Abstract
Background:
Several studies have described a clinical benefit of macrolides due to their immunomodulatory properties in various respiratory diseases. We aimed to assess the effect of macrolide therapy on mortality in patients hospitalized for Pseudomonas aeruginosa community-acquired pneumonia (CAP).
Methods:
We performed a retrospective population-based study of > 150 hospitals in the US Veterans Health Administration. Patients were included if they had a diagnosis of CAP and P aeruginosa was identified as the causative pathogen. Patients with health-care-associated pneumonia and immunosuppression were excluded. Macrolide therapy was considered when administered within the first 48 h of admission. Univariate and multivariable analyses were performed using 30-day mortality as the dependent measure.
Results:
We included 402 patients with P aeruginosa CAP, of whom 171 (42.5%) received a macrolide during the first 48 h of admission. These patients were older and white. Macrolide use was not associated with lower 30-day mortality (hazard ratio, 1.14; 95% CI, 0.70-1.83; P = .5). In addition, patients treated with macrolides had no differences in ICU admission, use of mechanical ventilation, use of vasopressors, and length of stay (LOS) compared with patients not treated with macrolides. A subgroup analysis among patients with P aeruginosa CAP in the ICU showed no differences in baseline characteristics and outcomes.
Conclusions:
Macrolide therapy in the first 48 h of admission is not associated with decreased 30-day mortality, ICU admission, need for mechanical ventilation, and LOS in hospitalized patients with P aeruginosa CAP. Larger cohort studies should address the benefit of macrolides as immunomodulators in patients with P aeruginosa CAP.
Macrolides are antibiotics widely used to treat respiratory infections. Current North American and European guidelines recommend their use in hospitalized patients with community-acquired pneumonia (CAP).1,2 In addition to their action against atypical microorganisms, a large variety of immunomodulatory effects have been related to macrolide use.3 Various studies have shown that macrolides may influence leukocyte function, cytokine expression, apoptosis, and mucus production.4,5 In clinical practice, observational studies have shown beneficial effects of macrolide treatment in patients with bacteremic pneumococcal pneumonia,6‐8 in patients hospitalized for CAP and severe CAP,9‐11 and for infection due to a macrolide-resistant pathogen.9,11
Pseudomonas aeruginosa is a macrolide-resistant pathogen associated with poor clinical outcomes in CAP. Although it is a rare pathogen in CAP,12 its presence is associated with higher morbidity and mortality.13,14 Some authors demonstrated that macrolides may reduce adherence, inhibit mobility, and decrease biofilm formation,3 which are all virulence factors of P aeruginosa. Several studies have shown a benefit of macrolide use in bronchiectasis,15 cystic fibrosis,16 COPD,17 and diffuse panbronchiolitis.18 However, no data are available regarding the impact of macrolide therapy in clinical outcomes in patients hospitalized for P aeruginosa CAP.
The aim of the present study, therefore, was to assess the effect of macrolide therapy on mortality in patients with CAP due to P aeruginosa. We hypothesized that treatment with macrolides would increase survival in hospitalized patients with Pseudomonas CAP.
Materials and Methods
We conducted a population-based cohort study using the administrative databases of the Department of Veterans Affairs (VA). The VA databases are repositories of clinical data from > 150 Veterans Health Administration (VHA) hospitals and 850 VHA clinics. The Institutional Review Board (number HSC20070783H) of The University of Texas Health Science Center at San Antonio and the South Texas Veterans Health Care System Research and Development Committee approved this study.
Patient Eligibility
We identified all patients admitted to one of the study hospitals between fiscal years 2002 and 2007 (October 1, 2001, to September 30, 2007) with a primary discharge diagnosis of pneumonia (International Classification of Diseases, Ninth Edition, Clinical Modification [ICD-9-CM], codes 480.0-483.99 or 485-487) or a secondary discharge diagnosis of pneumonia with a primary diagnosis of respiratory failure (518.81) or sepsis (038.xx). Eligible patients met the following inclusion criteria: (1) P aeruginosa was identified as the causative pathogen based on ICD-9-CM discharge diagnosis codes (482.1), (2) age was ≥ 65 years on admission, (3) patient had at least 1 year of VA outpatient care before admission, and (4) patient received at least one dose of antibiotics within 48 h of admission.
