Abstract
The role of phosphodiesterase (PDE) inhibitors in central nervous system has been investigated and shown to stimulate neuronal functions and increase neurogenesis in Alzheimer patients. The aim of this study is to investigate effect of PDE5 inhibitor zaprinast and PDE4 inhibitor rolipram on visual memory in novel object recognition (NOR) test, on olfactory memory in social transmission of food preference (STFP) test, and also on locomotion and anxiety in open field test in naive mice. Male Balb-c mice were treated intraperitoneally (i.p.) with zaprinast (3 and 10 mg/kg), rolipram (0.05 and 0.1 mg/kg), or physiological saline. Zaprinast (10 mg/kg) significantly increased cued/non-cued food eaten compared to control group, while rolipram had a partial effect on retention trial of STFP test. Zaprinast (10 mg/kg) and rolipram (0.05 and 0.1 mg/kg) significantly increased ratio index (RI) compared to control group in retention trial of NOR test. There was no significant effect of zaprinast and rolipram on total distance moved, speed, and center zone duration in open field test. Results of this study revealed that both zaprinast and rolipram enhanced visual memory in NOR test, however zaprinast exerted a significant memory-enhancing effect compared to rolipram in STFP test in mice.
Keywords: zaprinast, rolipram, memory, open field test, mice
Introduction
Accumulating evidence indicates that the inhibition of phosphodiesterase (PDE) activity may consist of a particularly interesting mechanism for memory enhancement.1,2 This is related to the substrates of PDEs: cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP). Both cyclic nucleotides play an important role in intracellular signaling3,4 and in processes of neuroplasticity, such as long-term potentiation (LTP). Inhibitors of PDEs are expected to increase cAMP and/or cGMP levels in neurons and hence might improve memory.
The underlying mechanism for PDE4 inhibitors for their cognitive-enhancing effects may involve modulation of activity within the cAMP/protein kinase A (PKA)/cAMP response element-binding (CREB) protein pathway. The prototypical PDE4 inhibitor most widely used in cognition studies is rolipram. It possesses significant brain penetration and has a half-life of one to three hours.5 In vitro studies showed that cAMP levels increased in hippocampal slices treated with rolipram.6 Rolipram attenuated deficits in spatial and non-spatial short-term memory and working memory in several behavioral tasks.7 Rolipram reversed the disruption in reference memory and/or working memory by the glutamate antagonist MK-801 in a radial-arm maze and reversed the effects of MK-801 in a passive avoidance task.8 Rolipram or HT0712 treatment of mice 20 minutes before training in the object recognition task improved retention performance 24 hours later.9 Similarly, the retention performance 24 hours after contextual fear learning was improved in mice treated with rolipram 30 minutes before training.10 PDE4 inhibition can reverse memory performance in different mouse disease models,9,11 providing strong support that this treatment approach may be effective in Alzheimer’s disease.
In 1997, PDE5 inhibition was first described to improve memory processes.12 PDE5 inhibitors (PDE5-I) such as sildenafil and vardenafil have not only been shown to be effective in the treatment of erectile dysfunction but are also candidate drugs for cognition enhancement. For instance, the specific PDE5-Is sildenafil and vardenafil have been shown to improve object recognition memory when injected immediately following the first trial.13 PDE5-Is are assumed to improve early processes of memory consolidation via either a presynaptic or postsynaptic mechanism. The presynaptic mechanism acts through the nitric oxide (NO)–cGMP signaling pathway and the postsynaptic mechanism through the cGMP/protein kinase G (PKG)/CREB protein signaling pathway.1
Zaprinast was used to inhibit PDE5 and improved the Long-tem memory (LTM) performance of rats in the object recognition task when given immediately after training at a dose of 10 mg/kg (intraperitoneally, i.p.).12 Zaprinast also inhibits PDE1, 9, 10, and 11. Previous studies also showed that zaprinast reversed the object memory deficits induced by the NOS inhibitor 7-nitroindazole in rats in the object recognition task.12 However, zaprinast was unable to reverse memory deficits in aged rats in this task.14 Animal studies indicate that PDE5 inhibitors have the potential to improve early consolidation processes of long-term memory, though this may exclude spatial information. This memory improvement is speculatively mediated by elevations in central cGMP levels.
