Abstract
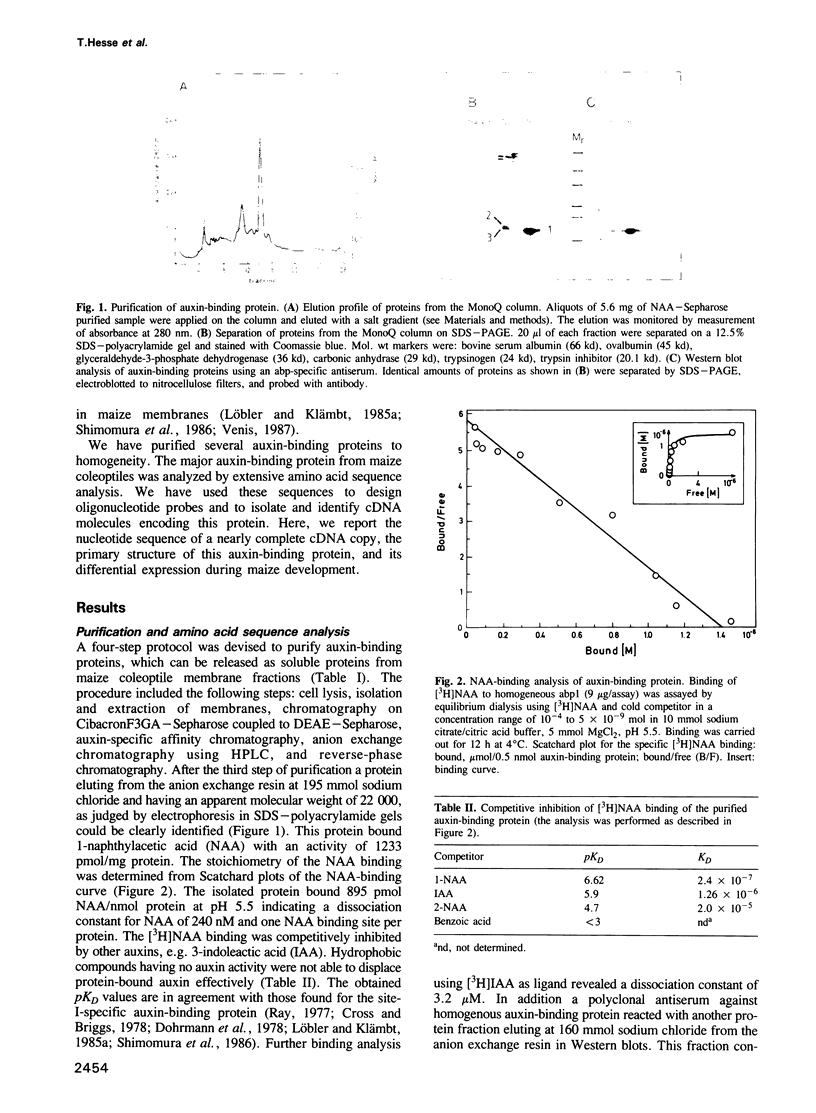
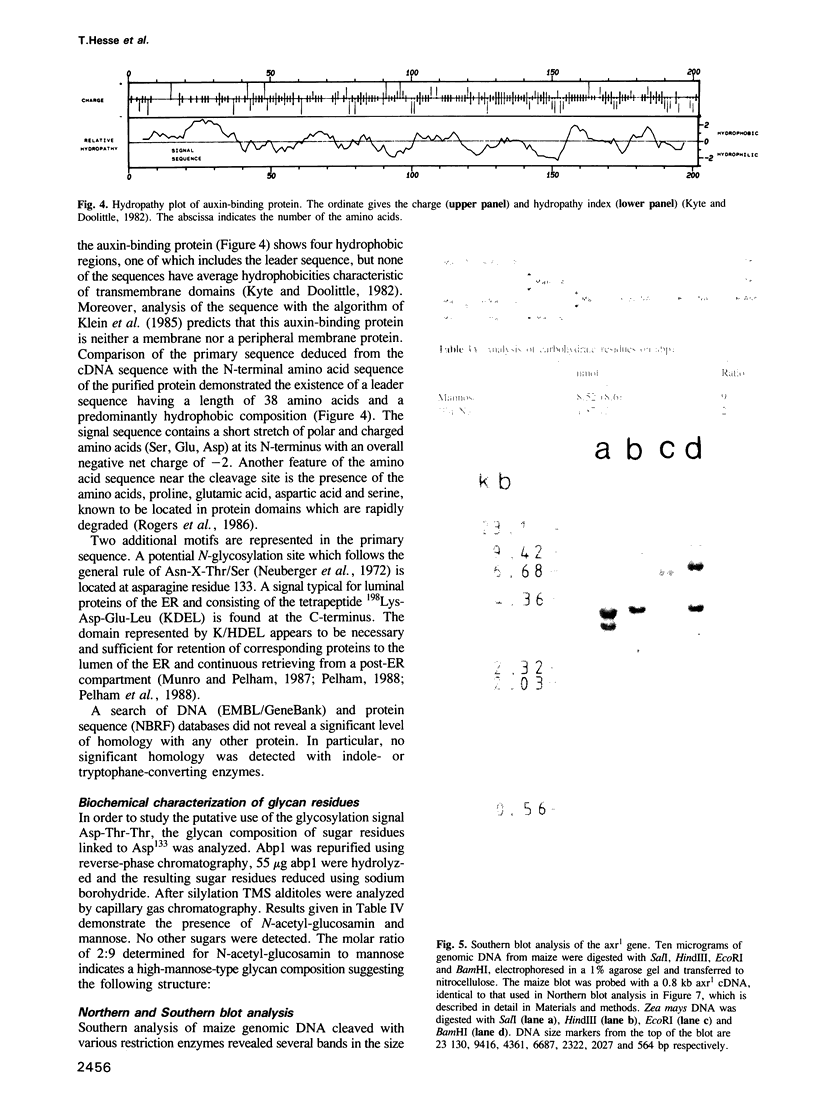
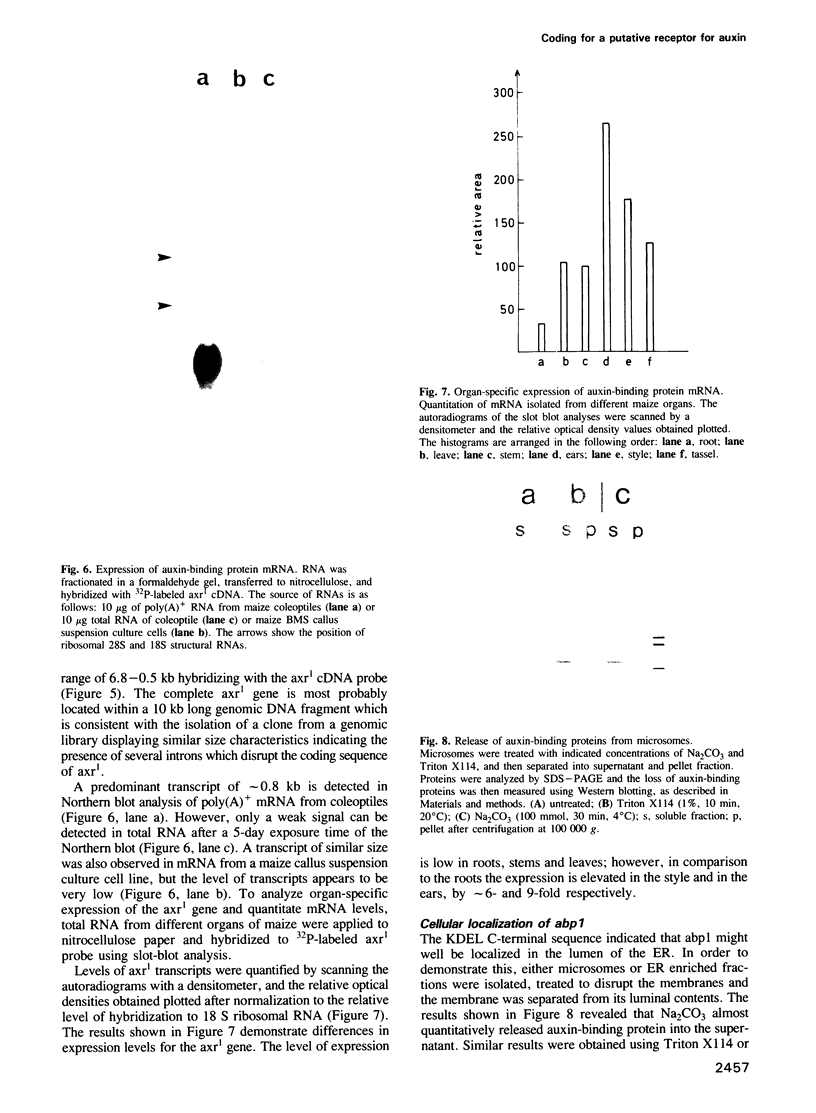
The major auxin-binding protein from maize coleoptiles was purified to homogeneity. The protein has an apparent mol. wt of 22 kd and binds 1-naphthylacetic acid with a KD of 2.40 x 10(-7) M. Additional antigenically related proteins, present in very low amounts, could be demonstrated in maize coleoptiles using immunodetection. Extensive protein sequence analysis of the major auxin-binding protein allowed the construction of several synthetic oligonucleotide probes which were used to isolate a cDNA coding for this protein. The cDNA corresponds to a mRNA with a 3'-poly(A)+ sequence and a single, long open reading frame of 603 bases. The open reading frame, starting 34 residues from the 5' end of the cDNA, predicts a 21,990 Dalton protein of 201 amino acids. Comparison of this deduced amino acid sequence with the partial amino acid sequences of purified auxin-binding protein, revealed a perfect match, involving a total of 53 amino acid residues. The primary amino acid sequence includes a 38-amino-acid-long N-terminal hydrophobic leader sequence which could represent a signal for translocation of this protein to the endoplasmic reticulum. An additional signal is located at the C-terminal end, consisting of the amino acids KDEL known to be responsible for preventing secretion of proteins from the lumen of the endoplasmic reticulum in eucaryotic cells. The primary sequence contains a N-glycosylation site (-asp133-thr-thr-). This site was found to be glycosylated by a high-mannose-type oligosaccharide.