To restrict the study to patients with CAP, patients who met one documented risk factor for health-care-associated pneumonia (HCAP) or were receiving immunosuppression were excluded. HCAP risk factors were defined as hospital admission in the previous 90 days, residence in a nursing home in the previous 90 days, receipt of outpatient IV antibiotics in the past 90 days, and hemodialysis. Immunosuppression was defined as presence of HIV, solid organ transplant, bone marrow transplant, and hematologic malignancy or reviving chemotherapy within 90 days of admission. The rationale for excluding patients with HCAP was to attempt to avoid bias with the a priori presumption of (1) higher mortality among patients with HCAP, (2) unclear need for atypical coverage among patients with HCAP, and (3) recommended use of anti-Pseudomonas coverage among all patients with HCAP compared with patients with CAP.
Baseline Characteristics
Baseline demographics were recorded at the time of admission, and comorbid illnesses were determined by ICD-9-CM codes from outpatient and inpatient care in accordance with the Charlson comorbidity scoring system as both an individual and a composite score.19 Patient race was recorded as white or black. Tobacco use was defined as nicotine dependence, a recorded visit to a VHA tobacco cessation clinic, a Current Procedural Terminology treatment code for smoking (99406, 99407), or an outpatient prescription for a smoking cessation product (buproprion, varenicline, nicotine inhalation system, nicotine replacement). Medication use in the 90 days before admission was documented for cardiovascular medications, diabetes medications, systemic corticosteroids (oral and injectable), pulmonary medications, and antibiotics.
Antibiotic Therapy
Antibiotic therapy received within the first 48 h of admission was evaluated. Macrolide therapy was defined if the patient received azithromycin, clarithromycin, or erythromycin. Antipseudomonal antibiotics included β-lactams (ceftazidime, cefepime, piperacillin, meropenem, imipenem), quinolones (ciprofloxacin, levofloxacin), aminoglycosides (gentamicin, amikacin, tobramycin), and monobactam (aztreonam).
Outcomes
Primary outcome was 30-day mortality. Secondary outcomes were ICU admission, use of invasive mechanical ventilation, use of vasopressors, and hospital length of stay (LOS). We chose to examine mortality at 30 days because previous research has demonstrated that 30-day mortality is primarily due to pneumonia rather than to comorbid conditions.20 Mortality was assessed through October 1, 2007, using the VA vital status file. Previous studies demonstrated that this methodology has a sensitivity of 98% for veterans’ death.21 LOS was calculated as the date of discharge minus the date of admission.
Statistical Analysis
For the statistical analyses, patients were stratified according to macrolide administration. Bivariate statistics were used to test the associations of demographics and clinical characteristics between both groups. Categorical variables were analyzed by χ2 test, and continuous variables were analyzed by Student t test. We defined statistical significance as a two-tailed P < .05. We performed a multivariable analysis by Cox proportional hazard model, with 30-day mortality as the dependent variable. Potential confounders were included in the multivariable analyses if P < 0.1 and a priori selection based on Charlson comorbidity score. A secondary analysis was performed, including only patients admitted or not admitted to the ICU. All analyses were performed with SPSS, version 19.0 for Windows (IBM) statistical software.
Results
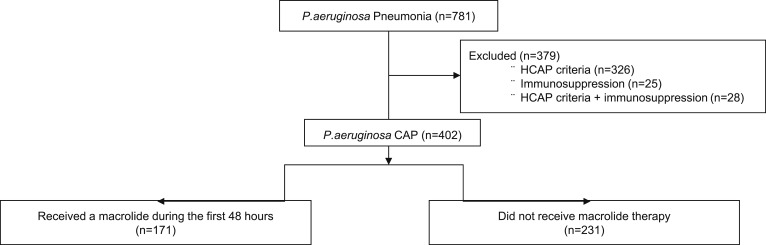
A total of 781 patients with P aeruginosa pneumonia were identified (Fig 1). Of these, 379 were excluded for HCAP (n = 326), immunosuppression (n = 25), or both (n = 28). The cohort included 402 patients with P aeruginosa CAP, of whom 42.5% (n = 171) received a macrolide during the first 48 h of admission and 57.5% (n = 231) did not receive macrolide therapy.
