Abstract
Background:
Human variations related to immune response and disease susceptibility is well-documented in Ayurveda. Prakriti (body constitution) is the basic constitution of an individual established at the time of birth and distinguishes variations, into three broad phenotype categories such as vata, pitta and kapha. Variation in immune response is often attributed to and measured from the difference in cluster differentiation (CD) markers expressed in lymphocytes. Currently, there are no reports available on the expression of CD markers related to prakriti.
Objective:
This is a pilot study performed to evaluate a panel of lymphocyte subset CD markers in dominant prakriti individuals.
Materials and Methods:
Immunophenotyping was carried out using whole blood from a total of healthy 222 subjects, who are grouped into kapha (n = 95), pitta (n = 57) and vata (n = 70) prakritis. CD markers such as CD3, CD4, CD8, CD14, CD25, CD56, CD69, CD71 and HLA-DR were analyzed using flow cytometry method. Differences between groups were analyzed using one-way ANOVA or Kruskal-Wallis analysis of variance (ANOVA) and multiple comparisons between groups were performed by Bonferroni or Mann-Whitney U test with corrections for type I error respectively. Significance was evaluated by ANOVA and Pearson's correlation.
Results:
We observed a significant difference (P < 0.05) in the expression of CD markers such as CD14 (monocytes), CD25 (activated B cells) and CD56 (Natural killer cells) between different prakriti groups. CD25 and CD56 expression was significantly higher in kapha prakriti samples than other prakriti groups. Similarly, slightly higher levels of CD14 were observed in pitta prakriti samples.
Conclusion:
Significant difference in the expression of CD14, CD25 and CD56 markers between three different prakriti is demonstrated. The increased level of CD25 and CD56 in kapha prakriti may indicate ability to elicit better immune response, which is in conformity with textual references in Ayurveda.
Keywords: Cluster differentiation 14, cluster differentiation 25, cluster differentiation 56, immunophenotyping, prakriti
INTRODUCTION
Variation in humans, in anatomical, physiological, immunological, psychological, disease susceptibility, disease prognosis and response to treatment forms the basic tenets of personalized medicine. Studies have indicated an increase in clinical potential of pharmacogenetics to identify genetic variants that affect treatment efficacy.[1] Recently, the studies on personalized medicine have relied on understanding of the molecular/genetic variations with respect to disease for the implementation of preventive health-care strategies and drug therapies to healthy individuals or during the various stage of the disease.[2] Thus, it aims to prevent diseases, maintenance of health, early detection and diagnosis of disease, customized screening for risk and treatment of the diseases is important for the development of tailored treatment to improve the quality-of-life after treatment.
Indian traditional medicinal system, Ayurveda largely represents personalized medicine by emphasizing on the concept of prakriti. Prakriti is the specific type of body constitution of an individual produced by the relative proportions of the three doshas (humors) such as vata, pitta and kapha, which is fixed at the time of conception and remains same throughout the life.[3,4,5] There are seven different types of prakriti manifested by different combination of these three doshas attributing to specific phenotypic characters and are documented in the ancient Ayurvedic literature viz., Charaka Samhita and Ashtanga Hridaya.[6,7,8,9,10] Ayurveda also explains the predisposition and susceptibility of human subjects with different prakriti to various diseases. For example, individuals with vata, pitta and kapha prakriti are generally predisposed to neurological disorders, digestive malfunctions and respiratory illness respectively.[8,11,12,13]
Circulating and resident immune cells collectively and coordinatively mount an immune response and depending on the number of factors, the extent of which may vary among individuals. These may also be attributed to predisposition and susceptibility of an individual to pathogen specific response.[14] Several reports are available on the enhancement of immunity by Ayurvedic herbal formulations.[15] Gautam et al.,[16] and Somarathna et al.,[17] have shown an increase in immune capacity by Ayurvedic herbal formulations through elevating the expression profile of cluster differentiation (CD) markers. Moreover, Ayurveda treats every individual as unique and the treatment is based on the concepts of prakriti. Studies by Bhushan et al.,[18] and Ghodke et al.,[19] have shown specific differences in genetic polymorphisms between different prakriti which in turn is related to drug metabolizing ability. Similarly, Prasher et al.,[20] have reported the variation in gene expression and biochemical profiles of the three constitutional prakriti types. Such reports are scanty and clear evidences to show the differences among the different prakritis are required. Hence, the present study was performed to evaluate the ability to initiate the immune response in dominant prakriti individuals by analyzing various CD markers and relate to the concepts laid down in Ayurveda.
MATERIALS AND METHODS
Ethical statement
Screening and sample collection was performed at the center named as Shri Dharmasthala Manjunatheshwara College of Ayurveda (SDMCA). Ethical consent was obtained from Institutional Ethical Committee of SDMCA, Udupi.
