdrawal to a sad film predicts subsequent recovery from depression. Psychophysiol. 2005;42:277–81 [DOI] [PubMed] [Google Scholar]
- 251.Porges S. The polyvagal theory: Phylogenetic substrates of a social nervous system. Int J Psychophysiol. 2001;42:123–46 [DOI] [PubMed] [Google Scholar]
- 252.Porges S. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage: A polyvagal theory. Psychophysiol. 1995;32:301–18 [DOI] [PubMed] [Google Scholar]
- 253.Jack A, Dawson A, Begany K, Leckie R, Barry K, Ciccia A, Snyder A. fMRI reveals reciprocal inhibition between social and physical cognitive domains. NeuroImage. 2012; doi:10.1016/j.neuroimage.2012.10.061 [DOI] [PMC free article] [PubMed]
- 254.Tversky A, Kahneman D. Judgment under uncertainty: Heuristics and biases. Science. 1974September7;185:1124–30 [DOI] [PubMed] [Google Scholar]
- 255.Campbell W, Sedikides C. Self-threat magnifies the self-serving bias: A meta-analytic integration. Rev Gen Psych. 1999;3(1):23–43 [Google Scholar]
- 256.Conlin M, O'Donoghue T, Vogelsang T. Projection bias in catalog orders. Ameri Econ Rev. 2007;97(4):1217–49 [Google Scholar]
- 257.Gross J. Emotion regulation in adulthood: timing is everything. Cur Dir Psych Sci. 2001;10;(6):214–19 [Google Scholar]
- 258.Greene J, Paxton J. Patterns of neural activity associated with honest and dishonest moral decisions. PNAS. 2009;106(30):12506–ii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Valdesolo P, DeSteno D. The duality of virtue: Deconstructing the moral hypocrite. J Exp Soc Psych. 2008;44:1334–8 [Google Scholar]
- 260.McRae K, Ochsner K, Gross J. The reason in passion: a social cognitive neuroscience approach to emotion regulation. In: Vohs K, Baumeister R, editors. Handbook of self-regulation: research theory and applications, 2nd ed.New York, NY: Guilford Press; 2011:186–203 [Google Scholar]
- 261.Barrett L, Russell J. The structure of current affect: Controversies and emerging consensus. Cur Dir Psych Sci. 1999;8(1):10–4 [Google Scholar]
- 262.Nagel E. The structure of science: problems in the logic of scientific explanation. London: Rutledge and Kegan Paul; 1962 [Google Scholar]
- 263.Barrett L, Mesquita B, Ochsner K, Gross J. The experience of emotion. Annu Rev Psychol. 2007;58:373–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Weiner B. An attributional theory of achievement motivation and emotion. Psychol Rev. 1995;92;(4):548–73 [PubMed] [Google Scholar]
- 265.Ekman P. Facial expression and emotion. Amer Psych. 1993;48(4); 384. [DOI] [PubMed] [Google Scholar]
- 266.Harre R, Parrott G. (Eds). The emotions: social, cultural and biological dimensions. London: Sage; 1996 [Google Scholar]
- 267.Stechler G. Affect: The heart of the matter. Psychoanal Dialog. 2003;13(5):711–26 [Google Scholar]
- 268.Zajonc R. On the primacy of affect. In Scherer K, Ekman P. (Eds) Approaches to emotion. 1984: Hillsdale, NJ: Lawrence; Erlbaum. 259–70 [Google Scholar]
- 269.Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci. 2003May15;23(10):4325–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Hahn R. The nocebo phenomenon: Concept, evidence and implications for public health. Prev Med. 1997;26:607–ii [DOI] [PubMed] [Google Scholar]
- 271.Lidstone S, de la Fuente-Fernandez R, Stoessl A.The placebo response as a reward mechanism. Seminars in Pain Medicine. 2005;3(1):37–42 [Google Scholar]
- 272.Michael R, Garry M, Kirsch I. Suggestion, cognition, and behavior. Cur Dir Psych Sci. 2012;21(3):151–6 [Google Scholar]
- 273.Katkin E, Wiens S, Ohman A. Nonconscious fear conditioning, visceral perception, and the development of gut feelings. Psych Sci. 2001;12(5):366–70 [DOI] [PubMed] [Google Scholar]
- 274.Bower G, Cohen P. Emotional influences in memory and thinking: Data and theory. In Clark M S., Fiske S. T. (Eds) Affect and cognition: The Seventh Annual Carnegie Symposium on Cognition 1982:291–331 [Google Scholar]