Figure 1.
Flow diagram illustrating the subjects included in the study. CAP = community-acquired pneumonia; HCAP = health-care-associated pneumonia; P. aeruginosa = Pseudomonas aeruginosa.
Patient Characteristics
Table 1 shows the characteristics of the subjects grouped by whether they received macrolide therapy. Patients who received macrolides were more likely to be older and white. No statistically significant differences were found in sex, marital status, drug abuse, prior prescribed medication use, preexisting comorbid conditions, or laboratory data between groups (Table 1). No significant differences were found in antibiotic coverage against P aeruginosa in the first 48 h of admission or in the various categories of antipseudomonal antibiotics.
Table 1.
—Demographics and Clinical Characteristics of Patients With Pseudomonas aeruginosa CAP (N = 402) With or Without Initial Macrolide Therapy
| Characteristic | Nonmacrolide (n = 231) | Macrolide (n = 171) | P Value |
| Age, y | 76.1 ± 6.5 | 77.4 ± 6.1 | .04 |
| Male sex | 227 (98.3) | 171 (100) | .1 |
| Married | 117 (50.6) | 93 (54.4) | .4 |
| Race | |||
| White | 186 (80.5) | 152 (88.9) | .02 |
| Black | 19 (8.2) | 11 (6.4) | .5 |
| Smoker | 89 (38.5) | 69 (40.4) | .7 |
| Alcohol abuse | 11 (4.8) | 7 (4.1) | .8 |
| Comorbid conditions | |||
| Myocardial infarction | 7 (3.0) | 5 (2.9) | .9 |
| Chronic heart failure | 35 (15.2) | 34 (19.9) | .2 |
| Peripheral vascular disease | 29 (12.6) | 19 (11.1) | .7 |
| Cerebrovascular disease | 35 (15.2) | 21 (12.3) | .4 |
| Dementia | 7 (3.0) | 10 (5.8) | .2 |
| COPD | 160 (69.3) | 121 (70.8) | .8 |
| Rheumatologic disease | 4 (1.7) | 5 (2.9) | .5 |
| Peptic ulcer | 5 (2.2) | 3 (1.8) | .9 |
| Cirrhosis | 2 (0.9) | 3 (1.8) | .6 |
| Diabetes mellitus | 50 (21.6) | 34 (19.9) | .7 |
| Diabetes with complications | 11 (4.8) | 14 (8.2) | .2 |
| Hemiplegia or paraplegia | 3 (1.3) | 1 (0.6) | .6 |
| Chronic renal disease | 3 (1.3) | 4 (2.3) | .4 |
| Neoplastic disease | 45 (19.5) | 37 (21.6) | .6 |
| Metastatic disease | 7 (3.0) | 6 (3.5) | .7 |
| Charlson comorbidity index score | 2.2 ± 1.9 | 2.4 ± 2.0 | .2 |
| Medication prescription within 90 d | |||
| Cardiovascular medications | 2.2 ± 1.2 | 2.1 ± 1.1 | .3 |
| Antidiabetic medications | 1.3 ± 0.6 | 1.3 ± 0.5 | .8 |
| Pulmonary medications | 3.9 ± 1.7 | 3.7 ± 1.8 | .4 |
| P aeruginosa antibiotic coverage | 205 (88.7) | 154 (90.1) | .6 |
| Category of antibiotics | |||
| β-Lactams | 171 (74.0) | 129 (75.4) | .7 |
| Aztreonam | 10 (4.3) | 7 (4.1) | .9 |
| Aminoglycosides | 71 (30.7) | 50 (29.2) | .7 |
| Fluoroquinolones | 150 (64.9) | 109 (63.7) | .8 |
Data are presented as mean ± SD or No. (%). CAP = community-acquired pneumonia.
