Abstract
Aberrant expression of the T-box transcription factor brachyury in human carcinomas drives the phenomenon of epithelial-mesenchymal transition (EMT), a phenotypic modulation that facilitates tumor dissemination and resistance to conventional therapies, including chemotherapy and radiation. By generating isogenic cancer cell lines with various levels of brachyury expression, we demonstrate that high levels of brachyury also significantly reduce the susceptibility of cancer cells to lysis by both antigen-specific T cells and natural killer cells. Our results indicated that resistance of brachyury-high tumor cells to immune-mediated attack was due to inefficient caspase-dependent apoptosis, manifested as inefficient nuclear lamin degradation in the presence of activated effector caspases. We correlated this phenomenon to loss of cell cycle kinase CDK1, which mediates lamin phosphorylation. In support of a causal connection, pre-treatment of tumor cells with a specific inhibitor of WEE1, a negative regulator kinase of CDK1, could counter the defective apoptosis of tumor cells expressing high levels of brachyury. Thus, our findings suggested that reconstituting CDK1 activity to threshold levels may be sufficient to restore immunosurveillance of mesenchymal-like cancer cells that have escaped previous immune detection or eradication.
Keywords: EMT, brachyury, immune resistance, CDK1, WEE1
Introduction
The progression of carcinomas remains a poorly understood phenomenon. A recently recognized mechanism involved in tumor metastasis is the epithelial-mesenchymal transition (EMT), a phenotypic modulation that promotes the loss of tumor epithelial features and the simultaneous acquisition of mesenchymal-associated traits, including tumor motility and invasiveness (1, 2). In addition to promoting metastasis, EMT has also been associated with the acquisition of resistance to cell death (3), a characteristic that may favor the survival of mesenchymal cancer cells not only as they disseminate from the primary tumor, but also in response to chemotherapy, radiation, and some small-molecule targeted therapies (4, 5). As a result, cancer therapies may select for tumor cells undergoing EMT, a phenomenon first demonstrated in vivo with breast cancer recurrences (6).
Our laboratory has characterized the T-box transcription factor brachyury as a driver of EMT in human carcinoma cells (7, 8). We have shown that various types of tumors overexpress brachyury (8, 9) and that its levels of expression positively correlate with resistance to chemotherapy or radiation (10, 11). We also demonstrated that circulating, brachyury-specific cytotoxic CD8+ T cells can be detected in the blood of carcinoma patients (12, 13), an observation that led us to propose a T-cell based immunotherapeutic approach, rather than conventional therapies, as a means to specifically target tumor cells undergoing brachyury-mediated EMT.
In recent years the role of the immune system in tumor eradication and prognosis has gained increased recognition (14, 15) and immune-evasion is now included as an “emerging hallmark of cancer” (16). To date, however, it is not clearly understood whether EMT contributes to the escape of tumors from host immune-surveillance and immune-mediated rejection. In the present study, human carcinoma cells undergoing EMT via brachyury overexpression were compared to their epithelial counterparts in terms of their susceptibility to immune-mediated attack. Our results demonstrate that high levels of brachyury reduce the susceptibility of carcinoma cells to either antigen-specific, CD8+ cytotoxic T lymphocytes (CTLs) or innate natural killer (NK) and lymphokine-activated killer (LAK) cells by decreasing the contribution to cell death of caspase-dependent pathways, while leaving unaffected the caspase-independent tumor cytolysis involving perforin. Analysis of apoptotic markers showed that resistance of brachyury-high tumor cells to caspase-mediated cell death is due to the absence of nuclear lamin degradation in the presence of normal levels of activated effector caspases, a defect that appears to be related to the loss of the cell-cycle dependent kinase 1 (CDK1, also known as p34cdc2), a kinase involved in lamin phosphorylation and subsequent caspase-mediated lysis (17, 18). Pre-treatment of tumor cells with a specific inhibitor of WEE1, a negative regulator kinase of CDK1, was shown to fully counter the defective apoptosis of tumor cells with high levels of brachyury in vitro, therefore indicating that reconstitution of CDK1 activity to a threshold level may be sufficient to improve immune-mediated attack of mesenchymal-like, brachyury-high tumor cells.
Materials and Methods
Immune effector cells
Peripheral blood from healthy donors and cancer patients was obtained under appropriate Institutional Review Board approval and informed consent. Antigen-specific, HLA-A2–restricted cytotoxic CD8+ T cells (CTLs) were expanded from peripheral blood mononuclear cells (PBMCs) from three different cancer patients utilizing the following HLA-A2 binding peptides as previously described: brachyury, WLLPGTSTL (12); mucin-1 (MUC1), ALWGQDVTSV (19) and carcinoembryonic antigen (CEA), YLSGADLNL (20). Briefly, dendritic cells (DCs) were prepared from PBMCs by culture in the presence of GM-CSF and IL-4, subsequently pulsed with the corresponding peptides and used to stimulate autologous T cells in vitro. CD8+ T cells were isolated with a magnetic CD8+ isolation kit (Miltenyi Biotech). Murine H-2Kb-restricted gp70-specific CTLs were established as previously described against peptide p15e604, KSPWFTTL (21). Natural killer cells were isolated from healthy donor PBMCs by using a magnetic NK cell isolation kit (Miltenyi Biotech). For generation of lymphokine activated killer (LAK) cells, purified NK cells were incubated overnight in RPMI media supplemented with 10% human AB sera and 2000 units/ml of recombinant human IL-2.
Cell culture
The following human carcinoma cell lines were obtained from American Type Culture Collection (ATCC): pancreatic PANC-1; lung A549 and H460; breast MDA-MB-231; and colon SW480. Cell lines were authenticated by short tandem repeat (STR) profiling. Expression vectors and transfection strategies were previously described (7). Silencing of CDK1 expression was achieved by utilizing an ON-TARGET SMARTpool siRNA; control cells were transfected with a non-targeting siRNA pool as per the manufacturer’s recommendations (Thermo Scientific). Cells were utilized 48 hours post-transfection.
Cytotoxicity assays
Cytotoxicity assays were performed as previously described (12). To inhibit the function of perforin/granzyme, NK or T cells were pre-incubated with 200 nM Concanamycin A (Sigma) for two hours at 37°C. Caspase-dependent lysis was inhibited by pre-incubating target cells with 50 μM Z-VAD-FMK (Calbiochem) for one hour prior to the assay. Where indicated, apoptosis was initiated by cross-linking of the FAS receptor with 12.5 μg/ml anti-CD95 (clone DX2) antibody (BD Pharmingen) and 0.5 μg/ml protein G (GE Healthcare) or incubation with 250 ng/ml of a recombinant, active multimeric Superkiller TRAIL (Enzo Life Sciences). When indicated, tumor cells were treated with 100 nM of the WEE1 inhibitor MK-1775 (ChemieTeck) for 72 hours prior to the assay.
