Abstract
Aim:
To appraise the efficacy of Vam3 (Amurensis H), a dimeric derivative of resveratrol, at inhibiting cigarette smoke-induced autophagy.
Methods:
Human bronchial epithelial cells were treated with cigarette smoke condensates, and a chronic obstructive pulmonary disease (COPD) model was established by exposing male BALB/c mice to cigarette smoke. The protein levels of the autophagic marker microtubule-associated protein 1A/1B-light chain 3 (LC3), Sirtuin 1 (Sirt1), and foxhead box O 3a (FoxO3a) were examined using Western blotting and Immunohistochemistry. LC3 punctae were detected by immunofluorescence. The levels of FoxO3a acetylation were examined by immunoprecipitation. The level of intracellular oxidation was assessed by detecting ROS and GSH-Px.
Results:
Vam3 attenuated cigarette smoke condensate-induced autophagy in human bronchial epithelial cells, and restored the expression levels of Sirt1 and FoxO3a that had been reduced by cigarette smoke condensates. Similar protective effects of Vam3, reducing autophagy and restoring the levels of Sirt1 and FoxO3a, were observed in the COPD animal model. Additionally, Vam3 also diminished the oxidative stress that was induced by the cigarette smoke condensates.
Conclusion:
Vam3 decreases cigarette smoke-induced autophagy via up-regulating/restoring the levels of Sirt1 and FoxO3a and inhibiting the induced oxidative stress.
Keywords: COPD, autophagy, Sirt1, FoxO3a, resveratrol, Vam3
Introduction
Chronic obstructive pulmonary disease (COPD) has become a major global public health problem. The World Health Organization has predicted that by 2020, COPD will be the fifth leading cause of morbidity, and the third leading cause of mortality. Cigarette smoke (CS) contains numerous oxidants, free radical and chemicals, which can induce oxidative stress in lungs, resulting in cell death and senescence. Although the mechanisms responsible for the pathogenesis of COPD remain unclear, there is no doubt that cigarette smoke is a major risk factor.
In the lung, a low level of autophagy eliminates damaged organelles and long-term proteins through the lysosomal degradation pathway, to maintain cellular homeostasis. However, dysregulation or excessive activation of autophagy would lead to cell death and an acceleration of age-related lung abnormalities. Recently, studies have shown that enhanced autophagy occurs in the lungs of patients with COPD and of mice exposed to CS, and may be involved in the pathogenesis of COPD1,2,3. Thus, protecting cells from autophagic death may be beneficial for the treatment of COPD.
Sirtuin 1 (Sirt1) is an NAD+-dependent deacetylase that plays a role in a wide range of biological processes, such as cell differentiation, cell death, cell senescence/aging, metabolism, inflammation, and stress resistance4,5. The function of Sirt1 is mainly achieved by deacetylation of histones and several important transcription factors such as forkhead box O3 (FoxO3), p53, nuclear factor-κB (NF-κB), and Ku706,7,8,9,10. The transcription factor FoxO3a belongs to the FoxO family, which regulates the expression of several genes that are involved in diverse biological processes, such as cell death, the cell cycle, senescence and oxidative stress resistance11,12,13. An increasing number of studies have shown that both Sirt1 and FoxO3a play important roles in autophagy11,14. Recently, both in vitro and in vivo experimental studies have demonstrated that the expression levels of Sirt1 and FoxO3 are decreased in response to CS-induced oxidative stress in the lung15,16,17, but the exact mechanisms have yet to be elucidated.
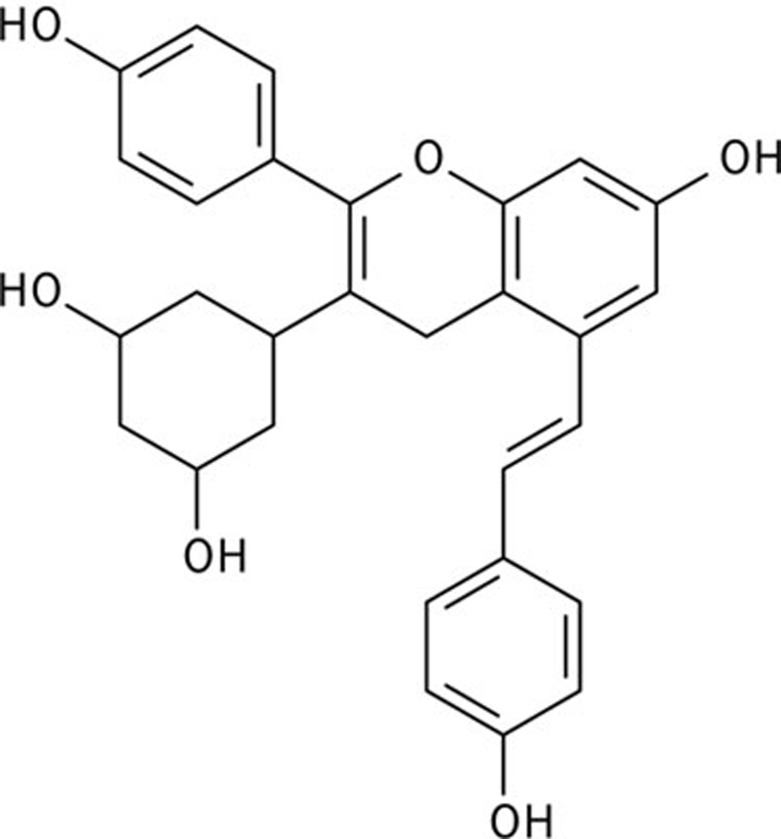
Resveratrol (3,5,4′-trihydroxystilbene, RES) is a polyphenolic compound found in grapes and wine. Mounting evidence strongly suggests that resveratrol has both anti-inflammatory and anti-oxidative functions that improve the outcomes of COPD18,19. Recent studies have also shown that resveratrol can inhibit autophagy induced by cigarette smoke20. Vam3 (amurensis H), a resveratrol dimer, was first isolated from ethanol extracts of Vitis amurensisRupr (Figure 1). Our previous in vivo and in vitro studies have found that Vam3 possesses clear anti-asthmatic21,22,23 and anti-COPD (unpublished) effects, achieved through a reduction in the synthesis of pro-inflammatory cytokines and leukotrienes. However, the molecular basis by which Vam3 protects against COPD is not clear. In this report, we have examined the role of Vam3 in CS-induced autophagy in vitro and in vivo, in an effort to develop a novel anti-autophagy agent for prevention of COPD progression.