Clinical Outcomes
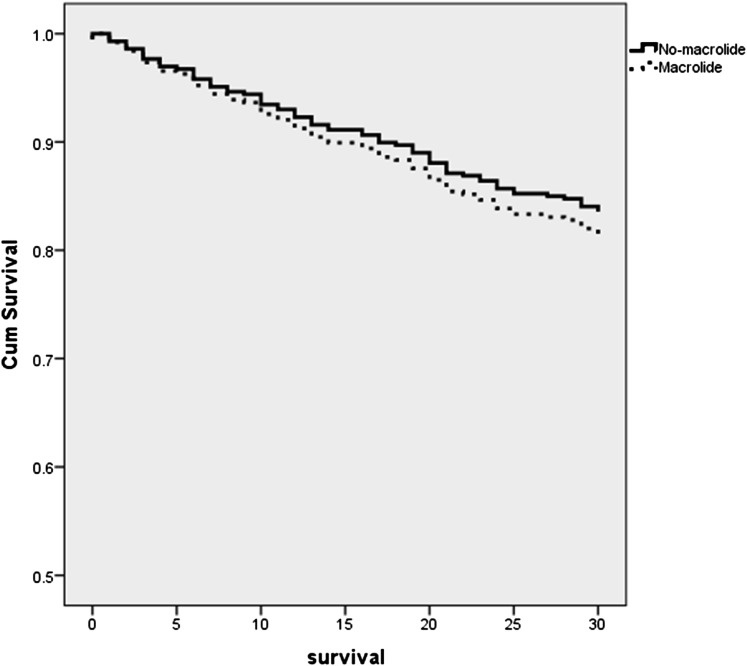
In the univariate analyses, patients with P aeruginosa CAP who received macrolides had no differences in 30-day mortality compared with patients who did not receive macrolides (Table 2). In addition, no differences were found between groups regarding ICU admission, need of mechanical ventilation, need of vasopressors, and LOS (Table 2). In the Cox proportional hazard analyses, after adjusting for Charlson comorbidity index score, the use of vasopressors was independently associated with higher 30-day mortality (hazard ratio, 2.13; 95% CI, 1.31-3.49; P = .002). However, macrolide antibiotic use was not independently associated with 30-day mortality (hazard ratio, 1.14; 95% CI, 0.70-1.83; P = .5) (Fig 2).
Table 2.
—Univariate Outcomes of Patients With P aeruginosa CAP Who Received Initial Macrolide vs Nonmacrolide Therapy
| Outcome | Nonmacrolide (n = 231) | Macrolide (n = 171) | P Value |
| 30-d mortality | 38 (16.5) | 32 (18.7) | .5 |
| Use of mechanical ventilation | 64 (27.7) | 49 (28.7) | .8 |
| Use of vasopressors | 38 (16.5) | 29 (17.0) | .8 |
| Need of ICU | 75 (32.5) | 61 (35.7) | .5 |
| Hospital LOS, d | 20.3 ± 33.1 | 18.9 ± 23.5 | .6 |
Data are presented as No. (%) or mean ± SD. LOS = length of stay. See Table 1 legend for expansion of other abbreviations.
Figure 2.
Cox survival curves of patients with P aeruginosa CAP treated with macrolides vs those not treated with macrolides (hazard ratio,1.14; 95% CI, 0.70-1.83; P = .5). Cum = cumulative. See Figure 1 legend for expansion of other abbreviation.
Subgroup Analyses
To assess the influence of severity of illness on baseline characteristics and outcomes, we performed an additional analysis stratified by ICU admission (n = 136) and no ICU admission (n = 266). No differences were found among baseline characteristics between groups, except that patients admitted to the ICU who received macrolide therapy had a higher rate of COPD (68.9% vs 48.0%, respectively) (Table 3). In addition, no differences in outcomes evaluated were found among groups with respect to the site of care (Table 3).
Table 3.