RNA isolation and RT-PCR
RNA isolation and real time PCR assays were performed as previously described (7) utilizing the probes listed in Table S1 (Applied Biosystems). Expression of CEA and gp70 mRNA was analyzed by PCR using a Titanium Taq PCR kit (Clontech) with 50 ng cDNA utlizing CEA or gp70-specific primers as listed in Table S1. PCR products were quantified with an Agilent Bioanalizer (Agilent Technologies).
Western blot
Commercial lysates from human lung and breast tumor biopsies were purchased from Imgenex (San Diego, CA). The following antibodies were used: pan-actin (clone Ab-5, Neo Markers), Beta-2 microglobulin (clone BBM.1, Santa Cruz), caspase 8 (clone Ab-3, Calbiochem), brachyury, lamin B1, lamin A/C (Abcam), CDK1 and caspase-3 (Cell Signaling Technology). Where indicated, protein lysates were run on a 7% acrylamide gel supplemented with 25 μM Phos-Tag (Wako Pure Chemical Industries), following the manufacture’s recommendations. All western blots were imaged and quantified using the Odyssey Infrared imaging system (LI-COR Biotechnology).
Immunofluorescence and immunohistochemistry
Carcinoma cells were grown on coverslips and stained as previously described (7) with monoclonal antibodies recognizing CDK1 (Cell Signaling), brachyury (Abcam) and MUC1 (clone DF3, 1/50 dilution). Where indicated, tumor cells were incubated with LAK cells at 37° C for one hour, and stained for F-actin utilizing Alexa Fluor 488® phalloidin as per the manufacturers’ recommendation. Images were captured utilizing a Leica Fluorescent microscope. Confocal images were obtained utilizing a Zeiss LSM 510 META Confocal Microscope.
Lung tumor tissue arrays were purchased from US Biomax, Inc. Sections of paraffin-embedded, formalin-fixed tissues were tested with a brachyury (Abcam) or a CDK1 antibody (EMD Millipore) as previously described (9), and counterstained with hematoxylin.
Murine studies
All animal studies were carried out in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Experimental studies were carried out under approval of the NIH Intramural Animal Care and Use Committee. Murine colon carcinoma MC38 cells were stably transfected with an empty vector (pCMV) or a brachyury-encoding (pBr) vector as previously described (22). Tumor cells (5×105 MC38-pCMV or MC38-pBr) were subcutaneously (s.c) implanted into female C57BL/6 mice on day 0. Beginning on day 4, animals were vaccinated weekly with either HBSS or 50 μg of a gp70 peptide (p15e) emulsified in Montanide ISA-51-VG (Seppic) at a 1:1 ratio. Where indicated, gp70-specific T cells were admixed with either 5×105 MC38-pCMV or MC38-pBr cells at a 2:1 ratio of effector cells to tumor cells and s.c. implanted into female C57BL/6 mice.
Statistical Analysis
Data was analyzed using GraphPad Prism (version 4) (GraphPad Software). Data points in graphs represent the mean ± SEM and p< 0.05 was considered significant.
Results
Brachyury expression associates with resistance to immune-mediated lysis
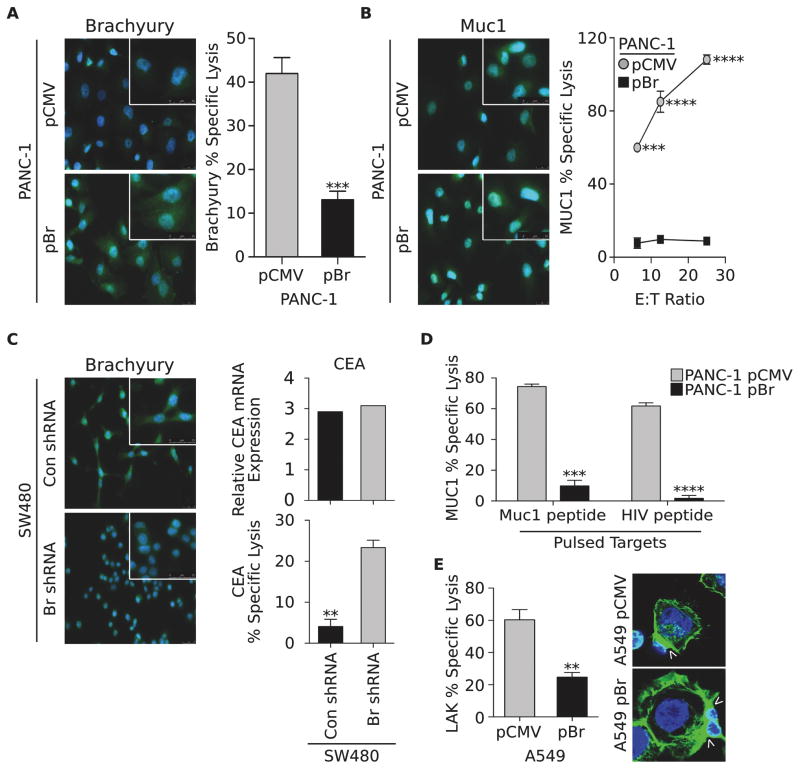
To investigate whether brachyury-mediated EMT could induce resistance to immune-mediated killing, an isogenic tumor cell pair was generated by stably transfecting pancreatic PANC-1 cells, characterized by low endogenous levels of brachyury, with a control vector (pCMV) or a vector encoding the full-length human brachyury protein (designated hereafter as pBr, Fig. 1A). Tumor lysis was evaluated utilizing an HLA-A2–restricted brachyury-specific CTL line established against an epitope of the brachyury protein as previously described (12). As shown in Fig. 1A, the cytotoxic lysis of PANC-1–pBr cells was significantly reduced compared to that of control PANC-1–pCMV cells, in spite of greater levels of the target brachyury being expressed by the PANC-1–pBr cells. Impaired tumor lysis was also observed with human CTLs directed against an HLA-A2–restricted epitope of the tumor antigen Mucin-1 (MUC1) (19). As shown in Fig. 1B, the levels of MUC1 were not decreased as a result of brachyury overexpression, yet the PANC-1–pBr cells were lysed less efficiently than PANC-1–pCMV cells at all effector-to-target cell ratios evaluated. Comparable results were obtained with breast MDA-MB-231 cancer cells that exhibited decreased MUC1-specific T-cell lysis after brachyury over-expression (Fig. S1A), or with PANC-1 cells induced into a mesenchymal phenotype by treatment with the EMT inducer TGF-β (Fig. S1B), compared to their epithelial cell counterparts.