As a result of our literature search, we found no study investigating the effects of zaprinast and rolipram on memory in the novel object recognition (NOR) test and social transmission of food preference (STFP) test. The aim of this study is to investigate the effect of PDE5 inhibitor zaprinast and PDE4 inhibitor rolipram on hippocampal-dependent visual memory in the NOR, on hippocampal-dependent olfactory memory in the STFP, and also on locomotion and anxiety in the open-field test in naive mice.
Methods
Animals
A total of 90 male inbred BALB/c ByJ mice (MAM TUBİTAK, Gebze, Kocaeli, Turkey) aged seven weeks upon arrival to the laboratory were used in this study. Animals (four to five per cage) were kept in the laboratory at 21 ± 1.5°C with 60% relative humidity under a 12-h light/dark cycle (light on at 8.00 p.m.) for two weeks before experimentation. All animals received food and water ad libitum. All procedures described in this paper were conducted in accordance with the European Community Council directive for the ethical treatment of animals (86/609/EEC) and with the ethical approval of the Kocaeli University Ethics Committee (number: AEK 9/4-2010, Kocaeli, Turkey).
STFP test
Hippocampus-dependent non-spatial olfactory memory15 was studied using the STFP task. In this test, mice are required to remember the scent of food smelled on the muzzle of a demonstrator mouse 24 hours earlier. When offered a choice of the flavored food eaten by the demonstrator mouse (cued food), or another novel-flavored food, mice with normal olfactory memory will eat a greater proportion of the familiar cued food than of the novel food.
The experiment was conducted in three phases: (i) habituation to flavored food, (ii) interaction between “demonstrator” and “observer” mice, and (iii) test of the food preference in the “observer” mice.15 Mice were housed at a ratio of three to four observer mice to one demonstrator. In the habituation phase, a demonstrator mouse was chosen from each cage. Demonstrators were housed singly in a cage separate from the colony for three hours with free access to water but not food. At the end of three hours, each demonstrator was allowed to eat powdered ground chow scented with either cinnamon (1%, w/w) or cocoa (2%, w/w).15 Half of the demonstrators received cocoa-flavored food, and the other half received cinnamon-flavored food. The demonstrators were allowed to eat the flavored food for two hours. The pellets were weighed before and after presentation to the demonstrators. The criterion for inclusion in the experiment was consumption of 0.2 g. All demonstrator mice tested met this criterion. Each demonstrator was then placed back with its observer cagemates for 30 minutes.15 Observers interacted with the demonstrator including sniffing the scent around the muzzle and on the breath of the demonstrator mouse.15 After the interaction period, the demonstrator mouse was removed from the interaction cage and returned to its individual cage.
In the final phase of the experiment, the food preference of the observer mice was tested 24 hours after the end of the interaction with the demonstrator. Five hours before the preference test, observer mice were caged individually with free access to water and food, in the same room where the interaction was performed. Three hours before the preference test, the food was removed from the observer’s cage. The two-hour preference test consisted of presenting each observer with a pair of weighed food pellets in the individual cage. One pellet contained the flavor of food eaten by the demonstrator (cued); the other contained the novel flavor of the pair (novel). Thus, half of the observers were tested with the cinnamon-flavored cued food eaten by their demonstrator versus the novel cocoa-flavored food and the other half were tested with the cocoa-flavored cued food eaten by their demonstrator versus the novel cinnamon-flavored food.15 After two hours, both food pellets were removed and weighed to quantify the food preference of the observer mice.
The ratio of the weight of the cued food eaten and the total weight of food eaten was used as a measure of food preference.15
NOR test
The protocol according to Ennaceur and Delacour16 was adjusted. The apparatus consisted of a circular open field 40 cm in diameter and 30 cm in height made of PVC with a black and white-striped cardboard pattern (30 × 20 cm) nailed on one of the walls. The floor was divided into six peripheral sections and one central section of the same dimension. The apparatus was placed in a sound-isolated room.16 A light bulb provided a constant illumination of about 100 lux above the central section.