Study design
In this cross-sectional pilot study, volunteers for prakriti assessment were recruited from various educational institutions from Udupi to Dakshina Kannada districts including Mangalore. Prakriti camps were conducted in various institutes with the consent from Head of the institutions. The students at these camps were made aware of prakriti determination by an Ayurvedic physician. Consenting volunteers were registered and informed consent was obtained. Prakriti assessment of each subject was performed in three stages. In the first stage, prakriti was assessed by senior vaidya (ayurvedic physician) based on his clinical skill and experience by applying classical Ayurvedic parameters of prakriti determination. In the second stage, another vaidya carried out same subject's prakriti assessment through Ayusoft prakriti assessment tool. Ayusoft is a logical tool, which consists of a comprehensive questionnaire and was formulated based on information from original Ayurvedic literature to qualitatively and quantitatively determine the prakriti.[21] Finally, a third vaidya who was blinded to the prakriti assessment of both senior vaidya and Ayusoft, compared the prakriti analysis. The role of third vaidya was to assess concordance amongst senior vaidya and Ayusoft assessments, since the prakriti assessment by vaidya and Ayusoft were performed independent of each other. Third vaidya also shortlisted the subjects, who showed prakriti percentage of 60% and above as per Ayusoft and in agreement with senior vaidya's assessment. Subjects who did not satisfy either criterion were excluded from the study. The criterion of 60% or above of single prakriti which was considered as dominant is primarily based on feasibility and consensus. It is well-recognized amongst Ayurvedic clinicians that single any dosha prakriti with very high percentage of one dosha, are extremely rare. Most of the individuals possess dual-dosha prakritis and therefore 60% as minimum cut off was maintained. To acquire the desired sample size with a much higher cut-off would be unviable due to (a) required sample size and (b) duration of the study.
Healthy, non-smoking, non-alcoholic male subjects between the age group of 20-30 were screened for the prakriti assessment (mean age 23 ± 4 years). Health status of the individual is considered on the basis of Ayurvedic literature as determined by Ayurvedic physicians. Accordingly, to consider one as healthy, individual should have signs of desire for food, easy digestion of ingested food, proper excretion of feces, urine, flatus, proper functioning of eyes, ears, nose, tongue and skin, comfortable sleep, easy awakening, attainment of strength, complexion and equilibrium of fire (both digestive and metabolic). Complete medical history including history of chronic illnesses or surgery, general physical examination with blood pressure, pulse rate, height, weight and routine auscultation was recorded to rule out overt cardiac or respiratory anomaly and history of infection within the past 3 months. The blood samples were collected following the World Health Organization (WHO) guidelines and international standard methodology. We have ensured the consistency in blood draw by following methodology reported in the literature.[22] Briefly, blood was drawn from selected individuals in the morning (7 AM-9 AM) before intake of any food in a BD Vacutainer® tubes (BD, Franklin Lakes, NJ, USA) by a senior technician as per WHO guidelines.[23] Samples were immediately transferred to the laboratory in an insulated container and immunophenotyping was performed using flow cytometry.
Immunophenotyping analysis
Immunophenotyping using fluorochrome labeled antibodies to CD3, CD4, CD8, CD14, CD19, CD25, CD56, CD69, CD71 and HLA-DR were carried out to analyze their distribution across different prakritis by employing fluorescence activated cell sorter (FACS Calibur; Becton Dickinson; BD, San Jose, CA). The analysis was performed following “lyse no wash” procedure as per the manufacturer's instructions. In brief, 50 μl of anti-coagulated whole blood in falcon tubes was mixed with fluorochrome labeled antibodies (20 μl) and incubated for 30 minute in dark at room temperature. Subsequently, red blood cell lysis buffer (1X: 450 μl) and liquid counting beads (20 μL of BD™ liquid counting beads, catalog number − 335925, BD, San Jose, CA) were added. The samples were mixed thoroughly and acquired after 12-15 minute of incubation at room temperature. Cell quest Pro software version 5.2 (BD, San Jose, CA) was used throughout for data acquisition and analysis.
Statistical analysis
The cell count distribution of each CD marker across the prakriti samples was statistically analyzed using the statistical package for the social sciences version 11.5 (IBM SPSS Statistics, USA) and GraphPad Prism version 5.00 for Windows, GraphPad Software (San Diego, California, USA). The differences in absolute count of all CD marker's expression were ascertained by applying parametric or non-parametric analysis of variance (ANOVA). The difference in expression of CD19, CD25, CD56, CD14 and CD69 between prakriti was assessed by Kruskal-Wallis ANOVA with Mann-Whitney U multiple comparison analysis corrected for type I error. Pearson's correlation analysis was performed using R version 2.15.2, Base graphing package to calculate the relation between the percentage of prakriti and absolute count of significant CD markers. The P ≤ 0.05 was considered as significant.