Figure 1.
Chemical structure of Vam3.
Materials and methods
Preparation of cigarette smoke condensate
The cigarette smoke condensate (CSC) was prepared from Honghe (15 mg tar, 1.2 mg nicotine), a popular type of cigarette in China, as described previously24,25. Briefly, a “water-pipe” smoking device was designed and operated to allow a stream of smoke to flow into a 2-L flask submerged in liquid nitrogen. The amount of smoke obtained was determined by the weight increase of the flask. CSC was prepared by dissolving the collected smoke particulates in dimethyl sulfoxide (DMSO) at a concentration of 500 mg/mL. The condensate was then divided into small vials and stored at −80 °C. On the day of the experiment, the CSC solution was diluted in M199 medium containing serum (Gibco, Grand Island, NY, USA), to the desired concentration, and used for cell treatment.
Cell culture and treatment
Human bronchial epithelial cells (Beas-2B) were grown in M199 medium (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS: HyClone, Logan, UT, USA). The cells were incubated at 37 °C in a humidified atmosphere with 5% CO2 and 95% air. During the logarithmic phase of growth, cells were pretreated with resveratrol (1 μmol/L, 0.1 μmol/L) or Vam3 (1 μmol/L, 0.1 μmol/L) for 2 h before treatment with CSC (300 mg/L) for 12 h. With a final concentration of 0.1 μmol/L, bafilomycin A1 had to be incubated for 2 h before the cells were harvested. All treatments were carried out in complete culture medium to avoid the induction of autophagy through the serum starvation pathway. Bafilomycin A1 and resveratrol were purchased from Fisher Scientific (Pittsburgh, PA, USA) and Sigma-Aldrich (St Louis, MO, USA), respectively, and Vam3 was produced by the Institute of Materia Medica of the Chinese Academy of Medical Sciences (Beijing, China).
Cytoplasmic and nuclear protein extraction
The cytoplasmic and nuclear fractions were prepared from Beas-2B cells (1×106−10×106) using NE-PER nuclear and cytoplasmic extraction reagents, according to the instructions of the manufacturer (Pierce Biotechnology Inc, Rockford, IL, USA). The resulting soluble protein and nuclear protein fractions were then stored at −80 °C until use.
Immunofluorescence
Cells were fixed in methanol at room temperature for 15 min, and then in cold-methanol at −20 °C for 15 min. After 3 washes with phosphate-buffered saline (PBS), the cells were incubated with blocking buffer [5 μL Tween 20 and 0.3 g bovine serum albumin (BSA), dissolved in 10 mL PBS] at room temperature for 30 min. The cells were then stained with an anti-LC3 antibody (Sigma-Aldrich, St Louis, MO, USA) at 4 °C overnight, and this was followed by 30 min incubation with fluorescein isothiocyanate (FITC)-labeled secondary antibody (Zhong Shan Golden Bridge Biotechnology Co, Ltd, Beijing, China). After washing, the cells were visualized with a fluorescence microscope (Olympus Optical, Tokyo, Japan), and images were captured with a digital camera (Kodak, NY, USA).
Western blotting and immunoprecipitation
For Western blot experiments, cells were lysed with a non-denaturing lysis buffer (Applygen Technologies Inc, Beijing, China) supplemented with a protease inhibitor cocktail (Thermo Fisher Scientific, Rockford, IL, USA). Cell extracts (60 μg/lane) were separated on a 10%–15% SDS polyacrylamide gel, and transferred onto PVDF membranes (Millipore, Bedford, MA, USA). The membranes were then blocked for 1 h at room temperature with 5% BSA or non-fat milk. Subsequently, the membranes were probed with specific primary antibodies for LC3, β-actin (Sigma-Aldrich, St Louis, MO, USA), Sirt1, FoxO3a, p53, acetyl-p53 (lysine 382) (Cell Signaling, Beverly, MA, USA), p62, GAPDH, and LaminB (Santa Cruz, CA, USA) at 4 °C overnight. After 3 washes, the membranes were incubated with a goat antibody linked to horseradish peroxidase (Santa Cruz, CA, USA) for 1–2 h, and antibody-antigen reactivity was detected using Western Blotting Substrate (Pierce Biotechnology Inc, Rockford, IL, USA).
For immunoprecipitation experiments, cell extracts were pre-cleared with protein A/G sepharose beads (Santa Cruz, CA, USA) and incubated with FoxO3a antibody for 6 h at 4 °C. Then, protein A/G sepharose beads were added, and the samples were incubated at 4 °C overnight to capture the immunocomplexes. Western blotting was performed with anti-acetylated-lysine antibody (Cell Signaling, Beverly, MA, USA) and anti-FoxO3a antibody.
Small interfering RNA transfection assay
Pre-validated small interfering RNAs (siRNAs) were purchased from Santa Cruz Biotechnology, and the cells were transfected using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions, with suitable scrambled siRNA controls. After being transfected for about 24 h, the cells were treated as described before.
