Abstract
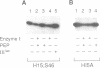
The bacterial phosphotransferase system (PTS) catalyzes the transport and phosphorylation of its sugar substrates. The protein-kinase-catalyzed phosphorylation of serine 46 in the phosphocarrier protein, HPr, inhibits PTS activity, but neither the mechanism of this inhibition nor its physiological significance is known. Site-specific HPr mutants were constructed in which serine 46 was replaced by alanine (S46A), threonine (S46T), tyrosine (S46Y) or aspartate (S46D). The purified S46D protein exhibited markedly lower Vmax and higher Km values than the wild-type, S46T or S46A protein for the phosphoryl transfer reactions involving HPr(His approximately P). Interactions of HPr with the enzymes catalyzing phosphoryl transfer to and from HPr regulated the kinase-catalyzed reaction. These results establish the inhibitory effect of a negative charge at position 46 on PTS-mediated phosphoryl transfer and suggest that HPr is phosphorylated on both histidyl and seryl residues by enzymes that recognize its tertiary rather than its primary structure. In vivo studies showed that a negative charge on residue 46 of HPr strongly inhibits PTS-mediated sugar uptake, but that competition of two PTS permeases for HPr(His approximately P) is quantitatively more important to the regulation of PTS function than serine 46 phosphorylation.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert C. A., Frank R., Stüber K., Deutscher J., Hengstenberg W. Phosphoenolpyruvate-dependent protein kinase enzyme I of Streptococcus faecalis: purification and properties of the enzyme and characterization of its active center. Biochemistry. 1985 Feb 12;24(4):959–964. doi: 10.1021/bi00325a023. [DOI] [PubMed] [Google Scholar]
- Band L., Yansura D. G., Henner D. J. Construction of a vector for cloning promoters in Bacillus subtilis. Gene. 1983 Dec;26(2-3):313–315. doi: 10.1016/0378-1119(83)90204-4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Bolshakova T. N., Dobrynina O. Y., Gershanovitch V. N. Isolation and investigation of the Escherichia coli mutant with the deletion in the ptsH gene. FEBS Lett. 1979 Nov 1;107(1):169–172. doi: 10.1016/0014-5793(79)80488-3. [DOI] [PubMed] [Google Scholar]
- Borthwick A. C., Holms W. H., Nimmo H. G. Amino acid sequence round the site of phosphorylation in isocitrate dehydrogenase from Escherichia coli ML308. FEBS Lett. 1984 Aug 20;174(1):112–115. doi: 10.1016/0014-5793(84)81087-x. [DOI] [PubMed] [Google Scholar]
- Breidt F., Jr, Hengstenberg W., Finkeldei U., Stewart G. C. Identification of the genes for the lactose-specific components of the phosphotransferase system in the lac operon of Staphylococcus aureus. J Biol Chem. 1987 Dec 5;262(34):16444–16449. [PubMed] [Google Scholar]
- Button D. K., Egan J. B., Hengstenberg W., Morse M. L. Carbohydrate transport in Staphylococcus aureus. IV. Maltose accumulation and metabolism. Biochem Biophys Res Commun. 1973 Jun 8;52(3):850–855. doi: 10.1016/0006-291x(73)91015-2. [DOI] [PubMed] [Google Scholar]
- Carter P., Bedouelle H., Winter G. Improved oligonucleotide site-directed mutagenesis using M13 vectors. Nucleic Acids Res. 1985 Jun 25;13(12):4431–4443. doi: 10.1093/nar/13.12.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Cozzone A. J. Protein phosphorylation in prokaryotes. Annu Rev Microbiol. 1988;42:97–125. doi: 10.1146/annurev.mi.42.100188.000525. [DOI] [PubMed] [Google Scholar]
- Deutscher J., Beyreuther K., Sobek H. M., Stüber K., Hengstenberg W. Phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus: factor IIIlac, a trimeric phospho-carrier protein that also acts as a phase transfer catalyst. Biochemistry. 1982 Sep 28;21(20):4867–4873. doi: 10.1021/bi00263a006. [DOI] [PubMed] [Google Scholar]
- Deutscher J., Kessler U., Hengstenberg W. Streptococcal phosphoenolpyruvate: sugar phosphotransferase system: purification and characterization of a phosphoprotein phosphatase which hydrolyzes the phosphoryl bond in seryl-phosphorylated histidine-containing protein. J Bacteriol. 1985 Sep;163(3):1203–1209. doi: 10.1128/jb.163.3.1203-1209.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J., Saier M. H., Jr ATP-dependent protein kinase-catalyzed phosphorylation of a seryl residue in HPr, a phosphate carrier protein of the phosphotransferase system in Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6790–6794. doi: 10.1073/pnas.80.22.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGAN J. B., MORSE M. L. CARBOHYDRATE TRANSPORT IN STAPHYLOCOCCUS AUREUS I. GENETIC AND BIOCHEMICAL ANALYSIS OF A PLEIOTROPIC TRANSPORT MUTANT. Biochim Biophys Acta. 1965 Feb 15;97:310–319. doi: 10.1016/0304-4165(65)90096-6. [DOI] [PubMed] [Google Scholar]