Figure 1. High levels of brachyury induce resistance to immune-mediated lysis.
(A) Immnofluorescent analysis of brachyury (green signal) in PANC-1 cells transfected with control pCMV or a vector encoding brachyury (pBr) and lysis of the same cells by brachyury-specific CTLs. Shown is a representative result of three similar experiments. (B) Expression of MUC1 (green signal) in the PANC-1 pair and lysis by MUC1-specific CTLs at indicated effector-to-target (E:T) ratios. Shown is a representative result of four similar experiments. (C) Immunofluorescent analysis of brachyury (green signal) in SW480 cells transfected with a control (Con shRNA) or a brachyury-specific shRNA (Br shRNA) (green signal). Right panels: CEA expression in the SW480 tumor pair (top) and lysis with CEA-specific CTLs (bottom). (D) Susceptibility of PANC-1 tumor cells pulsed with a MUC1 or a control HIV peptide to lysis by MUC1-specific CTLs. Shown is a representative result of two similar experiments. (E) LAK-mediated lysis of A549 cells transfected with pCMV vs. pBr, and corresponding Phalloidin staining of polymerized F-actin (green signal, arrows indicate the interacting surface). Original magnification of all images: 40X. Blue corresponds to DAPI stained nuclei. Error bars indicate the standard error of the mean (SEM) of triplicate measurements. ** p<0.01, *** p<0.001, **** p<0.0001.
Loss-of-function experiments were also conducted to examine the impact of reducing brachyury expression on the susceptibility of colon SW480 carcinoma cells to immune-mediated cytotoxicity. While brachyury silencing, indicated as Br shRNA, (Fig. 1C) had no significant impact on the level of expression of the tumor-associated antigen carcinoembryonic antigen (CEA, Fig. 1C), the susceptibility of SW480 cells to lysis by HLA-A2-restricted CEA-specific CTLs generated as previously described from the blood of a cancer patient (20) was greatly enhanced (Fig. 1C). These results indicated that, at high levels of expression, brachyury might impair T-cell mediated lysis regardless of the antigen specificity of the effector T cells.
Defective lysis of brachyury-high tumor cells is not due to defective antigen presentation
A well-recognized mechanism of tumor resistance to antigen-specific T-cell lysis is the downregulation of components of the antigen presentation and/or processing machinery. The PANC-1 tumor cell pair showed no differences regarding the levels of MHC-class I or beta-2 microglobulin (β2M) (Figs. S2A, B). Brachyury-high tumor cells also demonstrated a significant enhancement, rather than a reduction, in the expression of various components of the class I antigen-presentation machinery, including TAP1 and TAP2, tapasin, and the proteasome subunits LMP2 and LMP7 (Fig. S2C). Furthermore, the defective lysis of PANC-1–pBr cells by MUC1-specific CTLs was not reversed when antigen presentation was fully bypassed via the addition of exogenous MUC1 vs. control HIV peptide (Fig. 1D). Based on these results, it was evident that the reduced lysis of tumor cells with high levels of brachyury was not due to inefficient antigen presentation. This conclusion was further reinforced by the observation that reduced susceptibility of brachyury-high tumor cells to immune-mediated lysis was not restricted to antigen-specific T cells but also observed with antigen non-specific LAK cells. As shown in Figure 1E, LAK-mediated lysis of A459-pBr cells was significantly reduced compared with that of A549-pCMV cells, even if a stable engagement between the effectors (LAK) and the tumor cells occurred, irrespective of the level of brachyury, as demonstrated by equivalent polar actin polymerization observed at the interacting surface area (arrows, Fig. 1E). Similar results were observed with the PANC-1 tumor cell pair (not shown).
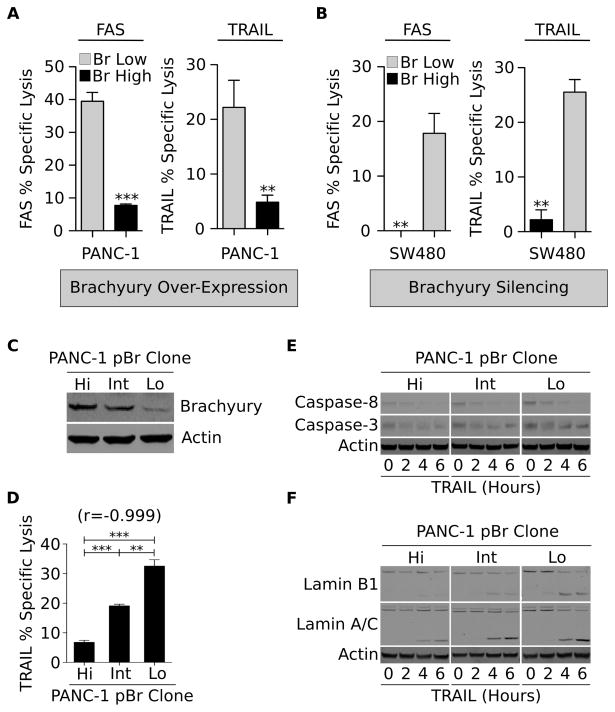
Degradation of nuclear lamins is defective in brachyury-high tumor cells
To understand the mechanism(s) involved in the resistance of brachyury-high cells to immune-mediated attack, lysis of tumor cells following engagement of the immune-related, surface-associated death receptors FAS and TRAIL-R was first evaluated. As shown in Fig. 2A, brachyury overexpression induced a profound resistance to FAS and TRAIL-mediated lysis in PANC-1 cells, while stable silencing of brachyury significantly enhanced the susceptibility of SW480 cells to both apoptotic triggers (Fig. 2B). This resistance was not related to the level of expression of functional or decoy FAS and TRAIL receptors (Figs. S2D, E), neither was associated with the expression of FAS-associated phosphatase-1 (FAP-1, Fig. S2F), a protein previously reported to be involved in the attenuation of FAS-mediated cell death (23).
Figure 2. Resistance to apoptosis in the presence of normal levels of activated caspases.