Measurement of intracellular ROS
Intracellular ROS generation was determined with the fluorescent probe dihydroethidium (DHE) according to the manufacturer's instruction (Vigorous, Beijing, China). Briefly, Beas-2B cells with or without RES and Vam3, after stimulation with CSC for 12 h, were incubated with DHE probe (final concentration 10 μmol/L) at 37 °C for 30 min in the dark. Then the cells were washed three times with fresh medium followed by measurement at an emission wavelength of 500 nm and an excitation wavelength of 600 nm using the fluorescent microplate reader (BioTek, Winooski, VT, USA).
Measurement of intracellular glutathione peroxidase (GSH-Px) vitality
Briefly, after stimulation with CSC for 12 h with or without RES and Vam3, cells lyses were measured with the Glutathione Peroxidase Detection Kit (Beyotime Institute of Biotechnology, Beijing, China) closely according to the user's instructions in 96-well plate. Then the plate was measured at 340 nm using the microplate reader (BioTek, Winooski, VT, USA). Finally, the amount of GSH-Px vitality was calculated by the formula U/mg=[A340/min (sample)-A340/min (blank)]/0.00622×dilution factor/protein concentration.
Animals and cigarette smoke exposure
The type of cigarette was described before. Specific pathogen free (SPF) male BALB/c mice (Beijing HFK Bioscience Co, Ltd, Beijing, China) were exposed to cigarette smoke (CS) or filtered air under identical conditions, beginning at 18–20 g. The cigarette smoke exposure was performed in a 4 L homemade plexiglass container which has a shelf in it according to equipment from Cao26. The container has a rent for each side above and there are many circular gaps in the shelf. Eight mice were placed on the shelf each time, below which there were 12 cigarettes. It would be taken for about 30 min until the smoke completely disappeared, and then repeated the process again. Mice were exposed for 30 min per exposure, three times a day for a period of 4 weeks. One hour before CS exposure, mice received either resveratrol (30 mg/kg) or Vam3 (50 mg/kg) by intragastric administration. About an hour after the last CS exposure and administration of resveratrol or Vam3, mice were sacrificed for harvesting of tissue. The study was designed to consist of 4 groups, containing 8 mice each: a control group (air-exposed mice), a model group (CS-exposed mice), an RES group (CS-exposed mice with 30 mg/kg resveratrol treatment) and a Vam3 group (CS-exposed mice with 50 mg/kg Vam3 treatment). Procedures followed for care and use of the animals were in accordance with institutional guidelines at the Experimental Animal Center of the Institute of Materia Medica, Beijing, China.
Immunohistochemistry
For hematoxylin and eosin (HE) staining, slices were deparaffinized in xylene and then rehydrated through graded alcohols to PBS, stained with hematoxylin and eosin, and then dehydrated again through graded alcohols. Finally, the slices were mounted with neutral gum and observed using a microscope. For immunohistochemistry staining, mouse lung tissues were fixed in 10% formaldehyde and embedded in paraffin. Lung slices were deparaffinized in xylene, rehydrated through graded alcohols to PBS, treated with 3% H2O2 for 10 min, and placed in an EDTA-antigen retrieval buffer in a microwave. The slices were blocked with sheep serum for 10 min, and then incubated with anti-LC3 (Abcam, Cambridge, MA, USA), anti-Sirt1 (Beijing Biosynthesis Biotechnology Co, Ltd, Beijing, China), and anti-FoxO3a (Cell Signaling, Beverly, MA, USA) antibodies; incubation with the primary antibodies was carried out at 4 °C overnight. The PV-9000 kit (Zhong Shan Golden Bridge Biotechnology Co, Ltd, Beijing, China) was applied to each section, according to the manufacturer's instructions. The stain was developed using ABC kit (Zhong Shan Golden Bridge Biotechnology Co, Ltd, Beijing, China), and this was followed by dehydration through graded alcohols. Finally, the slices were mounted with neutral gum, and assessment of immunostaining intensity was performed semi-quantitatively, using Image J software27.
Statistical analysis
Results are presented as mean±SD, calculated from at least 3 independent experiments. Differences in measured variables between experimental and control groups were assessed using Student's t-test for group comparisons. Statistically significant differences were accepted at P<0.05.