—Demographics, Clinical Characteristics, and Outcomes of Patients With P aeruginosa CAP by Admission to the ICU
| No ICU (n = 266) | ICU (n = 136) | |||||
| Characteristic or Outcome | Nonmacrolide (n = 156) | Macrolide (n = 110) | P Value | Nonmacrolide (n = 75) | Macrolide (n = 61) | P Value |
| Age, y | 76.2 ± 6.4 | 78.2 ± 6.3 | .01 | 75.8 ± 6.8 | 76.0 ± 5.3 | .8 |
| Male sex | 154 (98.7) | 110 (100) | .2 | 73 (97.3) | 61 (100) | .1 |
| Married | 84 (53.8) | 57 (51.8) | .7 | 33 (44.0) | 36 (59.0) | .08 |
| Race | ||||||
| White | 131 (84.0) | 98 (89.1) | .2 | 55 (73.3) | 54 (88.5) | .02 |
| Black | 9 (5.8) | 6 (5.5) | .9 | 10 (13.3) | 5 (8.2) | .3 |
| Smoker | 64 (41.0) | 46 (41.8) | .8 | 25 (33.3) | 23 (37.7) | .5 |
| Alcohol abuse | 8 (5.1) | 4 (3.6) | .5 | 3 (4.0) | 3 (4.9) | .7 |
| Comorbid conditions | ||||||
| Myocardial infarction | 4 (2.6) | 5 (4.5) | .3 | 3 (4.0) | 0 | .1 |
| Chronic heart failure | 25 (16.0) | 21 (19.1) | .5 | 10 (13.3) | 13 (21.3) | .2 |
| Peripheral vascular disease | 19 (12.2) | 11 (10.0) | .5 | 10 (13.3) | 8 (13.1) | .9 |
| Cerebrovascular disease | 22 (14.1) | 9 (8.2) | .1 | 13 (17.3) | 12 (19.7) | .7 |
| Dementia | 4 (2.6) | 6 (5.5) | .2 | 3 (4.0) | 4 (6.6) | .5 |
| COPD | 124 (79.5) | 79 (71.8) | .1 | 36 (48.0) | 42 (68.9) | .01 |
| Diabetes mellitus | 29 (18.6) | 20 (18.2) | .9 | 21 (28.0) | 14 (23.0) | .5 |
| Diabetes with complications | 4 (2.6) | 6 (5.5) | .2 | 7 (9.3) | 8 (13.1) | .4 |
| Neoplastic disease | 26 (16.7) | 26 (23.6) | .1 | 19 (25.3) | 11 (18.0) | .3 |
| Metastatic disease | 4 (2.6) | 3 (2.7) | .9 | 3 (4.0) | 3 (4.9) | .7 |
| Charlson comorbidity index score | 76.2 ± 6.4 | 78.2 ± 6.3 | .5 | 2.3 ± 2.3 | 2.6 ± 2.3 | .4 |
| P aeruginosa antibiotic coverage | 146 (93.6) | 100 (90.9) | .4 | 59 (78.7) | 54 (88.5) | .1 |
| 30-d mortality | 17 (10.9) | 12 (10.9) | .9 | 21 (28.0) | 20 (32.8) | .5 |
| Use of mechanical ventilation | … | … | … | 58 (77.3) | 44 (72.1) | .4 |
| Use of vasopressors | … | … | … | 35 (46.7) | 26 (42.6) | .6 |
| LOS | 12.0 ± 16.5 | 10.8 ± 10.4 | .5 | 37.7 ± 48.7 | 33.6 ± 32.2 | .5 |
Discussion
In this study, the use of macrolides in the first 48 h of hospital admission for CAP due to P aeruginosa was not associated with better clinical outcomes, including 30-day mortality. To our knowledge, this study is the first to evaluate the impact of macrolide therapy on mortality in a cohort of patients with P aeruginosa CAP.
The impact of macrolides on clinical outcomes in CAP is a matter for debate. Several observational studies have shown lower mortality rates for patients with bacteremic pneumococcal pneumonia6‐8 and severe CAP.9‐11 In a retrospective cohort study, Restrepo et al11 found a survival benefit of macrolide therapy in 30- and 90-day mortality rates for patients with sepsis after pneumonia. This effect was observed even in patients with macrolide-resistant pathogens. Similarly, Metersky et al9 carried out a retrospective analysis of bacteremic CAP of all etiologies and found lower mortality in patients treated with macrolides, even in infections caused by gram-negative pathogens, suggesting the presence of an immunomodulatory effect in addition to the antimicrobial properties of macrolides. However, other studies have not shown a benefit of macrolides used in combination with other antibiotics.22‐24 In a meta-analysis, Asadi et al25 showed that macrolide-based regimens were associated with lower mortality (22% reduction) compared with nonmacrolides in hospitalized patients with CAP. However, when the analysis was restricted to randomized controlled trials or to patients who received guideline-concordant antibiotics, this mortality benefit disappeared. In the present study, no differences in the 30-day mortality were found between groups according the use of macrolides in the first 48 h of admission.