The NOR task procedure consisted of three trials: habituation, training, and retention trials. Each mouse was individually habituated to the apparatus, with five minutes of exploration in the absence of objects (habituation trial).16 A mouse was always placed at the center section of the apparatus. In all, 30 minutes after the habituation trial, the mouse was placed in the apparatus for the first trial (T1) and two identical objects (moon or butterfly) were placed in a symmetrical position 10 cm above the side wall.16 A mouse could not displace the objects (they were sticked to the wall of the open field by patafix). The order of objects used per subject per trial was determined randomly. All combinations and locations of objects were used in a balanced manner to reduce potential biases because of preferences for particular locations or objects. A mouse was then placed in the central section of the box and the total time spent in exploring the two objects was recorded for five minutes by the experimenter. To avoid the presence of olfactory trails, the apparatus after each trial was thoroughly cleaned. Exploration of an object was defined as directing the nose to the object at a distance of maximum 1 cm and/or touching it with the nose. After the first exploration period, the mouse was put back in its home cage. Subsequently, after a predetermined retention interval (intertrial interval of one hour), the mouse was again placed in the apparatus for the second trial (T2, choice phase), but now with two dissimilar objects, a familiar one (the reference) and a new one.16 The object not used in the acquisition trial was used as the novel object in the recognition trial. The animals were then allowed to explore freely for five minutes, and the time spent exploring each object was recorded. If recognition memory was intact, the mice were expected to spend more time exploring the novel object.16
A ratio index (RI) was calculated as the time spent exploring the new object (N) divided by the total time exploring the objects (N + R) multiplied by 100. Higher RI is considered to reflect greater memory retention.16
Open field test
Treatment effects on animal locomotor activity were measured using the open field test. This test is also used to examine anxiety-like behaviors and is used to evaluate anxiolytic treatment.17 This experiment was performed as previously described.18 Briefly, the testing apparatus consisted of a wooden box (33 cm × 33 cm × 30 cm) with an indirect red light. An animal was placed in the center of test box, and total distance moved throughout the area, speed of animals, and time spent in center zone were recorded using EthoVision XT (Noldus) for five minutes.
Drug administration
Zaprinast and rolipram were purchased from Sigma Chemical Company (Sigma, St. Louis, MO) and were dissolved in saline supplemented with small amounts of DMSO. All drugs were freshly prepared and administered in a volume of 0.1 mL/10 g body weight. The control groups received the same volume of vehicle. Zaprinast (3 and 10 mg/kg) and rolipram (0.05 and 0.1 mg/kg) or vehicle were administered i.p. 60 and 30 minutes, respectively, before the retention sessions of NOR and STFP tests and before the open field test. Six animals were in each group. The effective dose of each drug was selected according to previous behavioral and neurochemical studies.19
Statistics
A one-way analysis of variance (ANOVA) post hoc Tukey’s test was used to analyze the RI of the animals in the NOR test: total distance moved, speed, and time spent in the center zone in the open field test. The Kruskal–Wallis post hoc Dunn’s test was used to analyze the cued food/total food% eaten and total food consumption in the STFP test. The data are expressed as mean ± SEM values. Statistical significance was set at P < 0.05.
Results
Effects of zaprinast and rolipram on olfactory memory in the STFP test
When zaprinast (3 and 10 mg/kg) and rolipram (0.05 and 0.1 mg/kg) were administered before the retention session of STFP test, there was a significant difference among the groups when the percentage of cued food per total food eaten was evaluated (H = 10.38, P = 0.03; Fig. 1a). Zaprinast (10 mg/kg) significantly increased percentage of cued food per total food eaten compared to the control group (P < 0.05; Fig. 1a), whereas rolipram had no significant effect. When the total food consumption in the STFP test was evaluated, there was no significant effect of drugs compared to control group (H = 3.28, P = 0.51; Fig. 1b).
Figure 1.
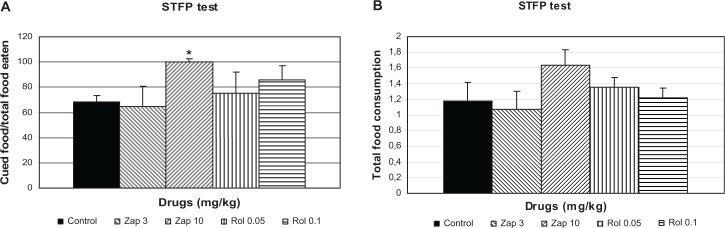
Effect of zaprinast (3 and 10 mg/kg) and rolipram (0.05 and 0.1 mg/kg) (n = 6) on (A) percentage of cued per non-cued food eaten and (B) total food consumption (in which zaprinast and rolipram were administered 60 and 30 minutes, respectively, before the retention trial) in the STFP test in mice. The data are expressed as the mean ± SEM values of animals.