Results
Cigarette smoke condensate (CSC) induces autophagy in a dose- and time-dependent manner
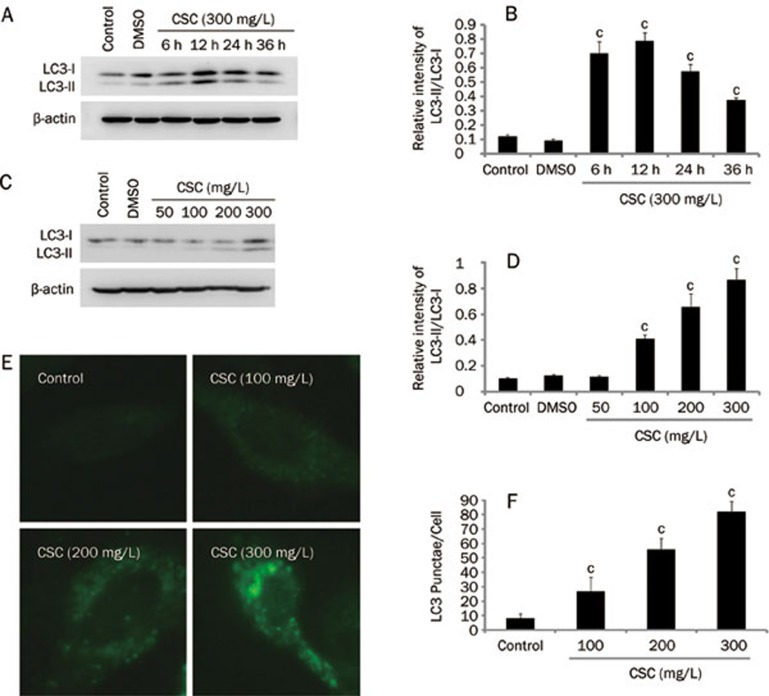
To investigate whether CSC affects the induction of autophagy, a time- and dose-effect analysis of this compound was carried out in Beas-2B cells. We found that CSC increased the accumulation of LC3-II and the conversion of LC3-I to LC3-II (Figures 2A–2D). At a CSC concentration of 300 mg/L, there was an approximately 6-fold increase in the ratio of LC3-II/LC3-I, compared to controls (Figure 2D). In addition, CSC (300 mg/L) increased the LC3-II/LC3-I ratio at 6 h post treatment, and a peak was reached at 12 h (Figure 2B). The effects of CSC on the formation of LC3 punctae in Beas-2B cells also appeared to be dose-dependent (Figure 2E and 2F), consistent with the immunoblotting analysis. These data clearly suggest that CSC induces autophagy in a dose- and time-dependent manner. Since LC3-II is subject to autophagic protein degradation, it also accumulates when the autophagosome-lysosome fusion is impaired28. In order to exclude this possibility, we observed LC3-II accumulation in the presence of a specific inhibitor of the vacuolar type H (+)-ATPase, bafilomycin A1. We observed that LC3-II accumulation increased in the presence of bafilomycin A1 and increased to an even greater extent when combined with cell exposure to CSC. These data indicate that the autophagic flux is intact in these cells. Addition ally, we examined the expression level of p62, also a marker of autophagy29, which was degraded when treatment with CSC (supplementary Figure A). Thus, combining the data showing LC3-II accumulation and p62 degradation with the autophagic flux analysis, we confirm that CSC can induce autophagy in Beas-2B cells.
Figure 2.
CSC-induced autophagy of Beas-2B cells is dose-and time-dependent. (A) Beas-2B cells were treated with CSC (300 mg/L), and cellular proteins were collected at the indicated time points and analyzed by Western blot. (C) Beas-2B cells were treated with CSC, at the indicated concentrations, for 12 h. Western blot was performed using anti-LC3 antibody. (B and D) The relative intensity of LC3-II/LC3-I was calculated by densitometry. (E) Beas-2B cells were treated with CSC, at the indicated concentrations, for 12 h. LC3 was detected by an immunofluorescence assay and examined using fluorescence microscopy, and representative cells were selected and photographed (×400). (F) Number of LC3 punctae per cell, for a total of 25 cells in 5 fields of view. Mean±SD. n=3. cP<0.01 vs control.
Vam3 attenuates CSC-induced autophagy and modulates the expression of Sirt1 and FoxO3a
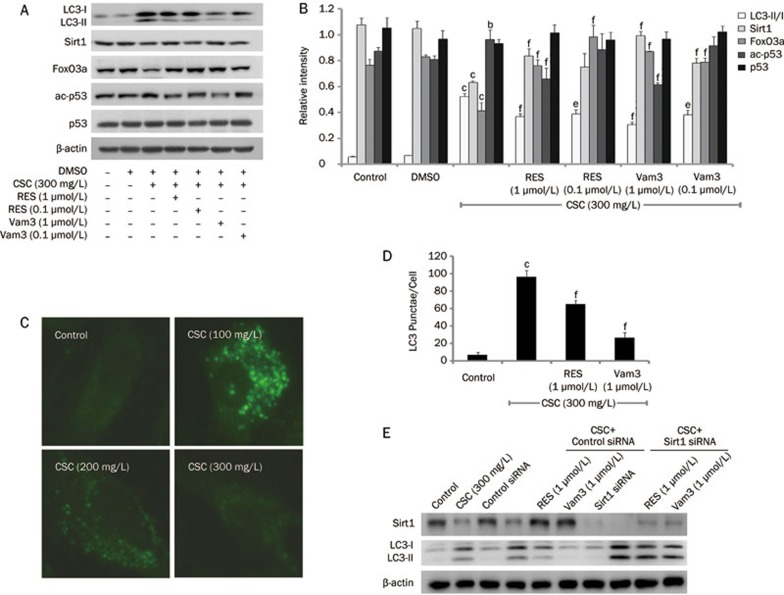
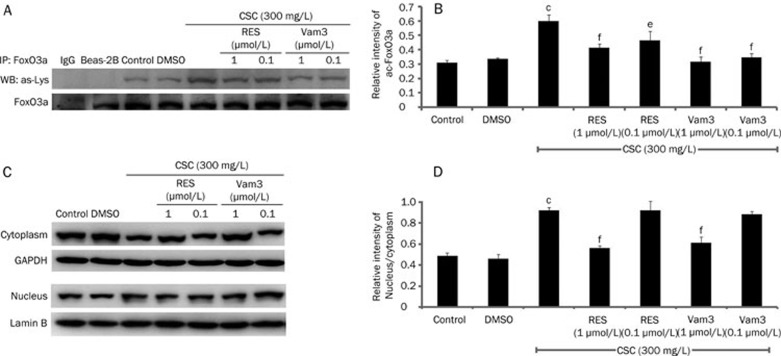
To investigate the involvement of Vam3 in CSC-induced autophagy, Beas-2B cells were pretreated with RES (1 μmol/L or 0.1 μmol/L) or Vam3 (1 μmol/L or 0.1 μmol/L) for 2 h, and this was followed by treatment with CSC (300 mg/L). The ratio of LC3-II/LC3-I was significantly increased in response to CSC alone, whereas pretreatment with RES or Vam3 prevented the increase in the LC3-II/LC3-I ratio. In addition, Vam3 seemed be much more effective than RES in attenuating this increase in the LC3-II/LC3-I ratio (Figure 3A and 3B). Pretreatment with RES and Vam3 also reduced the formation of LC3 punctae induced by CSC (Figure 3C and 3D). Furthermore, we found that RES and Vam3 could reverse the CSC-induced decrease of Sirt1 and FoxO3 expression (Figure 3A and 3B). Pretreatment with RES and Vam3 reduced acetylation of p53 on lysine 382, indicating that the deacetylase activity of Sirt1 had been restored (Figure 3A and 3B). We also assessed the activation of FoxO3a by detecting its translocation and the level of acetylation of lysine (Figure 4). FoxO3a, which translocated to the nucleus following treatment with CSC, could be retained in the cytoplasm by RES and Vam3 (Figure 4C and 4D). The level of acetylated FoxO3a was increased in response to CSC treatment, and was reduced to some degree by RES or Vam3 pretreatment (Figure 4A and 4B). These data indicate that Vam3 may attenuate the autophagy induced by CSC, through modulating the Sirt1/FoxO3a pathway.