- 275.Mischel W, Ayduk O. Willpower in a cognitive affective processing system: Dynamics of delayed gratification. In: Vohs K, Baumeister R, editors. Handbook of Self-Regulation: Research Theory and Applications, 2nd Ed. New York, NY: Guilford Press; 2011:83–105 [Google Scholar]
- 276.Egan L, Santos L, Bloom P. The origins of cognitive dissonance. Psych Sci. 2007;18(11):978–83 [DOI] [PubMed] [Google Scholar]
- 277.Moskowitz G, Li P, Kirk E. The implicit volition model: On the preconscious regulation of temporarily adopted goals. In Zanna M. P. (Ed) Adventures in Experimental Social Psychology. 2004;(36):317–413 [Google Scholar]
- 278.Wood W, Quinn J, Kashy D. Habits in everyday life: Thought, emotion and action. J Per Soc Psych. 2002;83:1281–97 [PubMed] [Google Scholar]
- 279.Bargh J, Gollwitzer P, Chai L, Barndollar K, Troetschel R. The automated will: Nonconscious activation and pursuit of behavioral goals. J Per Soc Psych. 2001;81:1014–27 [PMC free article] [PubMed] [Google Scholar]
- 280.Olson M, Fazio R. Implicit attitude formation through classical conditioning. Psych Sci. 2001;1(5):413–17 [DOI] [PubMed] [Google Scholar]
- 281.Panksepp J. Affective consciousness: Core emotional feelings in animals and humans. Cons Cog. 2005;14:30–80 [DOI] [PubMed] [Google Scholar]
- 282.O'Donoghue T, Rabin M. Doing it now or later. Amer Econ Rev. 1999;3:103–24 [Google Scholar]
- 283.Rabin M. Psychology and economics. J of Econ Literature. 1998March;36:ii–46 [Google Scholar]
- 284.Akerlof G. Nobel Prize lecture: Behavioral macroeconomics and macroeconomic behavior. http://www.nobelprize.org/nobel_prizes/economic-sciences/laureates/2001/akerlof-lecture.html Accessed February 20, 2014.
- 285.Aumann R, Sorin S. Cooperation and bounded recall. Games Econ Behav. 1989:1(1):5–39 [Google Scholar]
- 286.Cohen J, Blum K. Reward and decision. Neuron. 2002;36:193–8 [DOI] [PubMed] [Google Scholar]
- 287.Camerer C. Behavioral economics. World Congress of the Econometric Society. 2005August18–24; http://www.cemmap.com/papers/vol2_chap7.pdf Accessed February 19, 2014.
- 288.Sent E-M. Behavioral economics: How psychology made its (limited) way back into economics. Hist Pol Econ. 2004Win;36(4):735–60 [Google Scholar]
- 289.Bechara A, Damasio H, Tranel D, Damasio A. Deciding advantageously before knowing the advantageous strategy. Science. 1997February28;275:1293–4 [DOI] [PubMed] [Google Scholar]
- 290.Izard C. Basic emotions, natural kinds, emotion schemas, and a new paradigm. Pers Psych Sci. 2007;2(3):260–80 [DOI] [PubMed] [Google Scholar]
- 291.Izard C. Human emotions. New York, NY: Plenum; 1997 [Google Scholar]
- 292.Plutchik R. Emotions: A general psychoevolutionary theory. In: Scherer K, Ekman P, editors. Approaches to emotion. Hillsdale, NJ: Erlbaum; 1984. 197–219 [Google Scholar]
- 293.Clark J. Relations on homology between higher cognitive emotions and basic emotions. Biol Philos. 2010;25:75–94 [Google Scholar]
- 294.Arnold M. Psychological differentiation of emotional states. Psychol Rev. 1945;52:35–48 [Google Scholar]
- 295.Lazarus R. On the primacy of cognition. Am Psych. 1984;39(2);124–129 [Google Scholar]
- 296.Ortony A, Turner T. What's basic about basic emotions? Psychol Rev. 1990;97(3):315–31 [DOI] [PubMed] [Google Scholar]
- 297.Stenberg C, Campos J, Emde R. The facial expression of anger in 7-month-old infants. Child Dev. 1983;54(1):178–84 [DOI] [PubMed] [Google Scholar]
- 298.Lewis M. The emergence of human emotions. In: Lewis M, Haviland J, editors. Handbook of emotion. 1993:223–35
- 299.Han S, Lerner J, Keltner D. Feelings and consumer decision-making: The appraisal-tendency framework. J Consumer Psych. 2007;17(3):158–68 [Google Scholar]