Susceptibility of indicated tumor cell pairs obtained by either (A) overexpression or (B) silencing of brachyury in response to FAS or recombinant TRAIL. Experiments were repeated with at least two cell lines with similar results. (C) Western blot analysis of brachyury expression in single-cell derived PANC-1–pBr clones, and (D) corresponding sensitivity to TRAIL. Number in parentheses indicates the Pearson’s correlation coefficient calculated in relation to brachyury expression. Shown is a representative result of two similar experiments. (E) Tumor cells were left untreated or treated for indicated times with recombinant TRAIL; protein lysates were analyzed by western blot for caspase-8 and caspase-3, and (F) for cleavage of lamins B1 and A/C. Error bars indicate the SEM of triplicate measurements. Shown is a representative result of two similar experiments. ** p<0.01, ***p<0.001.
The association of brachyury with resistance to the extrinsic apoptotic pathway was further investigated with three single-cell derived populations of the PANC-1–pBr cell line (designated as PANC-1–pBr clones Hi, Int and Lo), characterized by high, intermediate and low levels of brachyury expression, respectively (Fig. 2C). As shown in Fig. 2D, a strong inverse correlation was observed between the level of brachyury and the tumor cells’ susceptibility to TRAIL-mediated lysis (r =−0.999). Unexpectedly, the degree of cleavage of both the initiator caspase-8 and the effector caspase-3 was equivalent among the clones, regardless of the level of brachyury expression (Fig. 2E). The degradation of nuclear lamins, however, was markedly different among the clones. As shown in Fig. 2F, cleavage of lamin B1 and, to a lower extent lamin A/C, was profoundly defective in tumor cells with high levels of brachyury (PANC-1–pBr clone Hi), compared to that observed in the brachyury-low clone (PANC-1–pBr clone Lo).
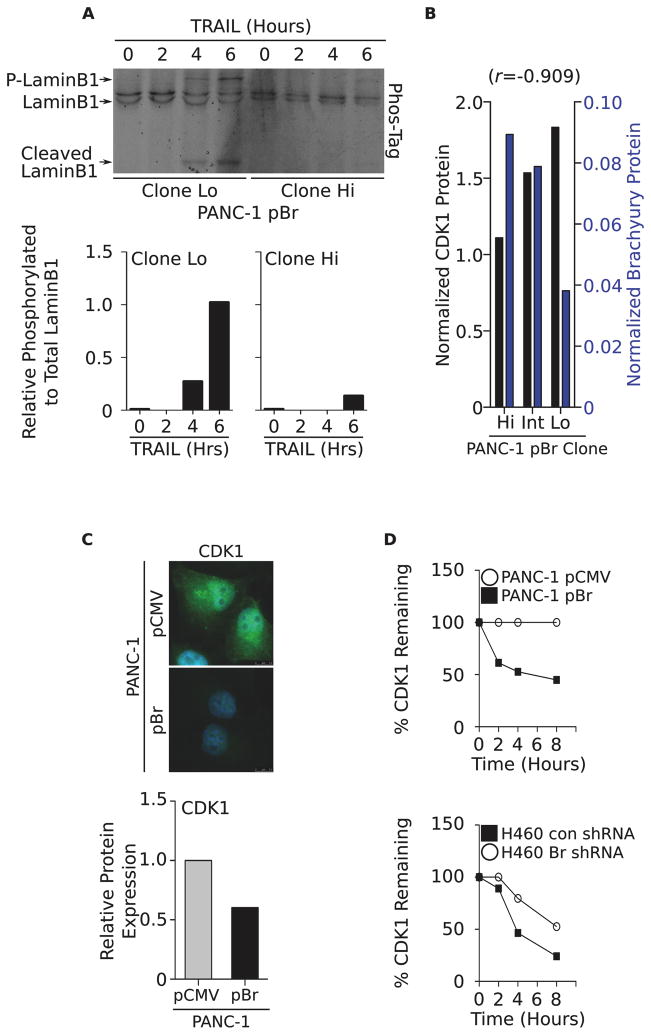
Defective lysis of brachyury-high cells associates with loss of CDK1 and can be restored by WEE1 inhibition
Previous reports have demonstrated that lamin phosphorylation is a required step for degradation of the nuclear lamina to take place during mitosis and apoptosis (18). We therefore hypothesized that inadequate lamin phosphorylation in brachyury-high tumor cells could be responsible, at least in part, for the inefficient degradation of the nuclear lamins, even in the presence of normal levels of activated effector caspases. As shown in Figure 3A, TRAIL treatment of tumor cells with low levels of brachyury (PANC-1–pBr clone Lo) resulted in efficient phosphorylation and concurrent cleavage of lamin B1, an effect that was not observed in the brachyury-high clone (PANC-1–pBr clone Hi).
Figure 3. Resistance of brachyury-high tumor cells is associated with loss of CDK1.
(A) Phosphorylation status of lamin B1 in TRAIL-treated PANC-1–pBr Lo and Hi clones evaluated on a Phos-Tag gel and corresponding quantification (bottom panels). Shown is a representative result of two similar experiments. (B) Expression of CDK1 (black bars) and brachyury protein (blue bars) in the various PANC-1–pBr clones relative to actin protein. Number in parentheses indicates the Pearson’s correlation coefficient calculated in relation to brachyury protein expression. Shown is a representative result of two similar experiments. (C) Immunofluorescent analysis (top panel) and western blot quantification (bottom panel) of CDK1 in the PANC-1 tumor pair; green and blue signal correspond to CDK1 and DAPI-stained nuclei, respectively. Original magnification: 100X. (D) CDK1 protein stability was evaluated in the PANC-1 and H460 tumor cell pairs following addition of 100 μg/ml cycloheximide for indicated time points. Protein levels were normalized to 0 hours (100%).
One of the kinases known to be involved with the phosphorylation of nuclear lamins is the cell-cycle dependent kinase-1 CDK1 (24). Western blot analysis of CDK1 expression in the single-cell clonal populations of PANC-1–pBr (Fig. 3B) demonstrated a strong inverse correlation between the levels of brachyury and CDK1 protein (r= −0.909). Additional immunofluorescence and western blot analyses conducted with the PANC-1 tumor cell pair corroborated the marked reduction of CDK1 protein in brachyury-high tumor cells (Fig. 3C). This decrease in CDK1 protein was not associated with changes in mRNA levels (Fig. S3A), but instead with a marked reduction of CDK1 protein stability, as demonstrated by the rapid degradation of CDK1 in brachyury-high cells (PANC-1–pBr and H460 Con shRNA) as compared to brachyury-low cells (PANC-1–pCMV and H460 Br shRNA, respectively, Fig. 3D and Fig. S3B).