- Eisermann R., Deutscher J., Gonzy-Treboul G., Hengstenberg W. Site-directed mutagenesis with the ptsH gene of Bacillus subtilis. Isolation and characterization of heat-stable proteins altered at the ATP-dependent regulatory phosphorylation site. J Biol Chem. 1988 Nov 15;263(32):17050–17054. [PubMed] [Google Scholar]
- Friedman S. A., Hays J. B. Initial characterization of hexose and hexitol phosphoenolpyruvate-dependent phosphotransferases of Staphylococcus aureus. J Bacteriol. 1977 Jun;130(3):991–999. doi: 10.1128/jb.130.3.991-999.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzy-Tréboul G., Steinmetz M. Phosphoenolpyruvate:sugar phosphotransferase system of Bacillus subtilis: cloning of the region containing the ptsH and ptsI genes and evidence for a crr-like gene. J Bacteriol. 1987 May;169(5):2287–2290. doi: 10.1128/jb.169.5.2287-2290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzy-Tréboul G., Zagorec M., Rain-Guion M. C., Steinmetz M. Phosphoenolpyruvate:sugar phosphotransferase system of Bacillus subtilis: nucleotide sequence of ptsX, ptsH and the 5'-end of ptsI and evidence for a ptsHI operon. Mol Microbiol. 1989 Jan;3(1):103–112. doi: 10.1111/j.1365-2958.1989.tb00109.x. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hengstenberg W., Penberthy W. K., Hill K. L., Morse M. L. Phosphotransferase system of Staphylococcus aureus: its requirement for the accumulation and metabolism of galactosides. J Bacteriol. 1969 Aug;99(2):383–388. doi: 10.1128/jb.99.2.383-388.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbitzer H. R., Hengstenberg W., Rösch P., Muss P., Bernsmann P., Engelmann R., Dörschug M., Deutscher J. HPr proteins of different microorganisms studied by hydrogen-1 high-resolution nuclear magnetic resonance: similarities of structures and mechanisms. Biochemistry. 1982 Jun 8;21(12):2879–2885. doi: 10.1021/bi00541a012. [DOI] [PubMed] [Google Scholar]
- Klevit R. E., Waygood E. B. Two-dimensional 1H NMR studies of histidine-containing protein from Escherichia coli. 3. Secondary and tertiary structure as determined by NMR. Biochemistry. 1986 Nov 18;25(23):7774–7781. doi: 10.1021/bi00371a073. [DOI] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. I. Isolation of a phosphotransferase system from Escherichia coli. J Biol Chem. 1971 Mar 10;246(5):1393–1406. [PubMed] [Google Scholar]
- LaPorte D. C., Koshland D. E., Jr Phosphorylation of isocitrate dehydrogenase as a demonstration of enhanced sensitivity in covalent regulation. Nature. 1983 Sep 22;305(5932):286–290. doi: 10.1038/305286a0. [DOI] [PubMed] [Google Scholar]
- MORSE M. L., ALIRE M. L. An agar medium indicating acid production. J Bacteriol. 1958 Sep;76(3):270–271. doi: 10.1128/jb.76.3.270-271.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mimura C. S., Poy F., Jacobson G. R. ATP-dependent protein kinase activities in the oral pathogen Streptococcus mutans. J Cell Biochem. 1987 Mar;33(3):161–171. doi: 10.1002/jcb.240330303. [DOI] [PubMed] [Google Scholar]
- Oskouian B., Stewart G. C. Cloning and characterization of the repressor gene of the Staphylococcus aureus lactose operon. J Bacteriol. 1987 Dec;169(12):5459–5465. doi: 10.1128/jb.169.12.5459-5465.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Novotny M. J., Hengstenberg W., Saier M. H., Jr Properties of ATP-dependent protein kinase from Streptococcus pyogenes that phosphorylates a seryl residue in HPr, a phosphocarrier protein of the phosphotransferase system. J Bacteriol. 1984 Oct;160(1):333–340. doi: 10.1128/jb.160.1.333-340.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Novotny M. J., Panos C., Saier M. H., Jr Mechanism of inducer expulsion in Streptococcus pyogenes: a two-step process activated by ATP. J Bacteriol. 1983 Oct;156(1):354–361. doi: 10.1128/jb.156.1.354-361.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Novotny M. J., Stuiver I., Saier M. H., Jr Regulation of glycerol uptake by the phosphoenolpyruvate-sugar phosphotransferase system in Bacillus subtilis. J Bacteriol. 