Note: *P < 0.05 compared to control group.
Effects of zaprinast and rolipram on visual memory in the NOR test
When zaprinast (3 and 10 mg/kg) and rolipram (0.05 and 0.01 mg/kg) were administered before the retention session of NOR test, there was a significant difference among the groups when the RI was evaluated [F(4, 29) = 5.94, P = 0.0017; Fig. 2]. Zaprinast (10 mg/kg) (P < 0.001) and rolipram (0.05 and 0.1 mg/kg) (P < 0.05) significantly increased the RI compared to the control group (Fig. 2).
Figure 2.
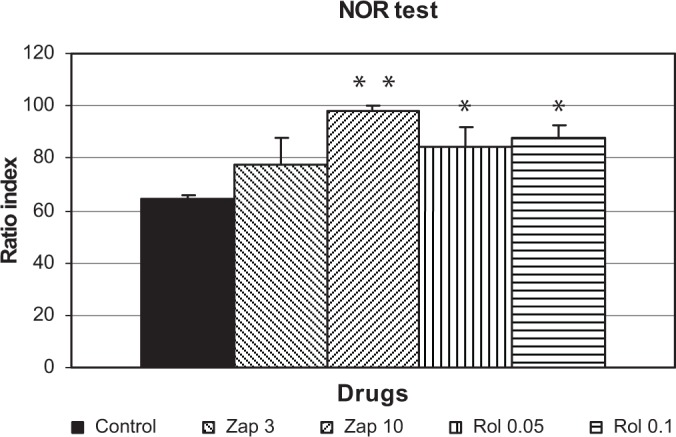
Effect of zaprinast (3 and 10 mg/kg) and rolipram (0.05 and 0.1 mg/kg) on RI (n = 6) (in which zaprinast and rolipram were administered 60 and 30 minutes, respectively, before the retention trial) in the NOR test in mice. The data are expressed as the mean ± SEM values of animals.
Notes: *P < 0.05, **P < 0.001 compared to control group.
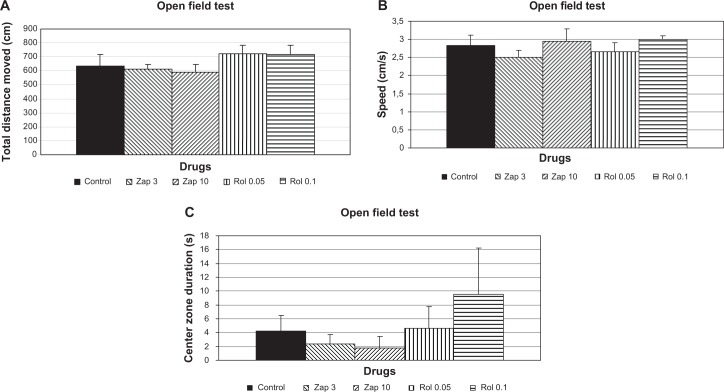
Effects of zaprinast and rolipram on locomotion and anxiety in the open field test
There was no significant difference between zaprinast (3 and 10 mg/kg), rolipram (0.05 and 0.01 mg/kg), and control groups about the total distance moved [F(4, 29) = 0.90; P > 0.05], speed [F(4, 29) = 0.70; P > 0.05], and center zone duration [F(4, 29) = 0.73; P > 0.05] in the open field test evaluation (Fig. 3).
Figure 3.
Effect of zaprinast (3 and 10 mg/kg) and rolipram (0.05 and 0.1 mg/kg) (n = 6) administration on locomotion and anxiety in the open field test. Drugs were injected 60 and 30 minutes, respectively, before testing. The data are expressed as the mean ± SEM values. (A) Total distance moved, (B) speed, and (C) center zone duration in the open field test.
Discussion
This study revealed that both PDE5 inhibitor zaprinast (10 mg/kg) and PDE4 inhibitor rolipram (0.05 and 0.1 mg/kg) increased the RI in the NOR test. Concerning the STFP test, zaprinast (10 mg/kg) enhanced percentage of cued/non-cued food eaten, while rolipram had a partial effect, although it did not reach a significant level. Both zaprinast and rolipram had no significant effect on total food consumption in the STFP test. Both drugs had no significant effect on total distance moved, speed and center zone duration in the open field test.
