Abstract
Recent studies have implicated the innate immunity system in the pathogenesis of myelodysplastic syndromes (MDS). Toll-like receptor (TLR) genes encode key innate immunity signal initiators. We recently identified multiple genes, known to be regulated by TLRs, to be overexpressed in MDS bone marrow (BM) CD34+ cells, and hypothesized that TLR signaling is abnormally activated in MDS. We analyzed a large cohort of MDS cases and identified that TLR1, 2 and 6 to be significantly overexpressed in MDS BM CD34+ cells. Deep-sequencing followed by Sanger-resequencing of TLR1, 2, 4 and 6 genes uncovered a recurrent genetic variant, TLR2-F217S, in 11% of 149 patients. Functionally, TLR2-F217S results in enhanced activation of downstream signaling including NF-kB activity after TLR2 agonist treatment. In cultured primary BM CD34+ cells of normal donors, TLR2 agonists induced histone demethylase JMJD3 and interleukin-8 gene expression. Inhibition of TLR2 in BM CD34+ cells from patients with lower-risk MDS using shRNA resulted in increased erythroid colony formation. Finally, RNA expression levels of TLR2 and 6 as well as presence of TLR2-F217S are associated with distinct prognosis and clinical characteristics. These findings indicate that TLR2-centered signaling is deregulated in MDS and that its targeting may have potential therapeutic benefit in MDS.
Keywords: myelodysplastic syndromes, innate immunity, Toll like receptor, IL-8, JMJD3
INTRODUCTION
The myelodysplastic syndromes (MDS) are a complex group of malignant myeloid disorders arising from bone marrow (BM) hematopoietic stem and progenitor cells (HSPCs). 1 The pathophysiology of MDS is not fully understood. We recently performed a genome-wide CHIP-Seq analysis of H3K4me3 in MDS.2 This analysis identified multiple genes marked by increased H3K4me3 in BM CD34+ cells. A large majority of the genes identified are known to be involved in Toll-like receptor (TLR) mediated innate immunity signaling and NF-kB activation. 2 TLRs are a family of pattern-recognition receptors (PRRs) that function as key initiators of innate immunity signaling.3 In the same study, we also identified that the histone demethylase JMJD3 (KDM6B) is significantly overexpressed in MDS BM CD34+ cells and plays an important role in the regulation of expression of genes involved in innate immunity. 2 Prior work by De Santa et al in murine macrophages has indicated that JMJD3 expression is regulated by TLR mediated NF-kB activation. 4 These findings suggested a potential role for TLR function in MDS.
Based on this, we performed gene expression and mutational analysis of eight human TLRs in a large cohort of MDS. Several major findings are reported here. First, we identified that TLR1, 2, and 6 are significantly overexpressed in MDS BM CD34+ cells. Of importance, both TLR1 and TLR6 are known to form functional heterodimers with TLR2. Second, deep sequencing identified a rare genetic variant of TLR2 (F217S) present in 11% of bone marrow mononuclear cells (BM-MNCs) of patients with MDS. The incidence of TLR2-F217S in MDS is significantly higher than its known frequency in normal population. Third, we demonstrated that inhibition of TLR2 in cultured BM CD34+ cells from patients with lower-risk of MDS results in increased formation of erythroid colonies. Finally, clinical analysis indicated that RNA expression levels of TLR2 and TLR6 as well as the presence of TLR2-F217S were associated with distinct clinical characteristics. These results indicate that TLR2 mediated innate immune signaling has a role in the pathophysiology of MDS and that its targeting may have therapeutic potential.
MATERIALS AND METHODS
Isolation and culture of bone marrow CD34+ cells
Human samples were obtained following institutional guidelines. MDS bone marrow specimens were obtained freshly from patients referred to the Department of Leukemia at MD Anderson Cancer Center (MDACC) following institutional guidelines. Diagnosis was confirmed by a dedicated hematopathologist (CB-R) as soon as sample was obtained. Bone marrows from healthy individuals were obtained from All Cells (Emeryville, CA). Isolation of CD34+ cells was performed using MicroBead Kit (Miltenyi, Bergisch Gladbach, Germany). Primary BM CD34+ cells were cultured in IMDM (Invitrogen, Carlsbad, CA), 20% BIT 9500 (bovine serum albumin, insulin, transferin), human thrombopoietin (hTPO) 50 ng/ml, IL3 10 ng/ml, Stem cell factor (SCF) 100 ng/ml, FLT3L 100 ng/ml (Stem Cell Technology, Vancouver, CA). For colony forming assays, healthy and MDS BM CD34+ cells were seeded at 1000 cells/ml and 10000 cells/ml respectively in 3.5 cm round culture dishes with methocult GF H4434 (Stem Cell Technology, Vancouver, CA).
Cell lines and culture
293T cells were cultured in DMEM, 10% fetal calf serum, 1% penicillin-streptomycin, and 2 mM L-glutamine. OCI-AML3 cells were cultured in RPMI-1640, 10% fetal calf serum and 1% penicillin-streptomycin. All cells were obtained from ATCC (Manassas, VA).
Quantitative RT-PCR
Total cellular RNA was extracted using Trizol (Invitrogen, Carlsbad, CA) according to manufacturer’s protocol. 200 nanograms of total RNA were used for reverse transcription (RT) reactions using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA). For real-time PCR, primers and probes were purchased from Applied Biosystems and analyzed using an Applied Biosystems Prism 7500 Sequence Detection System. PCR reactions were performed using 20 x Assays-On-Demand Gene Expression Assay Mix and TaqMan Universal PCR Master mix according to the manufacturer’s protocol. GAPDH was used as internal control.
Capture deep sequencing of genomic DNA
Illumina paired-end libraries were generated according to the manufacturer’s protocol (Illumina San Diego, CA). 3 μg of pre-capture library DNA was used for each capture reaction. NimbleGen SeqCap EZ Hybridization and Wash Kits (Roche, Basel, Switzerland) were used following the manufacturer’s protocol. 14–17 cycles of PCR amplification were applied to the samples after hybridization, based on yield. After that, captured libraries were quantified by picogreen and sequenced on the Illumina HiSeq 2000 as 100-bp paired-end reads, following the manufacturer’s protocols. Sequencing data was stored as fastq format which contained reads and quality information. Fastq files were then mapped and aligned to human reference hg18 using BWA 5. To refine the alignment and adjust the quality score provided by sequencer, GATK was used to realign reads and recalibrate the quality score 6. SNPs were called using AtlasSNP2 (http://www.hgsc.bcm.tmc.edu/cascade-tech-software_atlas_snp-ti.hgsc). The posterior probability was set as 0.9 and the minimum number of variants reads as 3. To identify rare SNPs, candidate SNPs were filtered against dbSNP130, 1000 genome database, and the Human Genome Sequencing Center (Baylor College of Medicine) internal database. Indels were called using AtlasIndel (http://www.hgsc.bcm.tmc.edu/cascade-tech-software_atlas2_snp_indel_calling_pipeline-ti.hgsc).