Various potential mechanisms have been described to explain the macrolide benefit in CAP. One of these is that macrolides may treat unrecognized atypical pathogens.26 Another is antibiotic synergy, but this effect is not probed with penicillins or cephalosporins. In the present cohort, atypical pathogens were excluded, and β-lactams (including penicillins and cephalosporins) were the most frequently used antibiotics. In addition, in most of the studies where macrolide benefit was observed, patients had severe or bacteremic CAP.9,11 Although we performed a subanalysis with only the patients admitted to the ICU, the cohort did not recognize severity of illness at admission or the presence or absence of bacteremia. All these arguments may explain, in part, why we did not find differences between groups.
P aeruginosa is a pathogen that produces many virulence factors, including exotoxins, enzymes, and biofilm, that protect it from environmental elements, host antibodies, and phagocytes.27 Although P aeruginosa is not part of the antimicrobial spectrum of macrolides, data show that macrolides are potential inhibitors of its growth and may alter its virulence factors. Macrolides inhibit quorum sensing of P aeruginosa that allows creation of biofilms and firm adherence to the airways. In several studies of sepsis caused by multidrug-resistant P aeruginosa in animal models, macrolides achieve a considerable benefit of survival.28 However, clinical evidence that macrolides may effectively affect acute clinical infection is not available. All studies suggesting clinical benefit of macrolides in other pathologies, such cystic fibrosis or panbronchiolitis, evaluated long-term treatment.3 In the present study, we evaluated the impact of the initial therapy for CAP (in the first 48 h of hospitalization) rather than of long-term treatment with macrolides and found no differences in clinical outcomes.
This study has several limitations. First, it was a retrospective cohort study in a predominantly elderly male population and is subject to the inherent limitations of all retrospective research. Second, the use of ICD-9-CM codes to identify patients with pneumonia, pathogens, and baseline characteristics has limitations. For example, it is not possible to correct for the heterogeneity of sample collection and the inability to differentiate between possible colonization vs true P aeruginosa infection, particularly in patients with COPD. However, most of the published P aeruginosa CAP studies are limited by single-center design and relatively small sample sizes.13,29,30 The use of ICD-9-CM codes enabled us to obtain significant amounts of information from a large national cohort of patients in a closed health system, which is a major strength of this study. The process of medical coding introduces opportunities for human error, but the use of ICD-9-CM codes for selecting patients with pneumonia has favorable positive and negative predictive values (85.5% and 97.2%, respectively), indicating a relatively low likelihood of misclassification.31 Third, this study lacked detailed information regarding microbiology studies and bacterial susceptibility and antibiotic time of administration, duration, and dosage, which future studies should address. Finally, we did not attempt to assess the clinical effectiveness of macrolides among patients with risk factors for multidrug-resistant pathogens, as recommended by the HCAP definition and clinical practice guidelines, because of uncertainties and heterogeneity raised by several expert reviews and cohort studies.32,33 However, we recognize the importance of stratifying the use of appropriate and direct antimicrobial therapy for patients with Pseudomonas infection according to clinical practice guidelines.1
In conclusion, macrolide use in the first 48 h of hospital admission in patients with CAP due to P aeruginosa is not associated with survival benefit. Further studies are needed to better characterize patients, microbiology, mechanisms of action, and severity of illness to understand potential benefits of macrolides in CAP.
Acknowledgments
Author contributions: Dr Restrepo had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Laserna: contributed to the data analysis and interpretation, preparation of the manuscript, and final approval of the manuscript.
Dr Sibila: contributed to the data analysis and interpretation, preparation of the manuscript, and final approval of the manuscript.
Dr Fernandez: contributed to the data analysis and interpretation, preparation of the manuscript, and final approval of the manuscript.
Dr Maselli: contributed to the data analysis and interpretation, preparation of the manuscript, and final approval of the manuscript.