PDE enzymes may be involved in the etiology of a number of CNS diseases, including Alzheimer’s disease, schizophrenia, and affective disorders, and have recently been proposed as potential targets for therapeutic intervention.20,21 In addition, PDEs may be targeted for the cognitive enhancement, and inhibitors of PDEs have proven to be useful experimental tools in exploring mechanisms of learning and memory.1,22 Selective PDE inhibitors of at least five types and inhibitors of PDE2,23 PDE4,24 PDE5,25 and PDE926 have all been shown to enhance memory in different behavioral paradigms and in different species.19
These memory enhancements may be related to the subsequent increases in intracellular cGMP and/or cAMP levels after PDE inhibition, particularly, as both cGMP and cAMP are important intracellular second messenger molecules that have been observed in consolidation processes.27 Interestingly, selective PDE inhibitor treatments are in line with the sequence of molecular changes taking place in the hippocampus during memory consolidation, as recently described by Izquierdo et al.28 Possible underlying mechanisms of action for memory enhancement after PDE inhibition are closely related to electrophysiological theories of learning and memory. Thus, the cAMP/PKA/CREB pathway as well as the cGMP/PKG/CREB pathway are key candidates in providing the biochemical substrate of long-term memory effects. The activation of both pathways may lead to CREB phosphorylation and, consequently, de novo protein synthesis.
The involvement of cAMP- and cGMP-mediated signaling in learning and memory is well known, and both have been posited to be involved in hippocampal LTP formation.29 More recently, it has been suggested that hippocampal cGMP can enhance postsynaptic cAMP and PKA30 or regulate LTP via presynaptic cGMP and PKG.31
Ample evidence supports a role for the cAMP/PKA/CREB pathway in learning and memory processes.32 PDE4 inhibitors specifically inhibit cAMP, and the underlying mechanism for their cognitive-enhancing effects may involve modulation of activity within the cAMP/PKA/CREB pathway.33 The prototypical PDE4 inhibitor most widely used in cognition studies is rolipram. It possesses significant brain penetration and has a half-life of one to three hours.5 In vitro studies showed that cAMP levels increased in hippocampal slices treated with rolipram.6 Studies have shown that rolipram produces memory-enhancing effects in a number of models and has antidepressant-like activity in both preclinical34 and clinical models.35 In 1997, it was first described that PDE5 inhibition improves memory processes.12 Zaprinast was used to inhibit PDE5 and when given immediately after training at a dose of 10 mg/kg (i.p.), improved the LTM performance of rats in the object recognition task.12 However, zaprinast also inhibits PDE1, 9, 10, and 11.
The memory-improving effects of PDE5 inhibitors may also, or alternatively, be related to an increased blood flow and, consequently, increased glucose metabolism, as PDE5 inhibitors are known to result in vasodilatation, most likely via cGMP.36,37 A decrease in blood flow generally results in a decrease in blood pressure. It was observed that a dose of 10 mg/kg zaprinast administration (i.p.) slightly increased the mean arterial blood pressure in conscious rats from one to four hours, after which recovery occurred.12 This had been observed before, and the mechanism by which zaprinast elevates mean arterial blood pressure is not clear.36 Yet, a depressor response after systemic administration of zaprinast has been observed at doses above 10 mg/kg.36,37 As the doses of zaprinast we used in our study have no effect on mean arterial blood pressure,36,37 it is unlikely that effects on peripheral blood pressure after zaprinast treatment contributed to its memory improvement.
The NOR test for rodents was formulated by Ennaceur and Delacour16 to measure the spontaneous exploratory activity toward a novel object and a familiar object. This test does not involve rule learning or reinforcement and is thought to evaluate working and visual memory. This test has many useful applications to study the neurobiological mechanisms of learning and memory. In the NOR test, the failure to discriminate between familiar and novel objects can be related to either impaired memory of the object or the inability to use spatial information. Occasionally, the effect might be unrelated to an amnesic action, as mice display an inhibition of locomotion. However, such an explanation does not apply to zaprinast and rolipram at the doses used because they do not affect locomotion and anxiety in the open field test in our study. Therefore, the performance observed at the NOR test can be attributed to memory-enhancing effects in the NOR test.
Hall38 originally described the open field test for the study of rat emotion. The procedure consists of placing an animal in an unknown environment from which escape is prevented by the surrounding walls.39 The open field test is a very common procedure used in animal psychology.18 Rodents naturally prefer periphery of the apparatus to the middle area of the open field. Treatments that increase time spent in central area without impairment of locomotion are deemed anxiolytic like, whereas treatments that decrease these variables produce anxiogenic effects.