Recombinant retrovirus mediated shRNA transduction
pGFP-V-RS plasmids expressing the 29mer shRNA against human TLR2 or control shRNA were purchased from Origene (Rockville, MD). Recombinant retrovirus expressing shRNA were packaged in 293T (Invitrogen, Carlsbad, CA) and concentrated using the PEG-IT virus precipitation solution (System Biosciences, Mountain View, CA). Transduction of OCI-AML3 and bone marrow CD34+ cells with virus was performed by mixing virus with cell suspension followed by centrifugation at 30°C for 90 min with 4μg/ml polybrene in medium. After viral transduction, OCI-AML3 cells with stable expression of the shRNA against TLR2 or a control scrambled shRNA were established in the presence of puromycine (10 μg/ml) in medium.
Luciferase assay
293T cells were transfected with pGL4.32[luc2P/NF-κB-RE] (Promega, Madison, WI) or pAP1-Luc (Agilent, Santa Clara CA) together with pCMV6-AC-GFP-TLR2 wild type (Origene, Rockville, MD) or P217S mutant. pRL-TK vectors expressing the Renilla luciferase were cotransfected as internal control. Lipofectamine 2000 (Invitrogen, Carlsbad, CA) was used for transfection. One day after transfection, TLR2 agonist MALP2 (1 μg/ml) or PAM2CSK4 (0.3 μg/ml) (Invivogen, San Diego, CA) were added to medium. Luciferase assays were performed 8 hours later with the Dual-Luciferase Reporter Assay kit (Promega, Madison, WI) on 3010 luminometer (BD Biosciences, San Diego, CA). Luciferase activity was calculated using the ratio of firefly luminescence from the pGL4.32 [luc2P/NF-κB-RE] or pLuc-AP1 reporter to the renilla luminescence from the control pRL-TK vectors reporter.
Western blot and immunoprecipitation
293T cells were transfected with pCMV6-AC-GFP-TLR2 wild type (Origene, Rockville, MD) or P217S mutant. TLR2 agonist MALP2 (1 μg/ml) or PAM2CSK4 (0.3 μg/ml) (Invivogen, San Diego, CA) were added into medium one day after transfection. Cells were collected 5–30 min afterwards. Cells were lysed using RIPA lysis buffer (Cell Signaling, Danvers, MA) or processed for immunoprecipitation using crosslink IP kit (Thermo Scientific, Rockford, IL). Antibodies used include phospho-p38 and IRAK1 (4511 and 4504, Cell Signaling, Danvers, MA), phospho-IRAK1 (S376) (PAB0497, Abnova, Walnut, CA), phospho-IRAK1 (T209) (ab61799, Abcam, Cambridge MA), poly-Ub-K63 (05-1308, Millipore, Billerica, MA), TLR2 (TA306125, Origene, Rockville, MD), β-actin (A5441 Sigma, St. Louis, MO).
Multicolor flow cytometry
All monoclonal antibodies, including CD34, CD117, CD64, CD14, CD71, CD45, HLA-DR, were obtained from BD Biosciences (San Jose, CA). Samples were incubated with monoclonal antibodies for 25 min at 4°C, and acquired on FACSCanto II instruments (BD Biosciences, San Diego, CA). Data were analyzed using FCS Express software (De Novo Software, Los Angeles, CA).
CHIP-PCR
Immunoprecipitated DNA was analyzed by quantitative real time PCR (Q-PCR) using the Quanti-Tect SYBR Green PCR kit (Qiagen, Valencia, CA). The amount of DNA fragment co-precipitated with antibody was calculated and compared to the amount of the same genomic fragment in total input DNA, resulting in percent input.
Statistical Methods
Overall survival was defined from date of sample to date of death or date last known alive; patients last known alive were censored for this analysis. To investigate associations between gene expression and overall survival, we considered splitting expression level at the 25th, 50th, and 75th percentiles, generating three possible binary variables. Associations with clinical and demographic features at time of sample were assessed only at the median expression level. For ordered clinical features such as IPSS, the Kruskal-Wallis test was used; for binary features, such as gender, the Fisher exact test was used. For continuous variables such as percentage bone marrow blasts or hemoglobin, the Wilcoxon rank sum test was used.
Other Reagents
Human lymphoblastoid cell DNA was purchased from Sigma-Aldrich (St Louis, MI). According to manufacturer, cells were collected from UK Caucasian blood donors. The DNA was then extracted from lymphoblastoid cell lines derived by Epstein Barr Virus (EBV) that can be continuously propagated in culture.