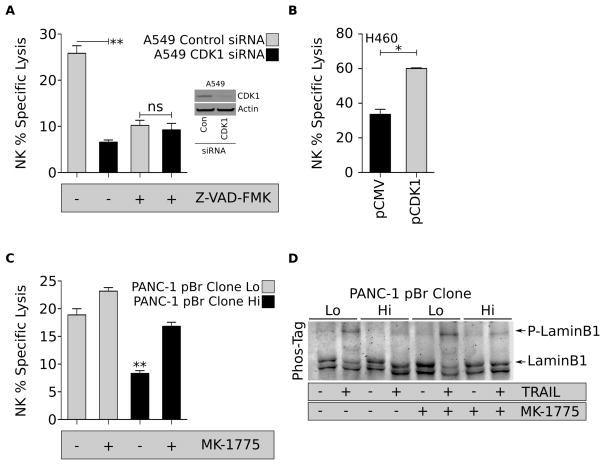
The role of CDK1 loss in the acquisition of resistance of brachyury-high tumor cells to immune-mediated attack was further investigated by evaluating the impact of CDK1 silencing on the tumor’s susceptibility to lysis by NK cells. As shown in Figure 4A, silencing of CDK1 led to a significant reduction in the susceptibility of A549 cells to NK-mediated lysis. As this effect was not seen with tumor cells pre-treated with the pan-caspase inhibitor Z-VAD-FMK, the results suggested that reduced levels of CDK1 impair caspase-dependent apoptosis. In additional experiments, CDK1 was overexpressed in tumor cells with high levels of brachyury (H460-pCDK1), a manipulation that resulted in enhanced NK-mediated lysis, compared to control H460-pCMV cells (Fig. 4B).
Figure 4. Lysis of brachyury-high cells can be restored by WEE1 inhibition.
(A) Lysis of A549 transfected with control or CDK1 siRNA by NK cells and pre-treated with Z-VAD-FMK prior to plating in the CTL assay. Insert shows a western blot analysis of CDK1 protein in transfected A549 cells. (B) NK-mediated lysis of H460 cells over-expressing CDK1, compared to control pCMV cells. (C) NK-mediated lysis of PANC-1–pBr Lo and Hi clones pre-treated with the WEE1 inhibitor MK-1775. Cells were plated at a 20:1 effector to target ratio, and percent lysis was assessed at 16 hours. Error bars indicate the SEM of triplicate measurements. (D) Phosphorylation status of lamin B1 in TRAIL-treated PANC-1-pBr clones left untreated or pre-treated with MK-1775. Shown is a representative result of two similar experiments. * p<0.05, ** p<0.01.
Based on the above results, we postulated that tumor sensitization to immune-mediated attack could be achieved by restoring the activity of remaining CDK1 protein to an adequate level for efficient nuclear apoptosis to proceed. The approach was investigated by inhibiting the WEE1 kinase, a cell cycle kinase that negatively regulates CDK1 activity. As demonstrated in Figure 4C, pre-treatment of PANC-1–pBr tumor cells with MK-1775, a WEE1-specific small molecule inhibitor, was sufficient to fully reconstitute the lysis of PANC-1–pBr clone Hi cells to the levels observed with the PANC-1–pBr clone Lo. We observed similar results in the ability of MK-1775 to restore the lysis of A549-pBr to levels comparable to A549-pCMV cells in response to NK- and TRAIL-mediated cell death (Fig. S4). As expected, pre-treatment with MK-1775 was associated with increased phosphorylation of laminB1 in PANC-1–pBr clone Hi cells treated with TRAIL (Fig. 4D).
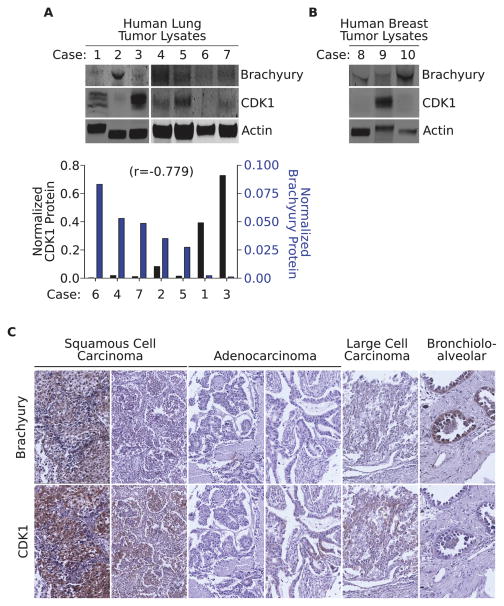
Expression of brachyury and CDK1 in tumor tissues
The expression of brachyury and CDK1 protein was compared by western blot analysis in commercial protein lysates derived from lung (cases 1–7) and breast (cases 8–10) primary carcinoma tissues. As shown in Figure 5A, there was an inverse correlation (r= −0.779) between the level of brachyury expression and that of CDK1 protein in lung tumor tissues. A trend was also observed in two out of three breast cancer tissues evaluated (Fig. 5B) that showed low levels of CDK1 expression in correspondence with high levels of brachyury. Additional lung cancer tissues were also analyzed by immunohistochemistry for the expression of brachyury and CDK1 protein. As shown in Figure 5C, an inverse correspondence between the levels of brachyury and those of CDK1 could be observed in 4 out of 6 lung carcinomas evaluated.
Figure 5. Expression of brachyury and CDK1 in tumor tissues.
(A) Western blot analysis of brachyury and CDK1 expression in commercially available protein lysates from lung tumor tissues. Actin was utilized as a loading control. Bottom panel: expression of CDK1 (black bars) and brachyury protein (blue bars) in the various tissues analyzed. Number in parentheses indicates the Pearson’s correlation coefficient calculated in relation to brachyury protein expression. (B) Brachyury and CDK1 expression in primary breast carcinomas. (C) Additional lung tumor tissue sections were stained for brachyury and CDK1 protein expression by immunohistochemistry (brown signal). Tissues were counterstained with hematoxylin; slides were digitally scanned with an Aperio ScanScope CS scanning system (Aperio Technologies Inc.). Original magnification: 40X.