- 300.Koole S, Van Dillen L, Sheppes G.The self-regulation of emotion. In: Vohs K, Baumeister R, editors. Handbook of Self-Regulation: Research Theory and Applications. 2nd ed.New York, NY: Guilford Press; 2011:22–40 [Google Scholar]
- 301.Baumeister R, Bratslavsky E, Finkenauer C, Vohs K. Bad is stronger than good. Rev Gen Psych. 2001;5(4):323–70 [Google Scholar]
- 302.Seligman M. Authentic happiness. New York, NY: Free Press; 2002 [Google Scholar]
- 303.Folkman S, Lazarus R, Gruen R, DeLongis A. Dynamics of a stressful encounter: Cognitive appraisal, coping, and encounter outcomes. J Per Soc Psych. 1986;50(5):992–1003 [DOI] [PubMed] [Google Scholar]
- 304.Pearlin H, Schooler C. The structure of coping. J Health Soc Beh. 1978;19:2–22 [PubMed] [Google Scholar]
- 305.Chen E. Protective factors for health among low-socioeconomic-status individuals. Cur Dir Psych Sci. 2012;21(3):189–93 [Google Scholar]
- 306.Haines J, Williams C. Coping and problem solving of self-mutilation. J Clin Psych. 1997;53(2):177–86 [DOI] [PubMed] [Google Scholar]
- 307.DeSteno D. Social emotions and intertemporal choice. Cur Dir Psych Sci. 2009;18(5):280–83 [Google Scholar]
- 308.Grant A, Sonnentag S. Doing good buffers against feeling bad: Prosocial impact compensates for negative task and self-evaluations. Org Beh Hum Dec Procs. 2010;111:13–22 [Google Scholar]
- 309.Ford D, Lerner R. Developmental Systems Theory: An integrative approach. 1993:88–98
- 310.Cacioppo J, Gardner W, Berntson G. The affect system has paralle and integrative processing components: Form follows function. J Pers Soc Psychol. 1999:76(5):839–55 [Google Scholar]
- 311.DeSteno D, Petty R, Rucker D, Wegener D. Beyond valence in the perception of likelihood: The role of emotion specificity. J Per Soc Psych. 2000;78(3):397–16 [DOI] [PubMed] [Google Scholar]
- 312.Morris W, Reilly N. Toward the self-regulation of mood: Theory and research. Mot Emo. 1987;11(3):25–249 [Google Scholar]
- 313.Thayer R, Newman J, McClain T. Self-regulation of Mood: Strategies for changing a bad mood, raising energy, and reducing tension. J Per Soc Psych. 1994;67(5):910–25 [DOI] [PubMed] [Google Scholar]
- 314.Izard C, Libero D, Putnam P, Haynes O. Stability of emotional experiences and their relations to traits of personality. J Per Soc Psych.1993;64(5):847–60 [DOI] [PubMed] [Google Scholar]
- 315.Lazarus R, Alfert E. Short-circuiting of threat by experimentally altering cognitive appraisal. J Abnor Soc Psych. 1964August;9(2):195–205 [DOI] [PubMed] [Google Scholar]
- 316.Seery M. Resilience: A silver lining to experiencing adverse life events? Cur Dir Psych Sci. 2001;20(6):390–4 [Google Scholar]
- 317.Tabibnia G, Satpute A, Lieberman M. The sunny side of fairness: Preference for fairness activates reward circuitry (disregarding unfairness activates selfcontrol circuitry). Psych Sci. 2008;19(4):339–47 [DOI] [PubMed] [Google Scholar]
- 318.Duckworth A, Seligman M. Self-discipline outdoes IQ in predicting academic performance of adolescents. Psych Sci. 2005;6(12):939–44 [DOI] [PubMed] [Google Scholar]
- 319.Duckworth A, Kirby T, Tsukayama E, Berstein H, Ericsson K. Deliberate practice spells success: Why grittier competitors triumph at the National Spelling Bee. Soc Psych Pers Sci. 2011;2(2):174–81 [Google Scholar]
- 320.Ramaprasad A. On the definition of feedback. Beh Sci. 1983;25;4–13
- 321.Swanson J, Rudman L, Greenwald A. Using the implicit association test to investigate attitude-behaviour consistency for stigmatized behavior. Cog Emo. 2001;5(2):207–30 [Google Scholar]
- 322.Osgood C, Tannenbaum P. The principle of congruity in the prediction of attitude change. Psychol Rev. 1955;62:42–55 [DOI] [PubMed] [Google Scholar]
- 323.Heider F. The psychology of interpersonal relations. New York, NY: Wiley; 1958. Epigenetic inheritance in evolution [Google Scholar]