Figure 3.
Vam3 attenuates CSC-induced autophagy in Beas-2B cells. Beas-2B cells were treated with CSC (300 mg/L) for 12 h, with or without RES (1 μmol/L, 0.1 μmol/L) or Vam3 (1 μmol/L, 0.1 μmol/L) pretreatment for 2 h. (A) Cell extracts were subjected to immunoblot analysis for detection of LC3, Sirt1, FoxO3a, p53, and acetylated p53 (lysine 382). (B) The relative intensity of LC3-II/LC3-I, Sirt1, FoxO3a, p53, and ac-p53 was calculated by densitometry. (C) LC3 was detected by an immunofluorescence assay and examined using fluorescence microscopy, and representative cells were selected and photographed (×400). (D) Number of LC3 punctae per cell, for a total of 25 cells in 5 fields of view. (E) After transfected with Sirt1 siRNA or scramble siRNA for 24 h, Beas-2B cells were treated with CSC (300 mg/L) for another 12 h, with or without RES (1 μmol/L, 0.1 μmol/L) or Vam3 (1 μmol/L, 0.1 μmol/L) pretreatment for 2 h. Then Western blot assays were performed with anti-LC3 and anti-Sirt1 antibodies. Mean±SD. n=3. bP<0.05, cP<0.01 vs control; eP<0.05, fP<0.01 compared to that in the absence of RES or Vam3. ac-p53, acetylated p53 (lysine 382).
Figure 4.
Vam3 inhibits the activation of FoxO3a, which is increased by CSC in Beas-2B cells. Beas-2B cells were treated with CSC (300 mg/L) for 12 h, with or without RES (1 μmol/L, 0.1 μmol/L) or Vam3 (1 μmol/L, 0.1 μmol/L) pretreatment for 2 h. (A) Cell extracts were immunoprecipitated with an anti-FoxO3a antibody, and the immune complexes were subjected to immunoblotting analyses with anti-acetyl-lysine antibody. IgG was used as the negative control. (B) The amounts of acetylated FoxO3a were calculated by densitometry and normalized to corresponding FoxO3a levels. (C) Equal amounts of cytoplasmic (cytoplasm) or nuclear (nucleus) protein extracts (60 μg/lane) were loaded and the membranes were probed with an anti-FoxO3a antibody. GAPDH served as the cytoplasmic control and LaminB was used as the nuclear control. (D) The relative intensity of nucleus/cytoplasm was calculated by densitometry. Mean±SD. n=3. cP<0.01 vs control; eP<0.05, fP<0.01 vs that in the absence of RES or Vam3.
Sirt1 is required in Vam3 effect on autophagy
To observe whether the Sirt1 pathway is required for the effect of Vam3 on autophagy, we knocked down Sirt1 with siRNA and found that RES and Vam3 were not able to inhibit autophagy induced by CSC, which indicated that the inhibitory effect of RES and Vam3 requires Sirt1 (Figure 3E).
Vam3 diminishes CSC-induced oxidative stress
Lots of free radicals and oxidants in CSC can induce oxidative stress in many types of cells and tissues30,31,32, and oxidative stress plays an important role in COPD development. Thus, whether Vam3 affecting oxidative and anti-oxidative balance or not makes us to detect the oxidative level in Beas-2B cells which were treated by CSC. We found that CSC up-regulated the generation of ROS (supplementary Figure B) and down-regulated the vitality of GSH-Px (supplementary Figure C), an enzyme protecting cells from free radicals damage by removing peroxides, whereas RES and Vam3 could reverse the alteration especially at 1 μmol/L.