There are controversial results for the effects of NO on anxiety. In the following studies, NOS inhibitors were known to possess anxiolytic effects,40 whereas NO donors had anxiogenic effects.41 Because inhibition of PDE had an opposite action compared to NOS inhibitors, they increase the formation of NO and can be expected to exert anxiogenic effects. In our study, both zaprinast and rolipram did not change center zone duration conferring no significant effect on anxiety.
STFP is a hippocampal-dependent olfactory memory test.15 In Figure 1a, we found statistically significant difference for 10 mg/kg zaprinast, showing that zaprinast at this dose increased percentage of cued food per non-cued food eaten ie enhancement of olfactory memory. Figure 1b reflects total food consumption, and zaprinast at this dose failed to affect this parameter ie zaprinast had no nonspecific effects on olfactory memory; it affected only cued food consumption. In this study, in the hippocampal-dependent NOR test, both zaprinast and rolipram enhanced visual memory; only zaprinast, at the higher dose, increased olfactory memory in the STFP test, and rolipram had a partial effect. Different explanations of this discrepancy can be proposed. Olfactory learning has no spatial components, as in the NOR test. However, it could be that distinct brain processes underlie spatial visual and olfactory memory formation.
There are also findings claiming that NO does not affect the retrieval of olfactory memory in adult sheep42 and that NO release is involved in the acquisition phase in an olfactory recognition test but does not affect post-acquisition recall.43
In conclusion, the present study demonstrates that both the PDE5 inhibitor zaprinast and the PDE4 inhibitor rolipram enhanced visual memory in the NOR test, whereas only zaprinast significantly enhanced olfactory memory in the STFP test. Both zaprinast and rolipram had no significant effect on locomotion and anxiety in the open field test. Future studies using different PDE inhibitors with different cognition methods can be performed to support our findings.
Footnotes
Author Contributions
FA, OM, IKC, and MHT conceived and designed the experiments. OM, EB, GU, and PT analyzed the data. FA, OM, and GU wrote the first draft of the manuscript. MHT, GU, and FE contributed to the writing of the manuscript. FA, OM, IKC, EB, MHT, GU, PT, and FE agreed with manuscript results and conclusions. FA, EB, GU, PT, and FE jointly developed the structure and arguments for the paper. FA, IKC, and EB made critical revisions and approved the final version. All authors reviewed and approved the final manuscript.
ACADEMIC EDITOR: Anuj Chauhan, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
DISCLOSURES AND ETHICS
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants.
REFERENCES
- 1.Prickaerts J, Sik A, van Staveren WC, et al. Phosphodiesterase type 5 inhibition improves early memory consolidation of object information. Neurochem Int. 2004;45:915–928. doi: 10.1016/j.neuint.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Fedele E, Raiteri M. In vivo studies of the cerebral glutamate receptor/NO/cGMP pathway. Prog Neurobiol. 1999;58:89–120. doi: 10.1016/s0301-0082(98)00077-x. [DOI] [PubMed] [Google Scholar]
- 3.Son H, Lu YF, Zhuo M, Arancio O, Kandel ER, Hawkins RD. The specific role of cGMP in hippocampal LTP. Learn Mem. 1998;5:231–245. [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey CH, Bartsch D, Kandel ER. Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci U S A. 1996;93:13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krause W, Kuhne G. Pharmacokinetics of rolipram in the rhesus and cynomolgus monkeys, the rat and the rabbit. Studies on species differences. Xenobiotica. 1988;18:561–571. doi: 10.3109/00498258809041693. [DOI] [PubMed] [Google Scholar]
- 6.van Staveren WC, Markerink-van Ittersum M, Steinbusch HW, de Vente J. The effects of phosphodiesterase inhibition on cyclic GMP and cyclic AMP accumulation in the hippocampus of the rat. Brain Res. 2001;888:275–286. doi: 10.1016/s0006-8993(00)03081-x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang HT, O’Donnell JM. Effects of rolipram on scopolamine-induced impairment of working and reference memory in the radial-arm maze tests in rats. Psychopharmacology. 2000;150:311–6. doi: 10.1007/s002130000414. [DOI] [PubMed] [Google Scholar]