Differential effect of brachyury on caspase-dependent vs. perforin-dependent tumor lysis
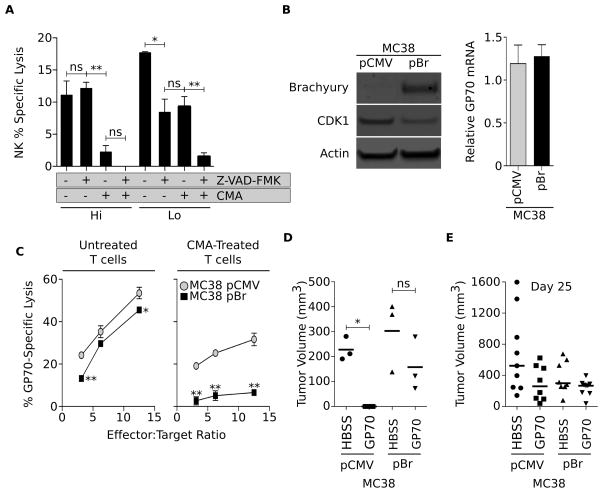
Immune effector cells have the capacity to kill target cells in absence of caspase activation through the perforin-dependent actions of granzymes. In light of the impact of brachyury expression on the caspase-dependent apoptotic pathway, we investigated whether high brachyury levels could also impair perforin-dependent lytic pathways. These studies were performed with two PANC-1–pBr clones (clone Hi vs. Lo with high vs. low levels of brachyury, respectively) that were left untreated or pre-treated with the pan-caspase inhibitor Z-VAD-FMK prior to the assay. Effector cells utilized in these assays were NK cells that were left untreated or pre-treated with CMA prior to the assay, a manipulation that ablates perforin-dependent lysis of the target cells. As shown in Figure 6A, single blockade of caspase activity significantly reduced the lysis of the PANC-1–pBr clone Lo but had no effect on the lysis of the PANC-1–pBr Hi clone. In contrast, blockade of perforin-dependent lysis via CMA treatment was able to significantly reduce the lysis of the PANC-1–pBr Hi but not that of the PANC-1–pBr Lo clone. Combined blockade of caspases and perforin activity, as expected, markedly reduced the lysis of both tumor cell lines. These results indicated that tumor cells with low levels of brachyury could be efficiently lysed after triggering of caspase-dependent pathways while lysis of tumor cells with high levels of brachyury appears to be exclusively dependent on the involvement of the perforin-mediated pathway.
Figure 6. Brachyury induces tumor immune resistance in vivo.
(A) Lysis of PANC-1–pBr Lo and Hi clones left untreated or pre-treated with the pan-caspase inhibitor Z-VAD-FMK by NK cells left untreated or pre-treated with CMA for inhibition of perforin-mediated lysis. (B) Western blot analysis of brachyury and CDK1 expression in MC38 cells transfected with pCMV vs. pBr, and expression of gp70 in the same cells (right panel). (C) Lysis of the MC38 tumor cell pair was conducted at indicated effector-to-target ratios with gp70-specific CTLs either untreated or pre-treated with CMA for inhibition of perforin-dependent lysis. Shown is a representative result of two similar experiments. (D) Indicated tumor cells were admixed in vitro with gp70-specific T cells and subsequently implanted s.c. into C57BL/6 mice. Shown is the tumor volume at day 16 post-implantation. (E) Tumor cells (MC38-pCMV vs. -pBr) were implanted s.c. on day 0; mice were vaccinated with gp70 peptide in adjuvant (vs. control HBSS) at days 4, 11 and 18. Shown is the tumor volume on day 25 post-tumor implantation. * p<0.05, ** p<0.01.
Tumor cells with high brachyury escape immune attack in vivo
To investigate whether high levels of brachyury expression would drive tumor resistance to effector lysis in vivo, the murine colon carcinoma cell line MC38 was stably transfected with a control vector (MC38-pCMV) or a vector encoding the full-length brachyury protein (MC38-pBr, Fig. 6B). Similarly to our observations with human carcinoma cells, overexpression of brachyury in MC38 cells concomitantly decreased the levels of CDK1 protein (Fig. 6B) without affecting the expression of gp70, the envelope protein of an endogenous murine retrovirus previously described as a tumor antigen in the MC38 model (21). The effect of brachyury overexpression on tumor lysis by gp70-specific CTLs was then evaluated in vitro. As shown in Fig. 6C, gp70-specific CTLs were able to lyse MC38-pBr cells less efficiently than control MC38-pCMV cells at all effector-to-target ratios utilized and, in agreement with the results with human carcinoma cells, the defective lysis of MC38-pBr cells was more pronounced after ablation of perforin-mediated cytotoxicity. To test whether brachyury could also induce resistance to immune effector cells in vivo, gp70-specific CTLs were admixed in vitro at a ratio of 2:1 with MC38-pBr or MC38-pCMV tumor cells and subsequently injected s.c. into syngeneic C57BL/6 mice. As shown in Fig. 6D, gp70-specific T cells were efficient at preventing growth of MC38-pCMV cells (tumors failed to grow in 3/3 mice) but failed to control the growth of MC38-pBr tumors in 3/3 animals. In additional experiments, MC38-pBr and MC38-pCMV cells were implanted s.c. in the flank of C57BL/6 mice. Four days after tumor implantation, mice were vaccinated with a gp70 peptide or saline admixed with adjuvant Montanide; vaccines were weekly administered for a total of 3 weeks. As shown in Figure 6E, gp70 vaccination was able to reduce (although not statistically significant) the growth of MC38-pCMV but not that of MC38-pBr tumors, compared to the corresponding control groups. These observations are in agreement with a report by Kudo-Saito et al (25) that demonstrated that B16 melanoma cells overexpressing snail were resistant to the anti-tumor effect of intratumoral injection of DCs pulsed with the gp70 peptide, as compared to parental B16 cells.
Discussion
The activation of the EMT program in tumors has been proposed as a mechanism by which cancer cells bearing a mesenchymal-like phenotype may survive conventional anti-neoplastic interventions. The studies reported here indicate that acquisition of a mesenchymal-like phenotype via expression of high levels of the EMT regulator brachyury could also mediate resistance to immune-mediated attack, potentially contributing to tumor ignorance and failure of immune rejection of human tumor cells.