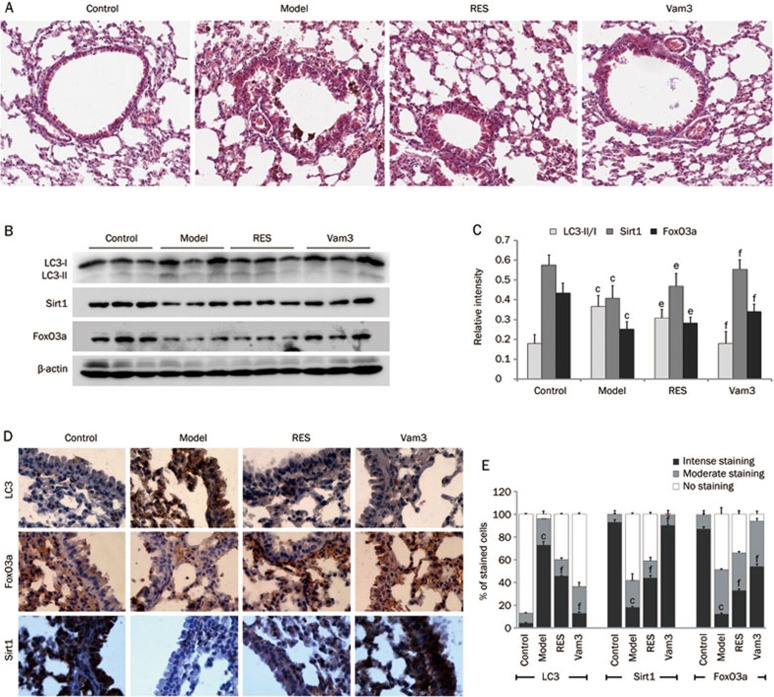
Vam3 attenuates autophagy in mouse lungs exposed to CS
In order to examine the efficacy of Vam3 as an inhibitor of autophagy in vivo, wild-type (WT) mice were exposed to CS, with or without RES (30 mg/kg) or Vam3 (50 mg/kg) for 4 weeks. We found that both RES and Vam3 inhibited, to varying degrees, the progression of pathology in mouse lungs exposed to CS, with Vam3 being somewhat superior to RES in this regard (Figure 5A). The levels and localizations of LC3, Sirt1, and FoxO3a were also examined. As shown in the immunoblots in Figure 5B and 5C, the conversion of LC3-I to LC3-II was increased in CS-exposed mouse lungs, but was decreased in lungs of mice pretreated with RES or Vam3. RES and Vam3 were also able to restore the expression levels of Sirt1 and FoxO3a that were reduced by CS. Immunohistochemical staining, presented in Figure 5D and 5E, indicated that RES and Vam3 could also reduce CS-induced up-regulation of LC3 and restore the CS-induced decrease in Sirt1 and FoxO3a.
Figure 5.
Vam3 attenuates CS-induced autophagy in the lungs of mice. Lung samples from each group (Control group: air-exposed mice; Model group: CS-exposed mice; RES group: CS-exposed mice with 30 mg/kg RES treatment; Vam3 group: CS-exposed mice with 50 mg/kg Vam3 treatment) were used for immunoblotting, HE staining and immunohistochemical analyses. (A) The lung sections were stained with hematoxylin and eosin, and the representative areas were captured by microscopy. (B) Expressions of LC3, FoxO3a, and Sirt1 in lung tissues from each group were determined by Western blotting. (C) The amounts of LC3, Sirt1 and FoxO3a were calculated by densitometry and normalized to corresponding actin levels (n=8 per group). (D) Expressions of LC3, FoxO3a, and Sirt1 in lung alveolar/airway epithelial cells from each group were determined using immunohistochemical staining (×400). (E) The number of stained cells from different levels (Intense staining level, Moderate staining level, No staining level) were calculated by ImageJ and normalized to corresponding total cells (n=4 per group). cP<0.01, compared to control; eP<0.05, fP<0.01, compared to the Model group.
Discussion
To date, there are no effective drugs that inhibit the progressive development of COPD. An increasing number of studies have shown that resveratrol may be superior to other drugs for COPD therapy18,19. Recently, Hwang et al showed that resveratrol could also attenuate autophagy induced by cigarette smoke in a variety of lung cells20. Since excessive autophagy leads to cell death, targeting autophagy may therefore have a beneficial outcome in the treatment of COPD. In this study, using in vitro cell culture techniques and in vivo animal models, we demonstrated that Vam3, like resveratrol, is able to attenuate CS-induced autophagy. In fact, we found Vam3 to be more effective than resveratrol at inhibiting autophagy induced by CS.
Recently, studies have shown that Sirt1, which acts in a protective role through the deacetylation of target genes, is highly inhibited in COPD patients15,16. Hwang et al reported that resveratrol attenuated CS-induced autophagy through prevention of Sirt1 reduction20. Hence, compounds that maintain or up-regulate Sirt1 levels and activities may be beneficial in attenuating CS-induced autophagy. In this study, we showed that the inhibitory effect of Vam3 on CS-induced autophagy appears to correlate with its ability to maintain Sirt1 expression and deacetylase activity.
FoxO3a has been demonstrated to regulate the expression of several genes that are involved in inflammation, oxidative stress resistance, and cellular senescence, and that are enhanced in the pathogenesis of COPD17. Therefore, FoxO3a may play an important role in the development of COPD. We found that the expression level of FoxO3a was decreased in CS-induced autophagy in vitro and in vivo, and that Vam3 could reverse this process. Brunet et al reported that FoxO3a is localized in the cytoplasm under normal conditions, and translocates to the nucleus in response to oxidative stress10. In our studies, CSC promoted FoxO3a translocation to the nucleus in Beas-2B cells, and Vam3 inhibited this process. Though the mechanism underlying CS-induced reduction and translocation of FoxO3a is unclear, it may be associated with post-translational modifications of FoxO3a, such as acetylation and phosphorylation33. Since the phosphorylation level of FoxO3 is mostly regulated by Akt activity34 and CS can induce Akt protein degradation35, we examined the Ser256 phosphorylation site in FoxO3a (data not shown). As we expected, the phosphorylation level of FoxO3 is decreased by treatment with CSC and up-regulated or restored by RES and Vam3, indicating that the Akt pathway might be involved in the regulatory role of Vam3 in FoxO3a. Furthermore, FoxO3a acetylation appears to be regulated via CREB-binding protein-mediated acetylation and Sirt1-mediated deacetylation10,36. Hwang et al found that the acetylation of FoxO3a was increased in the lungs of smokers as well as in the lungs of mice exposed to CS17. Consistent with this, our results showed that Sirt1 was reduced in CS-induced stress and that this was accompanied by increased levels of FoxO3a acetylation. Although many studies have shown that post-translational modifications of FoxO3a lead to the suppression of its transcriptional activity, some reports have indicated that FoxO3a acetylation increases target gene transcription10,37. Since LC3 is a direct target gene regulated by FoxO3a38, it is possible that CSC-induced acetylation of FoxO3a and its translocation to the nucleus enhanced the transcription of the LC3 gene to promote autophagy. Meanwhile, Vam3 can maintain Sirt1 level and activity, so it is likely that this would then reduce FoxO3a acetylation and LC3 up-regulation. But the exact mechanism should be further investigated and debated.