- 8.Zhang HT, Huang Y, Suvarna NU, et al. Effects of the novel PDE4 inhibitors MEM1018 and MEM1091 in models of memory and antidepressant sensitivity. Abstr Soc Neurosci. 2002;684:12. [Google Scholar]
- 9.Bourtchouladze R, Lidge R, Catapano R, et al. A mouse model of Rubinstein-Taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci U S A. 2003;100:10518–10522. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci U S A. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comery TA, Martone RL, Aschmies S, et al. Acute secretase inhibition improves cognition in the TG2576 mouse model of Alzheimer’s disease. Abstr Soc Neurosci. 2003;525:21. doi: 10.1523/JNEUROSCI.2693-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prickaerts J, Steinbusch HWM, Smits JFM, De Vente J. Possible role of nitric oxide-cyclic GMP pathway in object recognition memory: effects of 7-nitroindazole and zaprinast. Eur J Pharmacol. 1997;337:125–136. doi: 10.1016/s0014-2999(97)01301-0. [DOI] [PubMed] [Google Scholar]
- 13.Prickaerts J, van Staveren WC, Sik A, et al. Effects of two selective phosphodiesterase type 5 inhibitors, sildenafil and vardenafil, on object recognition memory and hippocampal cyclic GMP levels in the rat. Neuroscience. 2002;113:351–361. doi: 10.1016/s0306-4522(02)00199-9. [DOI] [PubMed] [Google Scholar]
- 14.Domek-Lopacinska K, Strosznajder JB. The effect of selective inhibition of cyclic GMP hydrolyzing phosphodiesterases 2 and 5 on learning and memory processes and nitric oxide synthase activity in brain during aging. Brain Res. 2008;1216:68–77. doi: 10.1016/j.brainres.2008.02.108. [DOI] [PubMed] [Google Scholar]
- 15.Bunsey M, Eichenbaum H. Selective damage to the hippocampal region blocks long-term retention of a natural and nonspatial stimulus-stimulus association. Hippocampus. 1995;5:546–556. doi: 10.1002/hipo.450050606. [DOI] [PubMed] [Google Scholar]
- 16.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1. Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 17.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 18.Belzung C. Measuring exploratory behavior. In: Crusio WE, Gerlai RT, editors. Handbook of Molecular Genetic Techniques for Brain and Behavior Research (Techniques in the Behavioral and Neural Sciences) Amsterdam: Elsevier; 1999. pp. 739–749. [Google Scholar]
- 19.Reneerkens OA, Rutten K, Steinbusch HW, Blokland A, Prickaerts J. Selective phosphodiesterase inhibitors: a promising target for cognition enhancement. Psychopharmacology (Berl) 2009;202:419–443. doi: 10.1007/s00213-008-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halene TB, Siegel SJ. PDE inhibitors in psychiatry—future options for dementia, depression and schizophrenia? Drug Discov Today. 2007;12:870–878. doi: 10.1016/j.drudis.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 21.Menniti FS, Chappie TA, Humphrey JM, Schmidt CJ. Phosphodiesterase 10A inhibitors: a novel approach to the treatment of the symptoms of schizophrenia. Curr Opin Investig Drugs. 2007;8:54–59. [PubMed] [Google Scholar]
- 22.Blokland A, Schreiber R, Prickaerts J. Improving memory: a role for phosphodiesterases. Curr Pharm Des. 2006;12:2511–2523. doi: 10.2174/138161206777698855. [DOI] [PubMed] [Google Scholar]
- 23.Rutten K, Prickaerts J, Hendrix M, van der Staay FJ, Sik A, Blokland A. Time-dependent involvement of cAMP and cGMP in consolidation of object memory: Studies using selective phosphodiesterase type 2, 4 and 5 inhibitors. Eur J Pharmacol. 2007;558:107–112. doi: 10.1016/j.ejphar.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 24.Rutten K, Prickaerts J, Blokland A. Rolipram reverses scopolamine-induced and time-dependent memory deficits in object recognition by different mechanisms of action. Neurobiol Learn Mem. 2006;85:132–138. doi: 10.1016/j.nlm.