Dr Mortensen: contributed to the study design, obtaining funding, coordination of the data analysis, preparation of the manuscript, and final approval of the manuscript.
Dr Anzueto: contributed to the study design, data acquisition and analysis, preparation of the manuscript, and final approval of the manuscript.
Dr Waterer: contributed to the data interpretation, preparation of the manuscript, and final approval of the manuscript.
Dr Restrepo: contributed to obtaining funding, data acquisition, preparation of the manuscript, and final approval of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The funding agencies had no role in the preparation, review, or approval of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Abbreviations
- CAP
community-acquired pneumonia
- HCAP
health-care-associated pneumonia
- ICD-9-CM
International Classification of Diseases, Ninth Edition, Clinical Modification
- LOS
length of stay
- VA
Department of Veterans Affairs
- VHA
Veterans Health Administration
Footnotes
Funding/Support: This research was supported by Howard Hughes Medical Institute faculty [Start-up Grant 00378-001] and a Department of Veterans Affairs Veterans Integrated Service Network 17 new faculty grant. Drs Laserna and Sibila are supported by Sociedad Espanola de Neumologia y Cirugia Toracica (SEPAR), Societat Catalana de Pneumologia (SOCAP), and Fundacio Catalana de Pneumologia (FUCAP). Dr Sibila is supported by Instituto de Salud Carlos III [BAE11/00102]. Dr Restrepo’s time is partially protected by K23-HL096054 from the National Heart, Lung, and Blood Institute.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodhead M, Blasi F, Ewig S, et al. ; European Respiratory Society; European Society of Clinical Microbiology and Infectious Diseases. Guidelines for the management of adult lower respiratory tract infections [published correction appears in Eur Respir J. 2006;27(2):439]. Eur Respir J. 2005;26(6):1138-1180 [DOI] [PubMed] [Google Scholar]
- 3.Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;23(3):590-615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giamarellos-Bourboulis EJ. Macrolides beyond the conventional antimicrobials: a class of potent immunomodulators. Int J Antimicrob Agents. 2008;31(1):12-20 [DOI] [PubMed] [Google Scholar]
- 5.Amsden GW. Anti-inflammatory effects of macrolides—an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother. 2005;55(1):10-21 [DOI] [PubMed] [Google Scholar]
- 6.Waterer GW, Somes GW, Wunderink RG. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch Intern Med. 2001;161(15):1837-1842 [DOI] [PubMed] [Google Scholar]
- 7.Martínez JA, Horcajada JP, Almela M, et al. Addition of a macrolide to a beta-lactam-based empirical antibiotic regimen is associated with lower in-hospital mortality for patients with bacteremic pneumococcal pneumonia. Clin Infect Dis. 2003;36(4):389-395 [DOI] [PubMed] [Google Scholar]
- 8.Baddour LM, Yu VL, Klugman KP, et al. ; International Pneumococcal Study Group. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170(4):440-444 [DOI] [PubMed] [Google Scholar]
- 9.Metersky ML, Ma A, Houck PM, Bratzler DW. Antibiotics for bacteremic pneumonia: improved outcomes with macrolides but not fluoroquinolones. Chest. 2007;131(2):466-473 [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez A, Mendia A, Sirvent JM, et al. ; CAPUCI Study Group. Combination antibiotic therapy improves survival in patients with community-acquired pneumonia and shock. Crit Care Med. 2007;35(6):1493-1498 [DOI] [PubMed] [Google Scholar]
- 11.Restrepo MI, Mortensen EM, Waterer GW, Wunderink RG, Coalson JJ, Anzueto A. Impact of macrolide therapy on mortality for patients with severe sepsis due to pneumonia. Eur Respir J. 2009;33(1):153-159 [DOI] [PubMed] [Google Scholar]
- 12.Fujitani S, Sun HY, Yu VL, Weingarten JA. Pneumonia due to Pseudomonas aeruginosa: part I: epidemiology, clinical diagnosis, and source. Chest. 2011;139(4):909-919 [DOI] [PubMed] [Google Scholar]