- 324.Maltz M. Psycho-cybernetics. New York, NY: Pocket Books; 1989 [Google Scholar]
- 325.Pribram K. Steps toward a neuropsychological theory. In Glass D. (Ed) Neurophysiology and Emotion. 1967:3–39
- 326.Higgins E. Self-discrepancy: A theory relating self and affect. Psychol Rev. 1987;94(3):319–40 [PubMed] [Google Scholar]
- 327.Turrigiano G. Homeostatic signaling: The positive side of negative feedback. Cur Opin Neurobio. 2007;7:318–24 [DOI] [PubMed] [Google Scholar]
- 328.Sanjuan P. Affect balance as meditating variable between effective psychological functioning and satisfaction with life. J Happiness Studies. 2011;12(3):373–84 [Google Scholar]
- 329.McCulloch W, Pitts W. A logical calculus of the ideas immanent in nervous activity. Bul Math Biophys. 1943;7:115–33 [PubMed] [Google Scholar]
- 330.Piaget J. The Origins of Intelligence in Children. New York, NY: International Universities Press; 1952 [Google Scholar]
- 331.Vaillant G. Adaptation to life: how the best and the brightest came of age. Boston, MA: Little, Brown; 1977 [Google Scholar]
- 332.Solomon R. The opponent process theory of acquired motivation. Amer Psychol. 1980;35:691–12 [DOI] [PubMed] [Google Scholar]
- 333.Diener E, Sandvik E, Larsen R. Age and sex effects for emotional intensity. Dev Psych. 1985May;21(3):542–6 [Google Scholar]
- 334.Freud A. The ego and the mechanisms of defense. London: Hogarth; 1937 [Google Scholar]
- 335.Mazar N, Amir O, Ariely D. The dishonesty of honest people: A theory of selfconcept maintenance. J Marketing Research. 2008;45(6);633–644 [Google Scholar]
- 336.Fredrickson B. What good are positive emotions? Rev Gen Psych. 1998;2(3):300–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Taylor S, Klein L, Lewis P, Gruenewald T, Gurung R, Updegraff J. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:441–20 [DOI] [PubMed] [Google Scholar]
- 338.Chen Y-H. Coping with suffering: The Buddhist perspective. In Wong P, Wong L. (Eds) Handbook of Multicultural Perspectives on Stress and Coping; International Cultural Psychology Series. 2006:73–89
- 339.Fredrickson B. Cultivating positive emotions to optimize health and wellbeing. Prevention Treatment. 2000March7;(3):Article 0001a [Google Scholar]
- 340.Richman L, Kubzansky L, Maselko J, Kawachi I, Choo P, Bauer M. Positive emotion and health: Going beyond the negative. Health Psych. 2005;24(4):422–9 [DOI] [PubMed] [Google Scholar]
- 341.Carstenson L, Mikels J. At the intersection of emotion and cognition: Aging and the positivity effect. Cur Dir Psych Sci. 2005;14(3):117 [Google Scholar]
- 342.Xu J, Roberts R. The power of positive emotions: It's a matter of life or death -subjective well-being and longevity over 28 years in a general population. Health Psych. 2010;29(1):9–19 [DOI] [PubMed] [Google Scholar]
- 343.Moffitt T, Arseneault L, Belsky D, et al. A gradient of childhood self-control predicts health, wealth, and public safety. PNAS 2011February15;108(7):2693–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Fredrickson B. Positivity: Groundbreaking research reveals how to embrace the hidden strength of positive emotions, overcome negativity, and thrive. New York, NY: Crown Archetype; 2009 [Google Scholar]
- 345.Erickson E. Identity: Youth and crisis. New York: W. W. Norton; 1968 [Google Scholar]
- 346.Leary M, Tate E, Adams C, Allen A, Hancock J. Self-compassion and reactions to unpleasant self-relevant events: Implications of treating oneself kindly. J Per Soc Psych. 2007;92(5):887–904 [DOI] [PubMed] [Google Scholar]
- 347.Heckman J. The economics, technology, and neuroscience of human capability formation. PNAS. 2007August14:104(33):13250–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Moore M. Coaching the multiplicity of mind: A strengths based model. Glob Adv Health Med. 2013;2(4):74–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Nelson C, Furtado E, Fox N, Zeanah C. The deprived human brain. Amer Scientist. 2009May-Jun:97:222–9 [Google Scholar]