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier-Brygoo H., Ephritikhine G., Klämbt D., Ghislain M., Guern J. Functional evidence for an auxin receptor at the plasmalemma of tobacco mesophyll protoplasts. Proc Natl Acad Sci U S A. 1989 Feb;86(3):891–895. doi: 10.1073/pnas.86.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauw G., De Loose M., Inzé D., Van Montagu M., Vandekerckhove J. Alterations in the phenotype of plant cells studied by NH(2)-terminal amino acid-sequence analysis of proteins electroblotted from two-dimensional gel-separated total extracts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4806–4810. doi: 10.1073/pnas.84.14.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cross J. W., Briggs W. R. Properties of a Solubilized Microsomal Auxin-binding Protein from Coleoptiles and Primary Leaves of Zea mays. Plant Physiol. 1978 Jul;62(1):152–157. doi: 10.1104/pp.62.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. H., Stratowa C., Rutter W. J. Isolation of full-length putative rat lysophospholipase cDNA using improved methods for mRNA isolation and cDNA cloning. Biochemistry. 1987 Mar 24;26(6):1617–1625. doi: 10.1021/bi00380a020. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Key J. L., Kroner P., Walker J., Hong J. C., Ulrich T. H., Ainley W. M., Gantt J. S., Nagao R. T. Auxin-regulated gene expression. Philos Trans R Soc Lond B Biol Sci. 1986 Nov 17;314(1166):427–440. doi: 10.1098/rstb.1986.0063. [DOI] [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Koch G. L. Reticuloplasmins: a novel group of proteins in the endoplasmic reticulum. J Cell Sci. 1987 May;87(Pt 4):491–492. doi: 10.1242/jcs.87.4.491. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lennarz W. J. Protein glycosylation in the endoplasmic reticulum: current topological issues. Biochemistry. 1987 Nov 17;26(23):7205–7210. doi: 10.1021/bi00397a001. [DOI] [PubMed] [Google Scholar]