CSC contains numerous free radicals and oxidants and induces oxidative stress, resulting in autophagy, apoptosis and cell senescence, all of which are important in the development of COPD32. Sirt1 and FoxO3a are also subjected to the effects of oxidative stress39,40. So we proposed that Vam3, like resveratrol, might also have an anti-oxidative effect on CSC-induced damage. By detecting the ROS level and GSH-Px vitality, we proved our hypothesis.
Taken together, we demonstrated that Vam3 inhibited autophagy and restored the expression and activity levels of Sirt1, as well as preserved the expression level of FoxO3a in CSC-treated Beas-2B cells and in CS-exposed mouse lungs. We also showed that Vam3 had an anti-oxidative effect. These findings indicate that Vam3 acts as an anti-autophagy agent by affecting Sirt1 and FoxO3a expression and activity, and possibly also through its anti-oxidative effect. Vam3 warrants further studies to investigate potential clinical applications, and it would be interesting to determine the underlying mechanisms by which Vam3 acts on Sirt1 and FoxO3a to inhibit autophagy.
Author contribution
Conceived and designed the experiments: Ji SHI, Qi HOU. Performed the experiments: Ji SHI, Ning YIN. Analyzed the data: Ji SHI, Ling-ling XUAN. Contributed reagents/materials/analysis tools: Chun-suo YAO, Ai-min MENG, and Qi HOU. Wrote the paper: Ji SHI.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (30772579) & National Drug Research Integrated Platform (2009ZX0 9301-003-13).
Footnotes
Supplementary figure is available at the Acta Pharmacologica Sinica website.
Supplementary Information
CSC induces induction of autophagy in Beas-2B cells.
Vam3 reverses CS-induced intracellular ROS generation in Beas-2B cells.
Vam3 reverses CS-induced reduction of GSH reductase vitality in Beas-2B cells.
References
- Chen ZH, Kim HP, Sciurba FC, Lee SJ, Feghali-Bostwick C, Stolz DB, et al. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS One. 2008;3:e3316. doi: 10.1371/journal.pone.0003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HP, Wang X, Chen ZH, Lee SJ, Huang MH, Wang Y, et al. Autophagic proteins regulate cigarette smoke-induced apoptosis: protective role of heme oxygenase-1. Autophagy. 2008;4:887–95. doi: 10.4161/auto.6767. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Choi AM. Autophagy in the lung. Proc Am Thorac Soc. 2010;7:13–21. doi: 10.1513/pats.200909-101JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys. 2010;501:79–90. doi: 10.1016/j.abb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Sinclair DA. Mammalian sirtuins: Biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–95. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahtola E, Louhelainen M, Forsten H, Merasto S, Raivio J, Kaheinen P, et al. Sirtuin1-p53, forkhead box O3a, p38 and post-infarct cardiac remodeling in the spontaneously diabetic Goto-Kakizaki rat. Cardiovasc Diabetol. 2010;9:5. doi: 10.1186/1475-2840-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol. 2007;292:L567–76. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- Nath KA. The role of Sirt1 in renal rejuvenation and resistance to stress. J Clin Invest. 2010;120:1026–8. doi: 10.1172/JCI42184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HY, Lavu S, Bitterman KJ, Hekking B, Imahiyerobo TA, Miller C, et al. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell. 2004;13:627–38. doi: 10.1016/s1097-2765(04)00094-2. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wang Y, Zhu WG. Applications of post-translational modifications of FoxO family proteins in biological functions. J Mol Cell Biol. 2011;3:276–82. doi: 10.1093/jmcb/mjr013. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Kitamura Y. The roles of PPAR, C/EBP and FoxO families in adipocyte differentiation and proliferation. Nihon Rinsho. 2011;69:259–63. [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Hou J, Shang YC. Oxidative stress: Biomarkers and novel therapeutic pathways. Exp Gerontol. 2010;45:217–34. doi: 10.1016/j.exger.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K. SIRT1: regulation of longevity via autophagy. Cell Signal. 2009;21:1356–60. doi: 10.1016/j.cellsig.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:861–70. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caito S, Hwang JW, Chung S, Yao H, Sundar IK, Rahman I. PARP-1 inhibition does not restore oxidant-mediated reduction in SIRT1 activity. Biochem Biophys Res Commun. 2010;392:264–70. doi: 10.1016/j.bbrc.2009.12.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JW, Rajendrasozhan S, Yao H, Chung S, Sundar IK, Huyck HL, et al. FOXO3 deficiency leads to increased susceptibility to cigarette smoke-induced inflammation, airspace enlargement, and chronic obstructive pulmonary disease. J Immunol. 2011;187:987–98. doi: 10.4049/jimmunol.1001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch J, Sibbing B, Jungck D, Lin Y, Urban K, Stoelben E, et al. Resveratrol impairs the release of steroid-resistant inflammatory cytokines from human airway smooth muscle cells in chronic obstructive pulmonary disease. J Pharmacol Exp Ther. 2010;335:788–98. doi: 10.1124/jpet.110.166843. [DOI] [PubMed] [Google Scholar]