- 13.Arancibia F, Bauer TT, Ewig S, et al. Community-acquired pneumonia due to gram-negative bacteria and Pseudomonas aeruginosa: incidence, risk, and prognosis. Arch Intern Med. 2002;162(16):1849-1858 [DOI] [PubMed] [Google Scholar]
- 14.von Baum H, Welte T, Marre R, Suttorp N, Ewig S; CAPNETZ study group. Community-acquired pneumonia through Enterobacteriaceae and Pseudomonas aeruginosa: diagnosis, incidence and predictors. Eur Respir J. 2010;35(3):598-605 [DOI] [PubMed] [Google Scholar]
- 15.Altenburg J, de Graaff CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA. 2013;309(12):1251-1259 [DOI] [PubMed] [Google Scholar]
- 16.Saiman L, Marshall BC, Mayer-Hamblett N, et al. ; Macrolide Study Group. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290(13):1749-1756 [DOI] [PubMed] [Google Scholar]
- 17.Albert RK, Connett J, Bailey WC, et al. ; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689-698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudoh S, Azuma A, Yamamoto M, Izumi T, Ando M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med. 1998;157(6):1829-1832 [DOI] [PubMed] [Google Scholar]
- 19.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245-1251 [DOI] [PubMed] [Google Scholar]
- 20.Mortensen EM, Coley CM, Singer DE, et al. Causes of death for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 2002;162(9):1059-1064 [DOI] [PubMed] [Google Scholar]
- 21.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aspa J, Rajas O, Rodriguez de Castro F, et al. ; Pneumococcal Pneumonia in Spain Study Group. Impact of initial antibiotic choice on mortality from pneumococcal pneumonia. Eur Respir J. 2006;27(5):1010-1019 [DOI] [PubMed] [Google Scholar]
- 23.Dwyer R, Ortqvist A, Aufwerber E, et al. Addition of a macrolide to a β-lactam in bacteremic pneumococcal pneumonia. Eur J Clin Microbiol Infect Dis. 2006;25(8):518-521 [DOI] [PubMed] [Google Scholar]
- 24.Paul M, Nielsen AD, Gafter-Gvili A, et al. The need for macrolides in hospitalised community-acquired pneumonia: propensity analysis. Eur Respir J. 2007;30(3):525-531 [DOI] [PubMed] [Google Scholar]
- 25.Asadi L, Sligl WI, Eurich DT, et al. Macrolide-based regimens and mortality in hospitalized patients with community-acquired pneumonia: a systematic review and meta-analysis. Clin Infect Dis. 2012;55(3):371-380 [DOI] [PubMed] [Google Scholar]
- 26.Waterer GW, Rello J, Wunderink RG. Management of community-acquired pneumonia in adults. Am J Respir Crit Care Med. 2011;183(2):157-164 [DOI] [PubMed] [Google Scholar]
- 27.Ma L, Conover M, Lu H, Parsek MR, Bayles K, Wozniak DJ. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 2009;5(3):e1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giamarellos-Bourboulis EJ, Adamis T, Laoutaris G, et al. Immunomodulatory clarithromycin treatment of experimental sepsis and acute pyelonephritis caused by multidrug-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2004;48(1):93-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang CI, Song JH, Oh WS, Ko KS, Chung DR, Peck KR; Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study Group. Clinical outcomes and risk factors of community-acquired pneumonia caused by gram-negative bacilli. Eur J Clin Microbiol Infect Dis. 2008;27(8):657-661 [DOI] [PubMed] [Google Scholar]
- 30.Falguera M, Carratalà J, Ruiz-Gonzalez A, et al. Risk factors and outcome of community-acquired pneumonia due to gram-negative bacilli. Respirology. 2009;14(1):105-111 [DOI] [PubMed] [Google Scholar]
- 31.Aronsky D, Haug PJ, Lagor C, Dean NC. Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319-328 [DOI] [PubMed] [Google Scholar]
- 32.Ewig S, Welte T, Chastre J, Torres A. Rethinking the concepts of community-acquired and health-care-associated pneumonia. Lancet Infect Dis. 2010;10(4):279-287 [DOI] [PubMed] [Google Scholar]
- 33.Attridge RT, Frei CR, Restrepo MI, et al. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia. Eur Respir J. 2011;38(4):878-887 [DOI] [PubMed] [Google Scholar]