1984 Jul;159(1):243–250. doi: 10.1128/jb.159.1.243-250.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Panos C. Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form. J Bacteriol. 1982 Jan;149(1):211–220. doi: 10.1128/jb.149.1.211-220.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Peterkofsky A., Romano A. H. Evidence for the presence of heat-stable protein (HPr) and ATP-dependent HPr kinase in heterofermentative lactobacilli lacking phosphoenolpyruvate:glycose phosphotransferase activity. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2041–2045. doi: 10.1073/pnas.85.7.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Saier M. H., Jr, Deutscher J., Grenier F., Thompson J., Hengstenberg W. The phosphoenolpyruvate:sugar phosphotransferase system in gram-positive bacteria: properties, mechanism, and regulation. Crit Rev Microbiol. 1988;15(4):297–338. doi: 10.3109/10408418809104461. [DOI] [PubMed] [Google Scholar]
- Romano A. H., Brino G., Peterkofsky A., Reizer J. Regulation of beta-galactoside transport and accumulation in heterofermentative lactic acid bacteria. J Bacteriol. 1987 Dec;169(12):5589–5596. doi: 10.1128/jb.169.12.5589-5596.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusina O. Iu, Gershanovich V. N. Kartirovanie mutatsii vnutri genov, kodiruiushchikh ferment I i belok Hpr fosfoenolpiruvatzavisimoi fosfotransferaznoi sistemy u Escherichia coli K-12. Soobshchenie II. Kartirovanie mutatsii vnutri gena ptsH. Genetika. 1983 Mar;19(3):397–405. [PubMed] [Google Scholar]
- Saier M. H., Jr Protein phosphorylation and allosteric control of inducer exclusion and catabolite repression by the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Microbiol Rev. 1989 Mar;53(1):109–120. doi: 10.1128/mr.53.1.109-120.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Simoni R. D. Regulation of carbohydrate uptake in gram-positive bacteria. J Biol Chem. 1976 Feb 10;251(3):893–894. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnierow B. J., Yamada M., Saier M. H., Jr Partial nucleotide sequence of the pts operon in Salmonella typhimurium: comparative analyses in five bacterial genera. Mol Microbiol. 1989 Jan;3(1):113–118. doi: 10.1111/j.1365-2958.1989.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Roseman S., Saier M. H., Jr Sugar transport. Properties of mutant bacteria defective in proteins of the phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6584–6597. [PubMed] [Google Scholar]
- Simoni R. D., Roseman S. Sugar transport. VII. Lactose transport in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):966–974. [PubMed] [Google Scholar]
- Sutrina S. L., Chin A. M., Esch F., Saier M. H., Jr Purification and characterization of the fructose-inducible HPr-like protein, FPr, and the fructose-specific enzyme III of the phosphoenolpyruvate: sugar phosphotransferase system of Salmonella typhimurium. J Biol Chem. 1988 Apr 15;263(11):5061–5069. [PubMed] [Google Scholar]
- Thorsness P. E., Koshland D. E., Jr Inactivation of isocitrate dehydrogenase by phosphorylation is mediated by the negative charge of the phosphate. J Biol Chem. 1987 Aug 5;262(22):10422–10425. [PubMed] [Google Scholar]
- Waygood E. B., Reiche B., Hengstenberg W., Lee J. S. Characterization of mutant histidine-containing proteins of the phosphoenolpyruvate:sugar phosphotransferase system of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1987 Jun;169(6):2810–2818. doi: 10.1128/jb.169.6.2810-2818.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yansura D. G., Henner D. J. Use of the Escherichia coli lac repressor and operator to control gene expression in Bacillus subtilis. Proc Natl Acad Sci U S A. 1984 Jan;81(2):439–443. doi: 10.1073/pnas.81.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]
- Zuber M., Patterson T. A., Court D. L. Analysis of nutR, a site required for transcription antitermination in phage lambda. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4514–4518. doi: 10.1073/pnas.84.13.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Kabbani O. A., Waygood E. B., Delbaere L. T. Tertiary structure of histidine-containing protein of the phosphoenolpyruvate:sugar phosphotransferase system of Escherichia coli. J Biol Chem. 1987 Sep 25;262(27):12926–12929. [PubMed] [Google Scholar]