- Wood LG, Wark PA, Garg ML. Antioxidant and anti-inflammatory effects of resveratrol in airway disease. Antioxid Redox Signal. 2010;13:1535–48. doi: 10.1089/ars.2009.3064. [DOI] [PubMed] [Google Scholar]
- Hwang JW, Chung S, Sundar IK, Yao H, Arunachalam G, McBurney MW, et al. Cigarette smoke-induced autophagy is regulated by SIRT1-PARP-1-dependent mechanism: implication in pathogenesis of COPD. Arch Biochem Biophys. 2010;500:203–9. doi: 10.1016/j.abb.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YT, Yao CS, Bai JY, Lin M, Cheng GF. Anti-inflammatory effect of amurensin H on asthma-like reaction induced by allergen in sensitized mice. Acta Pharmacol Sin. 2006;27:735–40. doi: 10.1111/j.1745-7254.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- Yang L, Yao CS, Wu ZY, Xuan LL, Bai JY, Cheng GF, et al. Effects of dihydroxy-stilbene compound Vam3 on airway inflammation, expression of ICAM-1, activities of NF-kappaB and MMP-9 in asthmatic mice. Yao Xue Xue Bao. 2010;45:1503–8. [PubMed] [Google Scholar]
- Huang KS, Lin M, Cheng GF. Anti-inflammatory tetramers of resveratrol from the roots of Vitis amurensis and the conformations of the seven-membered ring in some oligostilbenes. Phytochemistry. 2001;58:357–62. doi: 10.1016/s0031-9422(01)00224-2. [DOI] [PubMed] [Google Scholar]
- Yuan J, Ma J, Zheng H, Shi T, Sun W, Zhang Q, et al. Overexpression of OLC1, cigarette smoke, and human lung tumorigenesis. J Natl Cancer Inst. 2008;100:1592–605. doi: 10.1093/jnci/djn379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xiao T, Cheng S, Tong T, Gao Y. Cigarette smoke suppresses the ubiquitin-dependent degradation of OLC1. Biochem Biophys Res Commun. 2011;407:753–7. doi: 10.1016/j.bbrc.2011.03.095. [DOI] [PubMed] [Google Scholar]
- Cao J, Chen P, Yang Y, Ouyang RY, Peng H. Establishment and assessment of a mouse model of cigarette smoke-induced emphysema. Acta Lab Anim Sci Sin. 2010;18:278–82. [Google Scholar]
- Dunnill MS. Quantitative methods in the study of pulmonary pathology. Thorax. 1962;17:320–8. doi: 10.1136/thx.17.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Cuervo AM, Seglen PO. Methods for monitoring autophagy from yeast to human. Autophagy. 2007;3:181–206. doi: 10.4161/auto.3678. [DOI] [PubMed] [Google Scholar]
- Bjorkoy G, Lamark T, Pankiv S, Overvatn A, Brech A, Johansen T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 2009;452:181–97. doi: 10.1016/S0076-6879(08)03612-4. [DOI] [PubMed] [Google Scholar]
- Barreiro E, Del Puerto-Nevado L, Puig-Vilanova E, Perez-Rial S, Sanchez F, Martinez-Galan L, et al. Cigarette smoke-induced oxidative stress in skeletal muscles of mice. Respir Physiol Neurobiol. 2012;182:9–17. doi: 10.1016/j.resp.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Valenti VE, de Abreu LC, Sato MA, Ferreira C, Adami F, Fonseca FL, et al. Sidestream cigarette smoke effects on cardiovascular responses in conscious rats: involvement of oxidative stress in the fourth cerebral ventricle. BMC Cardiovasc Disord. 2012;12:22. doi: 10.1186/1471-2261-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhdar R, Denden S, Kassab A, Leban N, Knani J, Lefranc G, et al. Update in chronic obstructive pulmonary disease: role of antioxidant and metabolizing gene polymorphisms. Exp Lung Res. 2011;37:364–75. doi: 10.3109/01902148.2011.580416. [DOI] [PubMed] [Google Scholar]
- Hedrick SM. The cunning little vixen: Foxo and the cycle of life and death. Nat Immunol. 2009;10:1057–63. doi: 10.1038/ni.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Tindall DJ. Regulation of FOXO protein stability via ubiquitination and proteasome degradation. Biochim Biophys Acta. 2011;1813:1961–4. doi: 10.1016/j.bbamcr.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Lee JH, Huh JW, Ro JY, Oh YM, Lee SD, et al. Cigarette smoke induces Akt protein degradation by the ubiquitin-proteasome system. J Biol Chem. 2011;286:31932–43. doi: 10.1074/jbc.M111.267633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansen TB, Smits LM, van Triest MH, de Keizer PL, van Leenen D, Koerkamp MG, et al. Redox-sensitive cysteines bridge p300/CBP-mediated acetylation and FoxO4 activity. Nat Chem Biol. 2009;5:664–72. doi: 10.1038/nchembio.194. [DOI] [PubMed] [Google Scholar]
- van der Heide LP, Smidt MP. Regulation of FoxO activity by CBP/p300-mediated acetylation. Trends Biochem Sci. 2005;30:81–6. doi: 10.1016/j.tibs.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–71. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Rajendran R, Garva R, Krstic-Demonacos M, Demonacos C.Sirtuins: molecular traffic lights in the crossroad of oxidative stress, chromatin remodeling, and transcription J Biomed Biotechnol 2011. Published online 2011 September 7. doi: 10.1155/2011/368276 [DOI] [PMC free article] [PubMed]
- Storz P. Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid Redox Signal. 2011;14:593–605. doi: 10.1089/ars.2010.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CSC induces induction of autophagy in Beas-2B cells.
Vam3 reverses CS-induced intracellular ROS generation in Beas-2B cells.
Vam3 reverses CS-induced reduction of GSH reductase vitality in Beas-2B cells.