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Devan BD, Sierra-Mercado D, Jr, Jimenez M, et al. Phosphodiesterase inhibition by sildenafil citrate attenuates the learning impairment induced by blockade of cholinergic muscarinic receptors in rats. Pharmacol Biochem Behav. 2004;79:691–699. doi: 10.1016/j.pbb.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 26.van der Staay FJ, Rutten K, Bärfacker L, et al. The novel selective PDE9 inhibitor BAY 73-6691 improves learning and memory in rodents. Neuropharmacology. 2008;55:908–918. doi: 10.1016/j.neuropharm.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Izquierdo LA, Barros DM, Vianna MR, et al. Molecular pharmacological dissection of short- and long-term memory. Cell Mol Neurobiol. 2002;22:269–287. doi: 10.1023/A:1020715800956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izquierdo I, Bevilaqua LR, Rossato JI, Bonini JS, Medina JH, Cammarota M. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29:496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260(5114):1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto Y, Unoki S, Aonuma H, Mizunami M. Critical role of nitric oxide-cGMP cascade in the formation of cAMP-dependent long-term memory. Learn Mem. 2006;13(1):35–44. doi: 10.1101/lm.130506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhuo M, Hu Y, Schultz C, Kandel ER, Hawkins RD. Role of guanylyl cyclase and cGMP-dependent protein kinase in long-term potentiation. Nature. 1994;368(6472):635–639. doi: 10.1038/368635a0. [DOI] [PubMed] [Google Scholar]
- 32.Tully T, Bourtchouladze R, Scott R, Tallman J. Targeting the CREB pathway for memory enhancers. Nat Rev Drug Discov. 2003;2:267–277. doi: 10.1038/nrd1061. [DOI] [PubMed] [Google Scholar]
- 33.Nagakura A, Niimura M, Takeo S. Effects of a phosphodiesterase IV inhibitor rolipram on microsphere embolism-induced defects in memory function and cerebral cyclic AMP signal transduction system in rats. Br J Pharmacol. 2002;135:1783–1793. doi: 10.1038/sj.bjp.0704629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itoh T, Abe K, Tokumura M, Horiuchi M, Inoue O, Ibii N. Different regulation of adenylyl cyclase and rolipram sensitive phosphodiesterase activity on the frontal cortex and hippocampus in learned helpnessness rats. Brain Res. 2003;991:1–2. 142–149. doi: 10.1016/j.brainres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Scott AI, Perini AF, Shering PA, Whalley JL. In-patient major depression: is rolipram as effective as amitriptyline. Eur J Clin Pharmacol. 1991;40(2):127–129. doi: 10.1007/BF00280065. [DOI] [PubMed] [Google Scholar]
- 36.Dundore RL, Clas DM, Wheeler LT, et al. Zaprinast increases cyclic GMP levels in plasma and in aortic tissue of rats. Eur J Pharmacol. 1993;249:293–297. doi: 10.1016/0014-2999(93)90525-m. [DOI] [PubMed] [Google Scholar]
- 37.Dundore RL, Habeeb PG, Pratt PF, Becker LT, Clas DM, Buchholz RA. Differential hemodynamic responses to selective inhibitors of cyclic nucleotide phosphodiesterases in conscious rats. J Cardiovasc Pharmacol. 1992;19:937–944. doi: 10.1097/00005344-199206000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Hall CS. Emotional behavior in the rat: I. Defecation and urination as measures of individual differences in emotionality. J Comp Psychol. 1934;18:385–403. [Google Scholar]
- 39.Walsh RN, Cummins RA. The open field test: a critical review. Psychol Bull. 1976;83:481–504. [PubMed] [Google Scholar]
- 40.Yıldız F, Ulak G, Erden BF, Gacar N. Anxiolytic-like effects of 7-nitroindazole in the rat plus-maze test. Pharmacol Biochem Behav. 2000;65(2):199–202. doi: 10.1016/s0091-3057(99)00133-1. [DOI] [PubMed] [Google Scholar]
- 41.Caton PW, Tousman SA, Quock RM. Involvement of nitric oxide in nitrous oxide anxiolysis in the elevated plus-maze. Pharmacol Biochem Behav. 1994;48:689–692. doi: 10.1016/0091-3057(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 42.Kendrick KM, Guevara-Guzman R, Zorrilla J, et al. Formation of olfactory memories mediated by nitric oxide. Nature. 1997;388:670–674. doi: 10.1038/41765. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez-Andrade G, James BM, Kendrick KM. Neural encoding of olfactory recognition memory-review. J Reprod Dev. 2005;51:547–558. doi: 10.1262/jrd.17031. [DOI] [PubMed] [Google Scholar]