- Lin P. H., Selinfreund R. H., Wakshull E., Wharton W. Rapid isolation of plasmalemma from cultured A431 cells: characterization of epidermal growth factor receptors. Anal Biochem. 1988 Feb 1;168(2):300–305. doi: 10.1016/0003-2697(88)90322-3. [DOI] [PubMed] [Google Scholar]
- Löbler M., Klämbt D. Auxin-binding protein from coleoptile membranes of corn (Zea mays L.). I. Purification by immunological methods and characterization. J Biol Chem. 1985 Aug 15;260(17):9848–9853. [PubMed] [Google Scholar]
- Löbler M., Klämbt D. Auxin-binding protein from coleoptile membranes of corn (Zea mays L.). II. Localization of a putative auxin receptor. J Biol Chem. 1985 Aug 15;260(17):9854–9859. [PubMed] [Google Scholar]
- Maddy A. H., Dunn M. J., Kelly P. G. The characterisation of membrane proteins by centrifugation and gel electrophoresis. A comparison of proteins prepared by different methods. Biochim Biophys Acta. 1972 Nov 2;288(2):263–276. doi: 10.1016/0005-2736(72)90247-7. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Evidence that luminal ER proteins are sorted from secreted proteins in a post-ER compartment. EMBO J. 1988 Apr;7(4):913–918. doi: 10.1002/j.1460-2075.1988.tb02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Hardwick K. G., Lewis M. J. Sorting of soluble ER proteins in yeast. EMBO J. 1988 Jun;7(6):1757–1762. doi: 10.1002/j.1460-2075.1988.tb03005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Auxin-binding Sites of Maize Coleoptiles Are Localized on Membranes of the Endoplasmic Reticulum. Plant Physiol. 1977 Apr;59(4):594–599. doi: 10.1104/pp.59.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Specificity of Auxin-binding Sites on Maize Coleoptile Membranes as Possible Receptor Sites for Auxin Action. Plant Physiol. 