In recent years, numerous tumor antigens have been identified and antigen-specific CD8+ T-cell immune responses have been detected in the blood of cancer patients and in lymphocytic infiltrates of multiple types of tumors (15, 26). The acquisition of immune evasion mechanisms, however, allows cancer cells to grow and metastasize in spite of the presence of a measurable anti-tumor immune response (27, 28). Defects in the antigen processing and/or presentation machinery, including decreased expression of HLA molecules and reduced levels of tumor antigens are well-recognized mechanisms of immune evasion. These phenomena, however, cannot explain all instances of immunological resistance. Reports are now starting to implicate the phenomenon of EMT in tumor resistance to CTL-mediated lysis; Akalay et al (29), for example, recently demonstrated that EMT might induce resistance to CTL lysis through the induction of autophagy. In this report we have extended the understanding of the role of EMT in tumor resistance to immune attack by showing that brachyury decreases the ability of antigen-specific T cells to lyse tumor cells in the presence of effective levels of MHC, antigen or the various components of the antigen presentation machinery. Interestingly, tumor cells with high levels of brachyury showed an upregulation, rather than a downregulation, of the immunoproteosome subunits LMP2 and LMP7. This phenomenon, which is commonly seen in cellular responses to stress, may lead to enhanced generation and presentation of MHC-class I antigenic epitopes. Thus, deficient antigen presentation can be ruled out as the mechanism of resistance of brachyury-high cells. Furthermore, we show that the impairment of immune effector-mediated lysis of brachyury-high cells could also be observed during antigen-independent cytotoxicity mediated by NK or LAK cells.
The results from this study indicate that the poor killing associated with high levels of brachyury is mainly due to inefficient caspase-dependent apoptotic death, and not with perforin-mediated lysis involving granzymes. Previous studies have investigated the mechanisms by which other EMT transcription factors could drive resistance to cell death. For example, the overexpression of snail has been shown to impair apoptosis in response to TNF-α by decreasing the activity of initiator caspase-8 and effector caspase-3 (3). Contrasting with those studies, the resistance of brachyury-high tumor cells to caspase-mediated cell death takes place in the presence of normal levels of fully activated effector caspases. Instead, the major apoptotic defect identified here is the absence of degradation of nuclear lamins. The nuclear lamina is a protein mesh formed by the intermediate filament lamins A-type (lamins A, C) and B-type (lamins B1, B2) (30), closely associated with the inner nuclear membrane and the chromatin. In addition to playing a key role in maintaining the nuclear envelope integrity and nuclear architecture, disassembly of the lamina is a required step during mitosis as well as for the induction of caspase-dependent apoptosis (31). We demonstrate here a profound reduction in CDK1 levels in brachyury-high tumor cells which, in turn, results in deficient lamin phosphorylation and defective degradation by effector caspases (32). Although the mechanism involved in the reduction of CDK1 protein in brachyury-high cells has not been investigated here, we have previously shown that brachyury expression reduces the levels of the cell cycle regulator p21 (10) which, in turn, could promote the assembly and stabilization of cyclin B1/CDK1 kinase at the G2/M transition (33).
The WEE1 kinase inactivates CDK1 by phosphorylating Tyr15 (34). Previous reports have indicated a role for WEE1 in the decreased response of breast cancer cells to TRAIL-mediated apoptosis (35) or the resistance to radiotherapy in various types of tumor cells (36). Currently, a specific inhibitor of WEE1 is being tested in Phase II clinical trials for solid tumors in combination with chemotherapy. Our results demonstrate that WEE1 blockade by MK-1775 is able to fully revert the resistance of brachyury-high tumor cells to caspase-dependent cell death induced by TRAIL, NK or LAK effector cells. This observed reconstitution of susceptibility to cell death is presumably achieved by restoring threshold levels of activated CDK1 in brachyury-high cells, which might then allow for the proper phosphorylation of lamins and their subsequent targeting for degradation by activated caspases (Fig. S5).
To date, there is no clear understanding of what factors dictate the cytolytic mechanisms employed by CTLs to kill tumor cells, either in vivo or in vitro. A study with murine renal carcinoma cells, for example, has shown that tumors that present low levels of MHC class I-associated antigens to the effector T cells are preferentially lysed by the FasL pathway, whereas at high levels of peptide the CTLs lose their preference for effector pathway usage (37). We demonstrate here that efficient lysis of brachyury-high tumor cells could only be achieved by effector cells capable of lysing via the granule/perforin pathway. This observation is consistent with the idea that during perforin-dependent apoptosis, Granzymes A and B have been shown to directly cleave the nuclear lamins thus being able to promote the disruption of the nuclear membrane even in the absence of proper lamin phosphorylation (31).
A Phase I clinical trial of a recombinant yeast-brachyury vaccine (22) is currently ongoing in patients with advanced tumors (38). This vaccine is aimed at inducing a brachyury-specific T-cell immune response that could eliminate tumor cells undergoing brachyury-mediated EMT. The results from this study indicate that the effectiveness of an immune response against tumor cells that express high levels of brachyury could be further enhanced by (a) reconstituting caspase-dependent cell death via inactivation of the WEE1 kinase, or (b) by downregulating the expression of brachyury, which paradoxically can increase the susceptibility of the tumor to brachyury-specific (and other tumor-specific) T cells elicited by vaccination of patients with a brachyury-based vaccine. Although paradoxical, this strategy would allow for an alleviation of resistance mechanisms mediated by brachyury, and improve tumor lysis even in the presence of lower levels of the target antigen.
Supplementary Material
Acknowledgments
Financial support: This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
The authors thank Dr. Jeffrey Schlom for helpful discussions on the manuscript, Dr. James W. Hodge for the gp70-specific T cells, Dr. Yvona Ward and the CCR’s CCBB Microscopy Core Facility for their assistance in obtaining confocal images, Margie Duberstein, Marion Taylor and Bertina Gibbs for technical assistance, and Debra Weingarten for editorial assistance.
Footnotes
The authors have no conflict of interest to declare.
Authors’ contributions: DHH designed and performed experiments, collected and analyzed data and wrote the manuscript; BH and RIF performed experiments, collected and analyzed data; KYT provided vital reagents and CP designed experiments and supervised research, analyzed data and wrote the manuscript.