RESULTS
Overexpression of TLR2 and its functional partners TLR1 and 6 in bone marrow cells of MDS
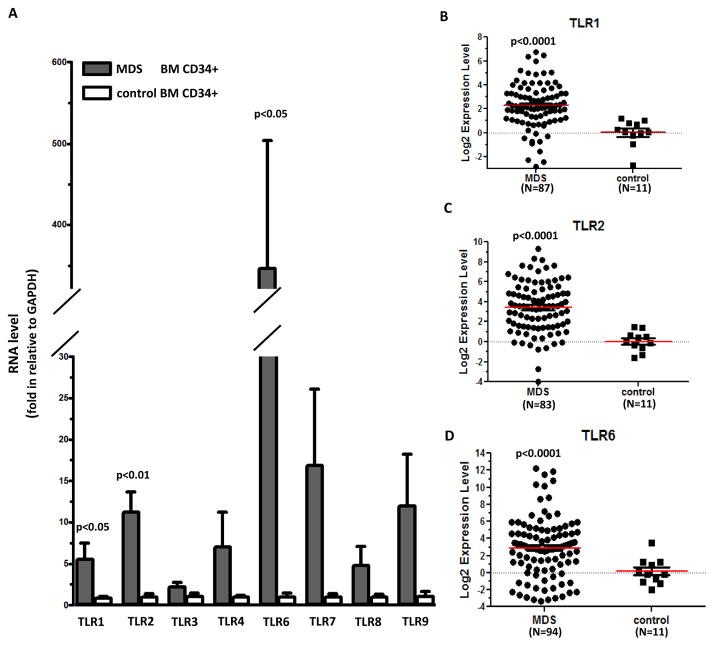
We first profiled RNA expression of 8 human TLRs (TLR 1–4 and 6–9) in a cohort of MDS BM CD34+ cells (N=40). RNA expression levels of TLRs in MDS in comparison to control BM CD34+ cells are shown in Figure 1A. This initial analysis indicated that TLR 1, 2 and 6 were significantly overexpressed in MDS samples compared to controls (Figure 1A). While the expression of TLR 4, 7, 8 and 9 was also increased in some MDS samples, the overall difference between MDS and controls was not significant (Figure 1A). We then expanded the analysis of RNA expression of TLR 1, 2 and 6 and summarized their expression in total 103 cases of MDS BM CD34+ samples analyzed. Summary of clinical characteristics of this sample cohort are in supplemental Table 1. Compared to controls, average expression levels in MDS samples were increased by 10-fold for TLR1 (74% over 2-fold increase, N=87, p<0.0001, Figure 1B), by 37-fold for TLR2 (82% over 2-fold increase, N=83, p<0.0001, Figure 1C), and by 168-fold for TLR6 (73% over 2-fold increase, N=94, p=0.0001, Figure 1D). This result is of potential biological significance because both TLR1 and TLR6 are known to form functional hetero-dimers with TLR2.7, 8
Figure 1. TLR RNA expression in primary MDS bone marrow CD34+ cells.
(a) Q-RTPCR analysis of RNA expression of 8 TLR genes in MDS and control BM CD34+ cells. (b–d) Logarithmic representation of Q-RTPCR results of TLR1, 2, and 6 in MDS and control CD34+ cells. Numbers of samples with evaluable RNA expression are marked underneath of each graph.
Identification of TLR2 mutation (F217S) in MDS BM mononuclear cells
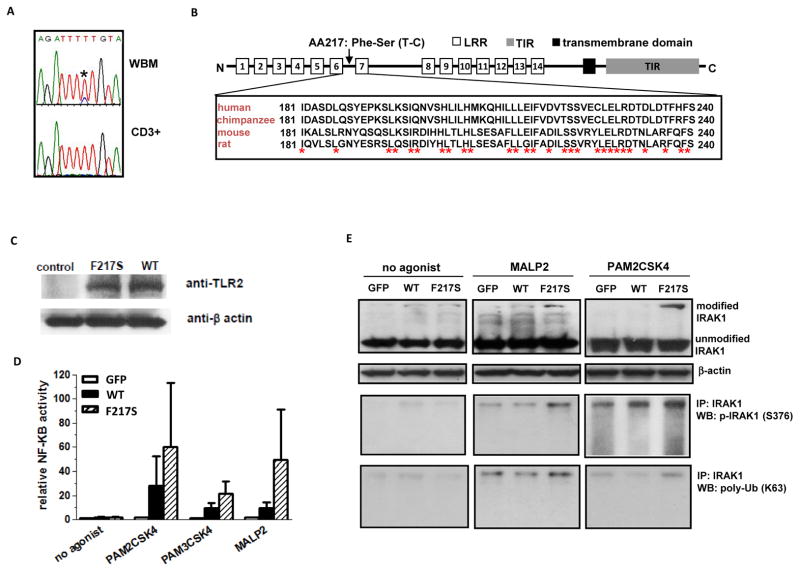
To further study the role of TLRs in MDS, we performed capture deep sequencing of genes encoding TLR1, 2, 4 and 6 in MDS bone marrow mononuclear cells (BM-MNC). We first studied a cohort of 40 patients and identified three rare single nucleotide variants (SNPs) located in the coding region of TLR2 and one SNP in TLR4 (supplemental Figure 1A). We subsequently analyzed these 4 SNPs using Sanger sequencing. One rare TLR2 SNP (rs139227237, g.154624709T>C) was detected as a heterogeneous alteration in 11% (N=17) of a cohort of 149 MDS BM-MNC samples (supplemental Table 2) (Figure 2A). At the protein level, this SNP results in a conserved hydrophobic phenyalanine (F217) located in the leucine-rich repeats of extracellular region to be converted into a hydrophilic serine (S) (Figure 2B). To evaluate whether TLR2-F217S is a somatic alteration in MDS, we analyzed corresponding bone marrow CD3+ cells available from 16 of the 17 patients known to carry this alteration in BM-MNC. Fourteen of these patients did not carry F217S in CD3+ cells (Figure 2A). Of interest, two CD3+ samples contained this SNP (supplemental Figure 1B). We also sequenced 20 CD3+ samples isolated from patients without TLR2-F217S. No TLR2-F217S was detected in any of these CD3+ cells (supplemental Figure 1C). We next analyzed a group of random normal control DNA samples (N=47) that were derived from human lymphoblastoid cells isolated from healthy donors. None of the normal control samples was positive for TLR2-F217S (data not shown).
Figure 2. Mutational analysis and identification of TLR2-F217S in MDS.
(a) Representative Sanger sequencing traces showing nucleotides around coding region of TLR2-F217 in whole bone marrow (WBM) DNA (Top) and CD3+ genomic DNA (Bottom) of one MDS sample. (b) Schema of TLR2 gene and sequence alignments between human and other species. (c) Western blots of GFP fusion protein expression of TLR2 WT and F217S in 293T cells. (d) Luciferase analysis of activation of NF-kB by WT and F217S TLR2 in transfected 293T cells. Cells were exposed to either no agonist or to PAM2CSK4, PAM3CSK4 or MALP2 known TLR2 agonists. Pooled data from three separate experiments. (e) Western blot characterization of IRAK1 modification in 293T cells transfected with either WT or F217S TLR2 and treated with no agonist, MALP2 or PAM2CSK4. Top, Western blot analysis of IRAK1; Middle, IRAK1 immunoprecipitation followed by phospho-IRAK1 Western blot; Bottom, IRAK1 immunoprecipitation followed by poly-ubiquitin (K63).