- 350.Beck A, Rush A, Shaw B, Emery G. (Eds). Cognitive therapy of depression. New York, NY: Guilford Press; 1979 [Google Scholar]
- 351.Ellis A. Reason and Emotion in Psychotherapy. New York, NY: Lyle Stuart; 1970 [Google Scholar]
- 352.Rozin P, Lowery L, Imada S, Haidt J. The CAD triad hypothesis; A mapping between three moral emotions (contempt, anger, disgust) and three moral codes (community, autonomy divinity). J Per Soc Psych. 1999:76^74–586 [DOI] [PubMed]
- 353.Tangney J, Stuewig J, Mashek D. Moral emotions and moral behavior. Annu Rev Psychol. 2007;58:345–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Mullen E, Nadler J. Moral spillovers: The effect of moral violations on deviant behavior. J Exp Soc Psych. 2008;44:1239–45 [Google Scholar]
- 355.DiGiuseppe R, Froh J. What cognitions predict state anger? J Rat-Emo Cog Beh Ther. 2002;20(2):133–49 [Google Scholar]
- 356.Scheff T, Retzinger S. Emotions and violence: shame and rage in destructive conflicts. Lexington, MA, USA: Lexington Books/D. C. Heath and Co; 1991 [Google Scholar]
- 357.Milligan R-J, Andrews B. Suicidal and other self-harming behaviour in offender women: The Role of Shame, Anger and Childhood Abuse. Leg Crim Psych. 2005;10(1):13–25 [Google Scholar]
- 358.Athens L. The Creation of Dangerous Violent Criminals. Chicago, IL: University of Illinois Press; 1992 [Google Scholar]
- 359.Gilligan J. Violence: Our deadly epidemic and its causes. New York: Grosset/Putnam; 1996 [Google Scholar]
- 360.Dickerson S, Kemeny M. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psych Bul. 2004;130(3):355–91 [DOI] [PubMed] [Google Scholar]
- 361.Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nature Neurosci. 2012May;15(5):663–8 [DOI] [PubMed] [Google Scholar]
- 362.Tsankova N, Renthal W, Kumar A, Nestler E. Epigenetic regulation in psychiatric disorders. Nature Revs Neurosci. 2007May:355–67 [DOI] [PubMed] [Google Scholar]
- 363.Hertzman C, Boyce T. How experience gets under the skin to create gradients in developmental health. Ann Rev Pub Health. 2010;31:329–47 [DOI] [PubMed] [Google Scholar]
- 364.Hyman S. How adversity gets under the skin. Nature Neurosci. 2009March3;12:241–3 [DOI] [PubMed] [Google Scholar]
- 365.Hara M, Kovacs J, Whalen E, et al. A stress response pathway regulates DNA damage through ^2-adrenoreceptors and |31-arrestin-1. Nature. 2011; 477(7364):349–53 doi:10.1038/nature10368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Kahnsari D, Murgo A, Faith R. Effects of stress on the immune system. Immunol Today. 1990;11(5):170–5 [DOI] [PubMed] [Google Scholar]
- 367.Burchinal M, Roberts J, Hooper S, Zeisel S. Cumulative risk and early cognitive development: A comparison of statistical risk models. Dev Psych. 2000;36:793–807 [DOI] [PubMed] [Google Scholar]
- 368.Boyce T, Quas J, Alkon A, Smider N, Essex M, Kupfer D. Autonomic reactivity and psychopathology in middle childhood. Brit J Psychiatry. 2001;79:144–50 [DOI] [PubMed] [Google Scholar]
- 369.Tsigos C, Chrousos G. Hypothalmic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Rsrch. 2002;53:865–71 [DOI] [PubMed] [Google Scholar]
- 370.Caspi A, McClay J, Moffitt T, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2012August2;297:851–4 [DOI] [PubMed] [Google Scholar]
- 371.Cummings E, Davies P. Effects of marital conflict on children: Recent advances and emerging themes in process-oriented research. J Child Psych Psychiatry Allied Disciplines. 2002;43:31–63 [DOI] [PubMed] [Google Scholar]
- 372.Essex M, Klein M, Cho E, Kalin N. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biol Psychiatry. 2002;52:776–84 [DOI] [PubMed] [Google Scholar]