References
- 1.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. 2011;27:347–76. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- 3.Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18:1131–43. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson S, Petti F, Sujka-Kwok I, Epstein D, Haley JD. Kinase switching in mesenchymal-like non-small cell lung cancer lines contributes to EGFR inhibitor resistance through pathway redundancy. Clin Exp Metastasis. 2008;25:843–54. doi: 10.1007/s10585-008-9200-4. [DOI] [PubMed] [Google Scholar]
- 5.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–59. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106:13820–5. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernando RI, Litzinger M, Trono P, Hamilton DH, Schlom J, Palena C. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J Clin Invest. 2010;120:533–44. doi: 10.1172/JCI38379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernando RI, Castillo MD, Litzinger M, Hamilton DH, Palena C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 2011;71:5296–306. doi: 10.1158/0008-5472.CAN-11-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roselli M, Fernando RI, Guadagni F, Spila A, Alessandroni J, Palmirotta R, et al. Brachyury, a driver of the epithelial-mesenchymal transition, is overexpressed in human lung tumors: an opportunity for novel interventions against lung cancer. Clin Cancer Res. 2012;18:3868–79. doi: 10.1158/1078-0432.CCR-11-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang B, Cohen JR, Fernando RI, Hamilton DH, Litzinger MT, Hodge JW, et al. The embryonic transcription factor Brachyury blocks cell cycle progression and mediates tumor resistance to conventional antitumor therapies. Cell death & disease. 2013;4:e682. doi: 10.1038/cddis.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larocca C, Cohen JR, Fernando RI, Huang B, Hamilton DH, Palena C. An autocrine loop between TGF-beta1 and the transcription factor brachyury controls the transition of human carcinoma cells into a mesenchymal phenotype. Mol Cancer Ther. 2013;12:1805–15. doi: 10.1158/1535-7163.MCT-12-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palena C, Polev DE, Tsang KY, Fernando RI, Litzinger M, Krukovskaya LL, et al. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin Cancer Res. 2007;13:2471–8. doi: 10.1158/1078-0432.CCR-06-2353. [DOI] [PubMed] [Google Scholar]
- 13.Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:501–8. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 15.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Peter M, Heitlinger E, Haner M, Aebi U, Nigg EA. Disassembly of in vitro formed lamin head-to-tail polymers by CDC2 kinase. The EMBO journal. 1991;10:1535–44. doi: 10.1002/j.1460-2075.1991.tb07673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao L, Perez D, White E. Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol. 1996;135:1441–55. doi: 10.1083/jcb.135.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsang KY, Palena C, Gulley J, Arlen P, Schlom J. A human cytotoxic T-lymphocyte epitope and its agonist epitope from the nonvariable number of tandem repeat sequence of MUC-1. Clin Cancer Res. 2004;10:2139–49. doi: 10.1158/1078-0432.ccr-1011-03. [DOI] [PubMed] [Google Scholar]
- 20.Salazar E, Zaremba S, Arlen PM, Tsang KY, Schlom J. Agonist peptide from a cytotoxic t-lymphocyte epitope of human carcinoembryonic antigen stimulates production of tc1-type cytokines and increases tyrosine phosphorylation more efficiently than cognate peptide. International journal of cancer Journal international du cancer. 2000;85:829–38. doi: 10.1002/(sici)1097-0215(20000315)85:6<829::aid-ijc16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 21.Gameiro SR, Higgins JP, Dreher MR, Woods DL, Reddy G, Wood BJ, et al. Combination therapy with local radiofrequency ablation and systemic vaccine enhances antitumor immunity and mediates local and distal tumor regression. PLoS One. 1371;8:2013. doi: 10.1371/journal.pone.0070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton DH, Litzinger MT, Jales A, Huang B, Fernando RI, Hodge JW, et al. Immunological targeting of tumor cells undergoing an epithelial-mesenchymal transition via a recombinant brachyury-yeast vaccine. Oncotarget. 2013;4:1777–90. doi: 10.18632/oncotarget.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schickel R, Park S-M, Murmann AE, Peter ME. miR-200c regulates induction of apoptosis through CD95 by targeting FAP-1. Mol Cell. 2010;38:908–15. doi: 10.1016/j.molcel.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ottaviano Y, Gerace L. Phosphorylation of the nuclear lamins during interphase and mitosis. J Biol Chem. 1985;260:624–32. [PubMed] [Google Scholar]
- 25.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 26.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 27.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nature reviews Immunology. 2006;6:715–27. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 28.Alexander S, Friedl P. Cancer invasion and resistance: interconnected processes of disease progression and therapy failure. Trends in molecular medicine. 2012;18:13–26. doi: 10.1016/j.molmed.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Akalay I, Janji B, Hasmim M, Noman MZ, Andre F, De Cremoux P, et al. Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis. Cancer research. 1158;73:2418–27. doi: 10.1158/0008-5472.CAN-12-2432. [DOI] [PubMed] [Google Scholar]
- 30.McKeon FD, Kirschner MW, Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986;319:463–8. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- 31.Zhang D, Beresford PJ, Greenberg AH, Lieberman J. Granzymes A and B directly cleave lamins and disrupt the nuclear lamina during granule-mediated cytolysis. Proc Natl Acad Sci U S A. 2001;98:5746–51. doi: 10.1073/pnas.101329598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peter M, Nakagawa J, Doree M, Labbe JC, Nigg EA. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990;61:591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- 33.Dash BC, El-Deiry WS. Phosphorylation of p21 in G2/M promotes cyclin B-Cdc2 kinase activity. Molecular and cellular biology. 2005;25:3364–87. doi: 10.1128/MCB.25.8.3364-3387.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker LL, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science. 1992;257:1955–7. doi: 10.1126/science.1384126. [DOI] [PubMed] [Google Scholar]
- 35.Garimella SV, Rocca A, Lipkowitz S. WEE1 inhibition sensitizes basal breast cancer cells to TRAIL-induced apoptosis. Molecular cancer research: MCR. 2012;10:75–85. doi: 10.1158/1541-7786.MCR-11-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bridges KA, Hirai H, Buser CA, Brooks C, Liu H, Buchholz TA, et al. MK-1775, a novel Wee1 kinase inhibitor, radiosensitizes p53-defective human tumor cells. Clin Cancer Res. 2011;17:5638–48. doi: 10.1158/1078-0432.CCR-11-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shanker A, Brooks AD, Jacobsen KM, Wine JW, Wiltrout RH, Yagita H, et al. Antigen presented by tumors in vivo determines the nature of CD8+ T-cell cytotoxicity. Cancer Res. 2009;69:6615–23. doi: 10.1158/0008-5472.CAN-09-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Open Label Study to Evaluate the Safety and Tolerability of GI-6301 a Vaccine Consisting of Whole Heat-Killed Recombitant Yeast Genetically Modified to Express Brachyury Protein in Adults With Solid Tumors. 2013 [cited 2013 Feb 5]; Available from: www.clinicaltrials.gov/ct2/show/NCT01519817.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.