Biological implications of TLR2-F217S in innate immunity signal activation
To characterize the impact of TLR2 mutation F217S on the activation of innate immunity signaling, we expressed green fluorescent protein (GFP) fused with wild-type or TLR2-F217S in 293T cells (Figure 2C) and analyzed NF-kB activity. 293T cells express endogenous TLR1 and 6 but not TLR2 and therefore are often used to characterize genetically altered forms of TLR2 9. In luciferase reporter assays, in the absence of TLR2 agonist, expression of wild-type or F217S mutant TLR2 led to similar levels of NF-kB activation (Figure 2D). When a TLR2 agonist (PAM2CSK4, PAM3CSK4 or MALP2) was added, NF-kB activation was increased in TLR2-F217S versus wild-type transfected cells (Figure 2D). Similar effects on AP1 activity and associated phospho-p38MAPK were also observed in TLR2-F217S with PAM2CSK4 treatment but not without TLR2 agonist (supplemental Figure 1D and 1E). To further assess the effect of TLR2-F217S on innate immunity signaling, we examined the status of IRAK1 protein, a key downstream signal mediator of TLRs.10 In the absence of TLR agonist, a slight increase of a slowly migrating IRAK1 band (~ 180KD) was observed in both wild-type and F217S transfected 293T cells (Figure 2E). After MALP2 and PAM2CSK4 treatment, the density of this high-molecular IRAK1 band was higher in TLR2 (F217S) than in wildtype transfected cells (Figure 2E). IRAK1 immunoprecipitation followed by immunobloting indicated that this higher molecular form of IRAK1 contained phospho- and polyubiquitin- IRAK1 (Figure 2E), two active isoforms of this protein. 11 Taken together, these results indicated that TLR2-F217S is a gain-of-function mutation that augments TLR2 mediated down-stream signaling.
TLR2 stimulation in BM CD34+ cells activates interleukin-8 and JMJD3
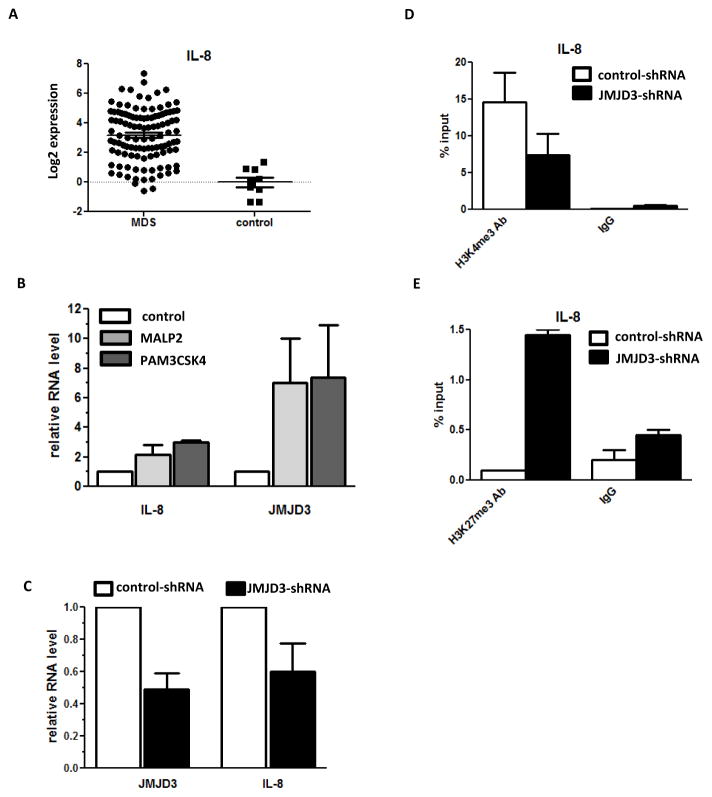
One major group of genes that are known to be regulated by TLR2 signals are inflammatory cytokines. 3 The levels of several of these cytokines are also known to be elevated in MDS bone marrows.12,13 We therefore investigated whether some of these cytokines were actively expressed in BM CD34+ cells and whether deregulation of TLR2 in MDS BM CD34+ cells could affect their expression. We first assessed basal expression of several these cytokines in normal BM CD34+ cells. Results indicated that basal RNA level of interleukin 8 (IL-8) was significantly higher than other inflammatory cytokines analyzed, including IL-1B, IL-4, IL-6, and TNF-α (Supplemental Figure 2A). Subsequently, we compared IL-8 RNA expression in MDS and control BM CD34+ cells. RT-PCR analysis of 109 MDS samples indicated that IL-8 expression was significantly elevated in MDS samples compared to normal CD34+ cells (Figure 3A and Supplemental Figure 2B). To evaluate whether TLR2 activation could contribute to the activation of IL-8 in BM CD34+ cells, we treated cultured primary BM CD34+ cells with MALP2 and PAM3CSK3. Both these TLR2 agonists induced higher IL-8 levels of RNA expression (Figure 3B).
Figure 3. Activation of IL-8 and JMJD3 gene expression by TLR2 signaling in primary BM CD34+ cells.
(a) Logarithmic representation of Q-RTPCR result of IL-8 gene in MDS and control CD34+ cells. (b) Q-RTPCR analysis of IL-8 and JMJD3 RNA expression in cultured BM CD34+ cells after treatment with TLR2 agonist MALP2 and PAM3CSK4. (c) Q-RTPCR analysis of IL-8 RNA expression in OCI-AML3 cells after JMJD3 knock-down. (d) H3K4me3 CHIP-PCR analysis of IL-8 promoter in the OCI-AML3 cells after JMJD3 knock-down. (e) H3K27me3 CHIP-PCR analysis of IL-8 promoter in OCI- AML3 cells after JMJD3 knock-down.