- 373.Patel V, Kleinman A. Poverty and common mental disorders in developing countries. Bul World Health Org. 2003;81(8):609–15 [PMC free article] [PubMed] [Google Scholar]
- 374.Masten A, Shaffer A. How families matter in child development: Reflections from research on risk and resilience. In: Clarke-Stewart A, Dunn J, editors. Families count: effects on child and adolescent development. New York, NY: Cambridge University Press; 2006:5–25 [Google Scholar]
- 375.Van Ijzendoorn M, Bakermans-Kranenburg M. DRD4 7-repeat polymorphism moderates the association between maternal unresolved loss or trauma and infant disorganization. Attach Hum Dev. 2006;8:291–307 [DOI] [PubMed] [Google Scholar]
- 376.Taylor S, Way B, Welch W, Hilmert C, Lehman B, Eisenberger N. Early family environment, current adversity, the serotonin transporter polymorphism, and depressive symptomatology. Biol Psychiatry. 2006;60:671–6 [DOI] [PubMed] [Google Scholar]
- 377.Bakermans-Kranenburg M, Van IJzendoorn M.Genetic vulnerability or differential susceptibility in child development: the case of attachment. J Child Psych Psychiatry. 2007;48:1160–73 [DOI] [PubMed] [Google Scholar]
- 378.Boyce T. A biology of misfortune: Stress reactivity, social context, and the ontogeny of psychopathology in early life. In: Masten A, editor. Multilevel dynamics in developmental psychopathology: Pathways to the future; 34th ed.Minneapolis, MN: University of Minnesota; 2007:45–82 [Google Scholar]
- 379.Kleinman A. Culture and depression. N Engl J Med. 2010;351(10, September2):951–3 [DOI] [PubMed] [Google Scholar]
- 380.Bell I. White paper: Neuropsychiatric aspects of sensitivity to low-level chemicals: A neural sensitization model. Tox Indust Health. 1994Jul-Oct;10(4-5):277–312 [PubMed] [Google Scholar]
- 381.Bell I, Schwartz G, Baldwin C, Hardin E. Neural sensitization and physiological markers in multiple chemical sensitivity. Reg Tox Pharm. 1996August;24(1Pt2):S39–47 [DOI] [PubMed] [Google Scholar]
- 382.Baron-Cohen S, Belmonte M. Autism: a window onto the development of the social and the analytic brain. Ann Rev Neurosci. 2005;28:10–126 [DOI] [PubMed] [Google Scholar]
- 383.Yunus M. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Seminars Arthrit Rheum. 2007June;36(6):339–56 [DOI] [PubMed] [Google Scholar]
- 384.Sorg B. Multiple chemical sensitivity: potential role for neural sensitization. Crit Revs Neurobio. 1999:13(3):283–316 [DOI] [PubMed] [Google Scholar]
- 385.Obradovic J, Bush N, Stamperdahl J, Adler N, Boyce W. Biological sensitivity to context: the interactive effect of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Dev. 2010Jan-Feb;81(1):270–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Crews D, Gillette R, Scarpino S, Manikkam M, Savenkova M, Skinner K. Epigenetic transgenerational inheritance of altered stress responses. PNAS. 2012June5;109(23):9143–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Qureshi I, Mehler M. Impact of nuclear organization and dynamics on epigenetic regulation in the central nervous system: Implications for neurological disease states. ANYAS. 2010:1204(Epigenetics and Neuropsychiatric Diseases), E20–E37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Panksepp J. Brain emotional circuits and psychopathologies. In: Clynes M, Panksepp J, editors. Emotions and psychopathology. New York, NY: Plenum Press,;1988: 37–76 [Google Scholar]
- 389.Pittenger C, Duman R. Stress, depression, and neuroplasty: A convergence of mechanisms. Neuropsychopharmacology. 2008;33: 88–109 [DOI] [PubMed] [Google Scholar]
- 390.Mehler M. Epigenetics and neuropsychiatric disease: introduction and meeting summary. ANYAS. 2010;1204 (Epigenetics and Neuropsychiatric Diseases):E1–E7 [DOI] [PubMed] [Google Scholar]