1977 Oct;60(4):585–591. doi: 10.1104/pp.60.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinard T., Jacobsen H. J. An inexpensive small volume equilibrium dialysis system for protein-ligand binding assays. Anal Biochem. 1989 Jan;176(1):157–160. doi: 10.1016/0003-2697(89)90286-8. [DOI] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Shimomura S., Sotobayashi T., Futai M., Fukui T. Purification and properties of an auxin-binding protein from maize shoot membranes. J Biochem. 1986 May;99(5):1513–1524. doi: 10.1093/oxfordjournals.jbchem.a135621. [DOI] [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüber K. Nucleic acid secondary structure prediction and display. Nucleic Acids Res. 1986 Jan 10;14(1):317–326. doi: 10.1093/nar/14.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson K. I., Farrell K. M., Ruderman J. V. The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes. Cell. 1986 Dec 26;47(6):861–870. doi: 10.1016/0092-8674(86)90801-9. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillmann U., Viola G., Kayser B., Siemeister G., Hesse T., Palme K., Löbler M., Klämbt D. cDNA clones of the auxin-binding protein from corn coleoptiles (Zea mays L.): isolation and characterization by immunological methods. EMBO J. 1989 Sep;8(9):2463–2467. doi: 10.1002/j.1460-2075.1989.tb08381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Walton J. D., Ray P. M. Evidence for Receptor Function of Auxin Binding Sites in Maize : RED LIGHT INHIBITION OF MESOCOTYL ELONGATION AND AUXIN BINDING. Plant Physiol. 1981 Dec;68(6):1334–1338. doi: 10.1104/pp.68.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. Protein transport. Signals and salvage sequences. Nature. 1987 May 7;327(6117):17–18. doi: 10.1038/327017a0. [DOI] [PubMed] [Google Scholar]