We have recently identified that the histone demethylase JMJD3 is overxpressed in BM CD34+ cells of MDS, which contributes to the abnormal epigenetic activation of multiple genes involved in innate immunity activation. 2 JMJD3 has been previously demonstrated to be regulated by TLRs in murine macrophages. 3 To examine if TLR2 activation can also activate JMJD3 in BM CD34+ cells, we assessed the effects of MALP2 and PAM3CSK3 on the expression of JMJD3. Results indicated that in cultured BM CD34+ cells, and in parallel to the increase in IL-8 expression, JMJD3 RNA levels also significantly increased after treatment with both TLR2 agonists (Figure 3B). We previously reported that in OCI-AML3 cells, knock-down of JMJD3 negatively affects the expression of multiple innate immunity genes. 2 We therefore examined if IL-8 RNA expression was also altered in OCI-AML3 cell line that constitutively expresses an shRNA against JMJD3. In parallel to JMJD3 knock-down, we observed a decrease of IL-8 RNA (Figure 3C). We next evaluated potential impact of JMJD3 knock-down on histone methylation by measuring the levels of H3K4me3 and H3K27me3 in the promoter of IL-8. CHIP-PCR revealed that there was a decrease in the level of H3K4me3 and an increase of H3K27me3 in IL-8 promoter (Figure 3D and 3E). These results indicated that TLR2 could activate the expression of both IL-8 and JMJD3. Furthermore, histone methylation of IL-8 promoter could be regulated by JMJD3. Consistent with the results from cultured cells, we also observed in primary MDS BM CD34+ cells (N=42) that RNA levels of IL-8 and JMJD3 were both positively correlated with TLR2 expression (R=0.88 and R=0.61 respectively, p<0.0001), and furthermore IL-8 level was also positively correlated with JMJD3 expression (R=0.66, p<0.0001).
TLR2 activation negatively regulates erythroid differentiation in cultured bone marrow CD34+ cells
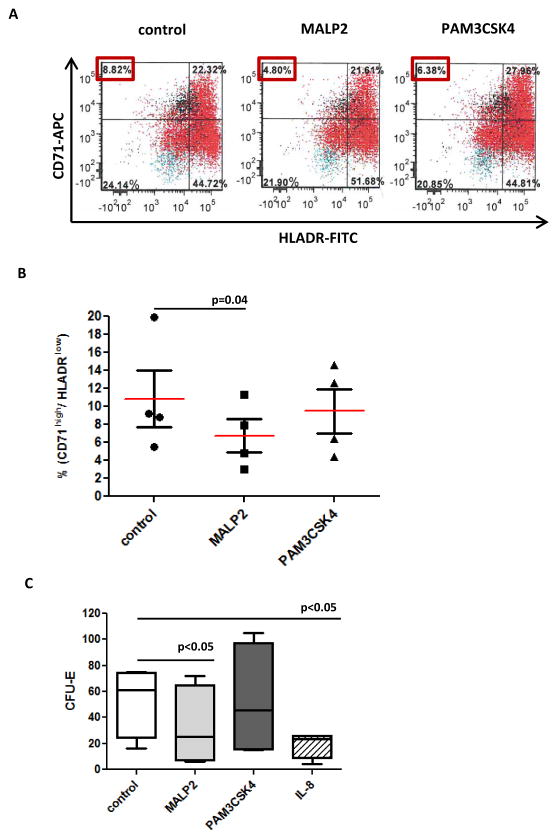
Stimulation of TLR2 has been shown to alter hematopoietic differentiation in cultured mouse HSPCs. 4 To explore the impact of TLR2 activation in human BM CD34+ cells, we treated BM CD34+ cells isolated from healthy individuals (N=4) with TLR2 agonists (MALP2 and PAM3CSK4) and analyzed their effects on hematopoietic differentiation: 48 hours after treatment, flow cytometry assays indicated that MALP2 caused a significant decrease of a erythroid precursor cell population, which was defined by strong CD71 expression and absence of HLA-DR (Figure 4A and 4B). PAM3CSK4 had similar but less significant effect (Figure 4A and 4B). The negative influence of TLR2 agonists on erythroid lineage was further confirmed by colony formation assays in methylcellulose medium (methocult). After 2 weeks in methocult culture, MALP2 treatment led to a 55% reduction in erythroid forming units (CFU-E) (Figure 4C). Consistent with the results of flow cytometry, PAM3CSK4 had a similar but less significant effect on CFU-E formation (Figure 4C). We also assessed the impact of IL-8 treatment in colony formation of normal BM CD34+ cells and observed similar negative effect on CFU-E formation as TLR2 agonist treatments (Figure 4C).
Figure 4. Effect of TLR2 activation in in vitro cultured primary normal bone marrow CD34+ cells.
(a) Flow cytometry analysis of normal CD34+ cells after being treated with MALP2 or PAM3CSK4 for 48 hours. Compared to control, treatment resulted in a decreased percentage of early erythroid progenitor cells marked with CD71+ and HLADR-. (b) Summary of flow cytometry analysis of CD71+ and HLADR- cells in primary BM CD34+ cells of four different donors. (c) Methocult medium supported colony formation assays revealed the decreased formation of erythroid colonies (CFU-E) from primary BM CD34+ cells treated by TLR2 agonist MALP2, PAM3CSK4 or IL-8.
Inhibition of TLR2 positively regulates CFU-E formation in MDS BM CD34+ cells
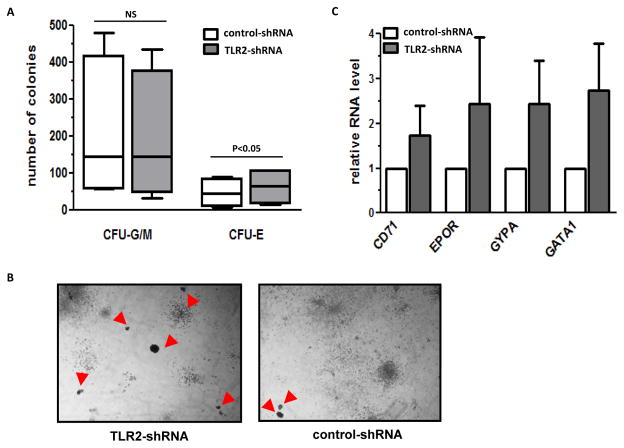
To evaluate the effect of TLR2 inhibition on haematological colony formation in cultured MDS BM CD34+ cells, we transduced primary BM CD34+ cells isolated from patients with newly diagnosed lower-risk MDS (N=4) and higher-risk MDS (N=3) with a recombinant retroviral mediated shRNA against TLR2. Patient characteristics are described in Supplemental Table 3. TLR2 shRNA resulted in reduced levels of TLR2 RNA expression in primary MDS BM CD34+ cells examined (supplemental Figure 3A). After 2 weeks of culture in methocult medium, all 4 lower-risk samples had an increased number of erythroid colonies (CFU-E) after TLR2 shRNA transduction (Figure 5A). On average, sixty-four CFU-E were formed per 104 MDS BM CD34+ cells plated in response to the transduction of TLR2-shRNA, which was a 35% increase compared to the number of CFU-E formed after control-shRNA transduction (Figure 5A). No significant effect on the formation of myeloid colonies (CFU-G/M) was observed after TLR2 inhibition (Figure 5A). Representative images of the methocult colonies formed from one low-risk sample are shown in Figure 5B. In contrast to lower-risk MDS, we did not observe a positive effect on the formation of erythroid or myeloid colonies in any of the three BM CD34+ cells isolated from patients with higher-risk MDS (supplemental Figure 3B). To further verify the effect of TLR2 inhibition in lower-risk samples, we measured transcripts of several genes known to be positively associated with erythroid differentiation, including Glycophorin-A (GYPA), CD71, EPOR and GATA1. In the cells collected from methocult colonies, expression of all four genes was increased after TLR2 inhibition (Figure 5C). The effect on gene expression further confirmed the positive impact of TLR2 inhibition on the differentiation of erythroid lineage in MDS BM CD34+ cells of lower-risk type of MDS.