- 391.Blair R. Moral reasoning and the child with psychopathic tendencies. Per Ind Difs. 997;22(5):731–9 [Google Scholar]
- 392.Blair R. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cog Sciences. 2007;11(9):388–92 [DOI] [PubMed] [Google Scholar]
- 393.Scaer R. The neurophysiology of dissociation and chronic disease. App Psychophys Biofeedback. 2001;26(1):73–91 [DOI] [PubMed] [Google Scholar]
- 394.Radulescu A. Schizophrenia - a parameters' game? J Theor Bio. 2008;254:89–98 [DOI] [PubMed] [Google Scholar]
- 395.Garay P, McAllister A. Novel roles for immune molecules in neural development: Implications for neurodevelopmental disorders. Front Synaptic Neurosci. 2010;2:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Carlat D. Unhinged: The trouble with psychiatry - a doctor's revelations about a profession in crisis. New York, NY: Free Press; 2010 [Google Scholar]
- 397.Whitaker R. Anatomy of an epidemic: magic bullets, psychiatric drugs, and the astonishing rise of mental illness in America. New York, NY: Crown Publishers; 2010 [DOI] [PubMed] [Google Scholar]
- 398.Kirsch I. The emperor's new drugs: exploding the antidepressant myth. New York, NY: Basic Books; 2011 [Google Scholar]
- 399.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: Amer Psychiatric Pub Inc; 1980 [Google Scholar]
- 400.Larsen R, Fredrickson B. Measurement issues in emotion research. In: Kahneman D, Diener E, Schwartz N, editors. Well-being: foundations of hedonic psychology). New York, NY: Russell Sage; 1999:40–60 [Google Scholar]
- 401.Averill J. Emotions are a many splendored thing. In: Eckman P, Davidson R, editors. The nature of emotion: fundamental questions. New York, NY: Oxford University Press; 1994:99–102 [Google Scholar]
- 402.Gross J, Barrett L. Emotion generation and emotion regulation: One or two depends on your point of view. Emo Rev. 2011;3(1):8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Fijda N. The laws of emotion. Amer Psych. 1988;43(5):349–58 [DOI] [PubMed] [Google Scholar]
- 404.Milan E, Iborra O, Hochel M, et al. Auras in mysticism and synesthesia: a comparison. Consci Cog. 2012;21:258–68 [DOI] [PubMed] [Google Scholar]
- 405.Phillips M, Drevets W, Rauch S, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–14 [DOI] [PubMed] [Google Scholar]
- 406.Berger J, Berry D. Statistical analysis and the illusion of objectivity. Amer Scientist. 1988Mar-Apr;76:159–65 [Google Scholar]
- 407.Raferty A. Baysian model selection in social research. Sociological Method. 1995;25:111–63 [Google Scholar]
- 408.Kording K, Wolpert D. Baysian integrations in sensorimotor learning. Nature. 2004January15;427:244–7 [DOI] [PubMed] [Google Scholar]
- 409.Barrett L. Was Darwin wrong about emotion? Cur Dir Psych Sci. 2011;20(6):400–6 [Google Scholar]
- 410.Darwin C. The expression of emotion in man and animals (Originally published 1872). New York, NY: Appleton; 2005:19 [Google Scholar]
- 411.Bain A. Mind and Body: The theories of their relation. New York, NY: Appleton Co.974:58–78 [Google Scholar]
- 412.van Praag H, Kempermann G, Gage F. Neural consequences of environmental enrichment. Nature Rev. Neurosci. 2000;1:191–8 [DOI] [PubMed] [Google Scholar]
- 413.Moore G. Principia ethics. Cambridge, MA: Cambridge University Press; 1903 [Google Scholar]
- 414.Hume D. A Treatise of Human Nature (Originally published 1739). London: Oxford University Press (Selby-Bigge); 1975 [Google Scholar]
- 415.Voorzanger B. No norms and no nature: The moral relevance of evolutionary biology. Biol Phil. 1987;2:253–70 [Google Scholar]
- 416.Seligman M, Csikszentmehalyi M. Positive Psychology: An introduction. Amer Psych. 2000;55(1):5–14 [DOI] [PubMed] [Google Scholar]
- 417.Deacon T. Incomplete Nature: How mind emerges from matter. New York, NY: W. W. Norton Company; 2011 [Google Scholar]