Figure 5. Effect of TLR2 shRNA transduction in cultured MDS bone marrow CD34+ cells.
(a) Numbers of myeloid colonies (CFU-G/M) and erythroid colonies (CFU-E) formed in methocult culture two weeks after transduction of TLR2-shRNA and control shRNA in BM CD34+ cells isolated from patients with lower-risk MDS (low-risk and intermediate-1). (b) Representative microphotographs of colonies formed in methocult plates after transduction of TLR2-shRNA (Left panel) and control shRNA (Right panel). Red arrows point to CFU-E. (c) Q-RTPCR analysis of the RNA levels of CD71, EPOR, GYPA and GATA1 in cells collected from total colonies after shRNA transduction and methocult assays.
Clinical implications of TLR in MDS
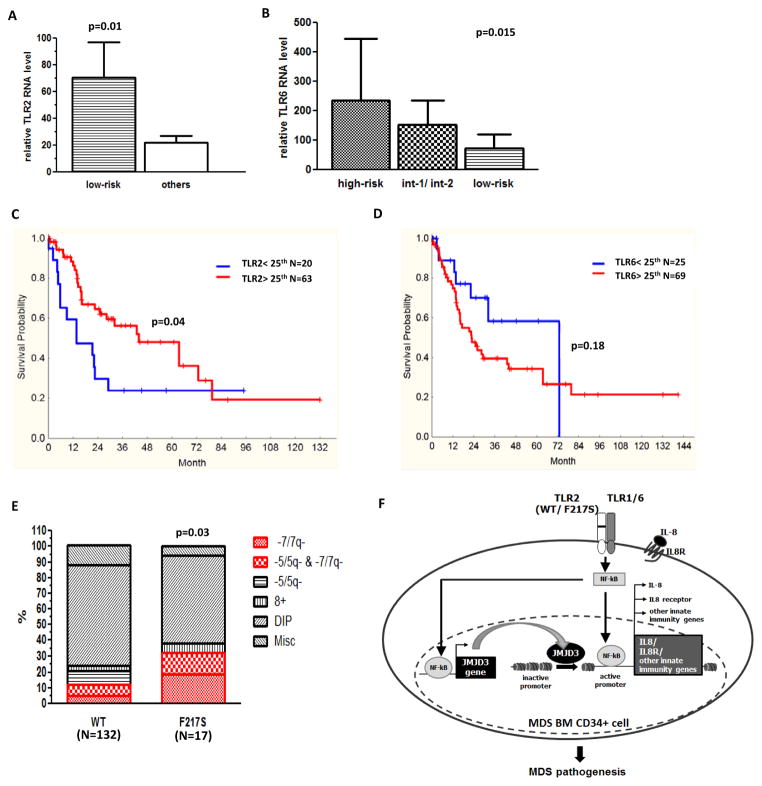
To further investigate implications of TLR1, 2 and 6 deregulation in MDS, we analyzed the associations of RNA expression in MDS BM CD34+ cells (N=103) with relevant clinical characteristics. Increased levels of TLR2 expression were associated with low-risk MDS by IPSS (p=0.01) (Figure 6A), diploid cytogenetic (p=0.04), and a diagnosis of chronic myelomonocytic leukemia (CMML) (p=0.04). In contrast, expression of TLR6 gradually increased with IPSS risk: high-risk patients had highest TLR6 expression, intermediate-1 and -2 patients had intermediate expression, and low-risk group had lowest TLR6 expression (p=0.015) (Figure 6B). Patients that had lowest TLR6 expression were associated with diploid cytogenetic (p=0.02) and a diagnosis of CMML (p=0.0004). Furthermore, patients with higher TLR6 expression (above median) had increased percentage of bone marrow blasts (7.8% v.s. 3.2% p<0.0001). In terms of overall survival (OS), we observed that patients with higher TLR2 expression (above 25% median) had significant longer survival than patients with lower TLR2 expression (43.9 v.s. 13.7 months, p=0.03) (Figure 6C). TLR6 expression had a tendency to be negatively correlated with OS (22.7 v.s. 72.8 months, p=0.18) (Figure 6D). Finally, patients with TLR2-F217S had a significant higher frequency of chromosome 7 deletion (p=0.03) (Figure 6E).
Figure 6. Clinical analysis and proposed model of TLR2 mediated innate immunity signaling in MDS.
(a) Higher TLR2 expression level in low-risk patients compared to others. (b) Distinct TLR6 expression levels in high-risk, intermediate-1 and 2, and low-risk patients. (c–d) Correlation of TLR2 and 6 with overall survival of patients. (e) Patients with TLR2-F217S are with higher frequency of 7-/7q-. (f) Proposed model of a potential TLR2-JMJD3-IL8 signaling axis is abnormally activated in BM CD34+ cells of MDS. This abnormal activation includes overexpression of TLR1, 2 and 6, as well as genetic alteration (F217S) of TLR2. This signal axis leads to consequent activation of JMJD3 and IL-8. JMJD3 can also positively regulate both genes of IL8 and its receptor in MDS BM CD34+ cells. Impact of this innate immunity signaling contributes to the pathogenesis of MDS.
DISCUSSION
Recent advance indicates that abnormal activation of innate immunity signals occurs in MDS. For instance, deregulation of TRAF-6, a key innate immunity signal mediator, together with the loss of miR-146a, is involved in the development of del5q MDS.14,15 We also recently reported that the histone demethylase JMJD3 and multiple innate immunity genes regulated by JMJD3 are overexpressed in BM CD34+ cells of MDS. 2 Of importance, expression of JMJD3 has been previously shown to be controlled by TLR mediated NF-kB activation in murine macrophage cells. 4 Based on this, we hypothesized that TLRs could be abnormally upregulated in MDS.
First, based on the analysis of RNA expression in a large cohort of patient samples, we document significant overexpression of TLR1, 2 and 6 in MDS BM CD34+ cells. Although TLRs are known to be present in differentiated hematopoietic cells, such as macrophages and dendritic cells, emerging evidence has recently indicated that functional TLRs are also actively expressed in primitive HSPCs, including hematopoietic stem cells and their early progenies such as multipotent progenitors (MPPs), common lymphoid progenitors (CLPs) and common myeloid progenitors (CMPs). 16, 17,18 It is known that both TLR1 and 6 bind to TLR2, 7, 8 and together they form TLR2 centered functional pattern-recognition receptor complexes that recognize conserved molecular patterns. These conserved molecular patterns are derived from Gram positive bacteria and mycobacteria, or from endogenous molecules released during tissue damage or cell death. 3, 19, 20 Therefore, the identification of overexpression of TLR1, 2 and 6 in MDS BM CD34+ cells is of potential biological significance. For instance, an autosomal syndrome with monocytopenia and increased susceptibility to mycobacteriosis has been reported in MDS.21 TLR2 has been demonstrated to be overexpressed in other types of neoplasms and has been shown to have oncogenic function independent of chronic inflammation in a gastric cancer model. 22 Other studies using smaller patient cohorts have previously reported overexpression of other TLRs such as TLR4 in MDS BM CD34+ cells.23
We have also identified a mutation of TLR2-F217S in 11% of patients. We demonstrate that TLR2-F217S induces more robust activation of NF-kB, p38MAPK, and IRAK1 modifications upon stimulation with TLR2 agonists in transfected 293T cells (Figure 2 and supplemental Fig XX). These results are in support of the central hypothesis that TLR2 signaling is abnormally activated in MDS. The question is whether TLR2-F217S is a somatic mutation in MDS. First, none of the 47 normal human control DNA samples had TLR2-F217S. This result is consistent with records in an annotated SNP database (1000 Genome project http://www.ncbi.nlm.nih.gov/projects/snp), which indicates that the incidence of TLR2-F217S in population is 0.3%. TLR2-F217S was found in 2 of the 19 CD3+ T cell specimens studied. One explanation is that T-cells emerged from the original MDS clone. Defective T-cells such as NKT cells have been previously reported in MDS. 24, 25 To further clarify this, we will need to analyse non-hematological controls such as germ line cells in large cohort of patients. A potential model is that TLR2-F217S can be a somatic mutation in patients with MDS but also a polymorphism that could be associated with increased risk for development of MDS. Large scale population analysis in both patients and healthy individuals, as well as detailed biological characterization in hematopoietic cells, will need to be performed to better define the implications of TLR2-F217S in the pathogenesis of MDS.
In this study, we also observed that stimulation of TLR2 in human BM CD34+ cells leads to activation of multiple genes known to be overexpressed in the same cell compartment of MDS. Emerging evidence has recently suggested a direct regulation of hematopoietic stem and progenitor cells (HSPC) by innate immunity signaling.26 In mouse HSPCs, acute TLR stimulation has been shown to alter myeloid/lymphoid ratios whereas chronic stimulation results in stem cell exhaustion and bone marrow failure.16, 17 Hyperactivation of TLR signaling has also been documented in bone marrow failure syndromes such as Fanconi anemia. 27, 28 In this report, we demonstrate that TLR2 activation in primary human BM CD34+ cells activates expression of histone demethylase JMJD3 and the inflammatory cytokine IL-8. We also demonstrate that JMJD3 can positively regulate expression of IL-8. In a recent study, we have previously shown that JMJD3 activates expression of the gene encoding IL-8 receptor (IL8RB), which is overexpressed in MDS BM CD34+ cells. 2 Together, these results suggest that in BM CD34+ cells of MDS, there is a potential TLR2-JMJD3-IL8 signaling axis. One molecular consequence of the deregulation of this signaling is overexpression IL-8. Overexpressed IL-8 then influences the fate of CD34+ HSPCs through its receptor (IL8RB), which is overexpressed in the same cell, forming a potential autocrine of IL-8 to contribute to the pathogenesis of MDS (Figure 6F).
Results of this study also have clinical implications. First, knock-down of TLR2 in MDS CD34+ cells resulted in increased erythroid differentiation. Although the precise biochemical mechanisms by which TLR2 inhibition promotes erythroid differentiation is still unclear, these observations have obvious therapeutic implications, suggesting that targeting TLR2 could improve hematopoiesis in patients with MDS. Second, RNA expression levels of TLR2 and 6, as well as the presence of genetic alteration of TLR2-F217S, were associated with several important clinical characteristics. Of interest, there is a potential discrepancy between the effect of TLR2 and TLR6 expression. This discrepancy potentially reflects the pathological and molecular heterogeneity of MDS. Mechanistically, this discrepancy suggests that TLR6 can potentially contribute to the pathogenesis of MDS through mechanisms independent of its interaction with TLR2. Indeed, recent studies have suggested that TLR6 can interact with innate immunity receptors other than TLR2 to sense microbial invading and stimulate host defensive response.29 The results of clinical analysis should be considered exploratory and need to be validated in a larger cohort of patients.
In summary, this study identifies that a TLR2 centered innate immunity pathway is deregulated in MDS, including both gene overexpression and genetic alteration. These results, together with our recent identification of JMJD3 and other genes that are involved in innate immunity regulation, further support a role of innate immunity signals in the bone marrow stem/progenitor cells of MDS. Further characterization of TLR2 associated innate immunity signals, particularly the potential TLR2-JMJD3-IL8 axis, may have prognostic and therapeutic benefits in MDS.
Supplementary Material
Acknowledgments
This work was supported by grant RP100202 from the Cancer Prevention & Research Institute of Texas (CPRIT), the DOD/Congressionally Directed Medical Research Programs, the MD Anderson Cancer Center Leukemia SPORE grant CA100632, the Evans Foundation, and the MD Anderson Cancer Center CCSG CA016672. IG-G was funded by the Regional Ministry of Education of Castilla-la Mancha, Spain, supported by the European Social Fund (ESF). We are thankful for Zhihong Fang, Ying Hu, and Michael Fernandez for technical help.
Footnotes
CONFLICT OF INTEREST
None of the authors have any conflict of interest with the data presented here.
Supplementary information is available at Leukemia’s website.
References
- 1.Tefferi A, Vardiman JW. Myelodysplastic syndromes. The New England journal of medicine. 2009;361(19):1872–85. doi: 10.1056/NEJMra0902908. [DOI] [PubMed] [Google Scholar]
- 2.Wei Y, Chen R, Dimicoli S, Bueso-Ramos C, Neuberg D, Pierce S, et al. Global H3K4me3 genome mapping reveals alterations of innate immunity signaling and overexpression of JMJD3 in human myelodysplastic syndrome CD34+ cells. Leukemia: official journal of the Leukemia Society of America Leukemia Research Fund, U.K. 2013 doi: 10.1038/leu.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 4.De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130(6):1083–94. doi: 10.1016/j.cell.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–60. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang JY, Nan X, Jin MS, Youn SJ, Ryu YH, Mah S, et al. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity. 2009;31(6):873–84. doi: 10.1016/j.immuni.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, et al. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169(1):10–4. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- 9.Merx S, Neumaier M, Wagner H, Kirschning CJ, Ahmad-Nejad P. Characterization and investigation of single nucleotide polymorphisms and a novel TLR2 mutation in the human TLR2 gene. Hum Mol Genet. 2007;16(10):1225–32. doi: 10.1093/hmg/ddm070. [DOI] [PubMed] [Google Scholar]
- 10.Janssens S, Beyaert R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol Cell. 2003;11(2):293–302. doi: 10.1016/s1097-2765(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 11.Conze DB, Wu CJ, Thomas JA, Landstrom A, Ashwell JD. Lys63-linked polyubiquitination of IRAK-1 is required for interleukin-1 receptor- and toll-like receptor-mediated NF-kappaB activation. Mol Cell Biol. 2008;28(10):3538–47. doi: 10.1128/MCB.02098-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kornblau SM, McCue D, Singh N, Chen W, Estrov Z, Coombes KR. Recurrent expression signatures of cytokines and chemokines are present and are independently prognostic in acute myelogenous leukemia and myelodysplasia. Blood. 2010;116(20):4251–61. doi: 10.1182/blood-2010-01-262071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sioud M, Floisand Y. TLR agonists induce the differentiation of human bone marrow CD34+ progenitors into CD11c+ CD80/86+ DC capable of inducing a Th1-type response. European journal of immunology. 2007;37(10):2834–46. doi: 10.1002/eji.200737112. [DOI] [PubMed] [Google Scholar]
- 14.Starczynowski DT, Kuchenbauer F, Argiropoulos B, Sung S, Morin R, Muranyi A, et al. Identification of miR-145 and miR-146a as mediators of the 5q- syndrome phenotype. Nat Med. 2009 doi: 10.1038/nm.2054. [DOI] [PubMed] [Google Scholar]
- 15.Rhyasen GW, Starczynowski DT. Deregulation of microRNAs in myelodysplastic syndrome. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2012;26(1):13–22. doi: 10.1038/leu.2011.221. [DOI] [PubMed] [Google Scholar]
- 16.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24(6):801–12. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esplin BL, Shimazu T, Welner RS, Garrett KP, Nie L, Zhang Q, et al. Chronic exposure to a TLR ligand injures hematopoietic stem cells. J Immunol. 2011;186(9):5367–75. doi: 10.4049/jimmunol.1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Luca K, Frances-Duvert V, Asensio MJ, Ihsani R, Debien E, Taillardet M, et al. The TLR1/2 agonist PAM(3)CSK(4) instructs commitment of human hematopoietic stem cells to a myeloid cell fate. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2009;23(11):2063–74. doi: 10.1038/leu.2009.155. [DOI] [PubMed] [Google Scholar]
- 19.Kawai T, Akira S. TLR signaling. Cell death and differentiation. 2006;13(5):816–25. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 20.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annual review of immunology. 2011;29:139–62. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinh DC, Patel SY, Uzel G, Anderson VL, Freeman AF, Olivier KN, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010;115(8):1519–29. doi: 10.1182/blood-2009-03-208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tye H, Kennedy CL, Najdovska M, McLeod L, McCormack W, Hughes N, et al. STAT3-Driven Upregulation of TLR2 Promotes Gastric Tumorigenesis Independent of Tumor Inflammation. Cancer Cell. 2012;22(4):466–78. doi: 10.1016/j.ccr.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Maratheftis CI, Andreakos E, Moutsopoulos HM, Voulgarelis M. Toll-like receptor-4 is up-regulated in hematopoietic progenitor cells and contributes to increased apoptosis in myelodysplastic syndromes. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13(4):1154–60. doi: 10.1158/1078-0432.CCR-06-2108. [DOI] [PubMed] [Google Scholar]
- 24.Fujii S, Shimizu K, Klimek V, Geller MD, Nimer SD, Dhodapkar MV. Severe and selective deficiency of interferon-gamma-producing invariant natural killer T cells in patients with myelodysplastic syndromes. British journal of haematology. 2003;122(4):617–22. doi: 10.1046/j.1365-2141.2003.04465.x. [DOI] [PubMed] [Google Scholar]
- 25.Chan AC, Neeson P, Leeansyah E, Tainton K, Quach H, Prince HM, et al. Testing the NKT cell hypothesis in lenalidomide-treated myelodysplastic syndrome patients. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2010;24(3):592–600. doi: 10.1038/leu.2009.279. [DOI] [PubMed] [Google Scholar]
- 26.Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2011;32(2):57–65. doi: 10.1016/j.it.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anur P, Yates J, Garbati MR, Vanderwerf S, Keeble W, Rathbun K, et al. p38 MAPK inhibition suppresses the TLR-hypersensitive phenotype in FANCC- and FANCA-deficient mononuclear phagocytes. Blood. 2012;119(9):1992–2002. doi: 10.1182/blood-2011-06-354647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanderwerf SM, Svahn J, Olson S, Rathbun RK, Harrington C, Yates J, et al. TLR8-dependent TNF-(alpha) overexpression in Fanconi anemia group C cells. Blood. 2009;114(26):5290–8. doi: 10.1182/blood-2009-05-222414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Almeida LA, Macedo GC, Marinho FA, Gomes MT, Corsetti PP, Silva AM, et al. Toll-Like Receptor 6 Plays an Important Role in Host Innate Resistance to Brucella abortus Infection in Mice. Infection and immunity. 2013;81(5):1654–62. doi: 10.1128/IAI.01356-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.