RESULTS
Estimation of CD markers in dominant prakriti
The distribution of dominant prakriti was found to be highest in kapha with 42.79% followed by vata 31.53% and pitta 25.67%. Immunophenotyping was carried out in whole blood from a total of 222 subjects, categorized into different dominant prakriti such as kapha (n = 95), pitta (n = 57) and vata (n = 70). In the present study, the total number of cells in a defined volume expressing CD3, CD4, CD8, CD19, CD14, CD25, CD56, CD69, CD71 and HLA-DR was analyzed. Statistical analysis revealed that there was a significant difference (P < 0.05) in the expression of CD markers such as CD14 (monocytes), CD25 (activated B-cells) and CD56 (Natural killer cells [NK cell]) between different prakriti groups of subjects [Figure 1]. There was a statistically significant difference between prakriti groups in absolute CD14 expressions (H[2] = 6.22, P = 0.044) with a mean rank of 99.08 (median = 114) for kapha, 119.96 (median = 147) for pitta and 121.46 (median = 139) for vata prakriti. The median expression for CD14 was found to be higher in pitta as compared to kapha and vata prakritis. However, Mann-Whitney U test multiple group comparison analysis showed insignificant P value (P > 0.017 [corrected α error]) in all possible comparison analysis. Kruskal-Wallis ANOVA revealed a significant (H[2] = 11.15, P = 0.003) difference in the expression of CD25 among prakritis. The median expression of CD25 in case of kapha found to be highest (136) with a mean rank of 127.48, followed with pitta prakriti (121) mean rank of (105.46) and it was least for vata prakriti (106.5) which showed a mean rank of 105. Further, multiple comparison analysis between kapha and vata prakritis revealed a significant difference in expression with Mann-Whitney test with U = 2343 (Z = −3.238) and P = 0.001 and this difference was observed to-be moderate (r = −0.21). The median CD56 expression also showed a high level of expression in the case of kapha (122.4) followed with pitta (111.65) and then by vata (96.81) samples. Between three dominant prakriti group comparison, a statistical significant difference was observed (H[2] = 6.318, P = 0.042). Mann-Whitney U test showed kapha prakriti with higher expression of CD56 as compared to vata with P = 0.016. On the other hand, there were no significant differences (P > 0.05) in the expression of CD3, CD4, CD8, CD19 CD69, CD71 and HLA-DR between different prakritis [Table 1]. The mean expression of different lymphocyte subset CD markers in total population (n = 222) was found to be comparable with the earlier studies, where reference ranges for different lymphocyte subset have been reported in healthy Indian population.[24,25,26]
Figure 1.
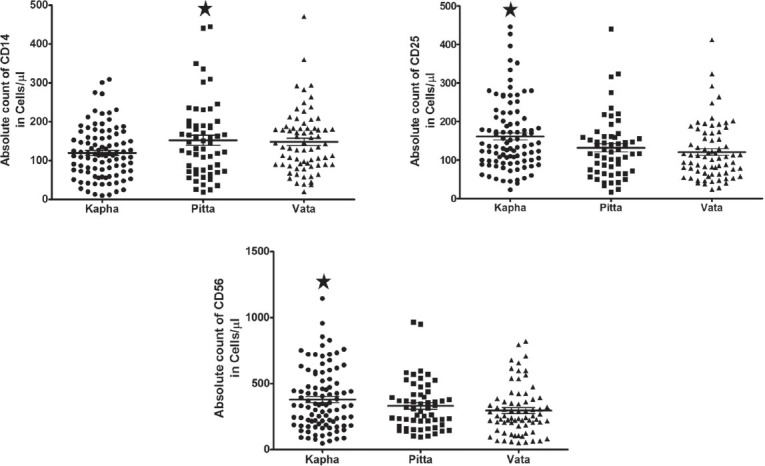
Expression of significant cluster differentiation markers across three prakriti. Scatter plot for expression of CD14, CD25 and CD56 which are significant in expression between three dominant prakriti (P < 0.05). Significance with the highest mean value was represented in * sign
Table 1.
The expression distribution of different CD markers in three dominant prakritis
The inference on the effect of doshas with respect to the percentage for expression of CD14, CD25 and CD56 was further evaluated by correlation analysis. Pearson correlation analysis is performed to assess the relationship between percentage values of doshas (kapha, pitta and vata) and CD marker expression. Analysis of CD25 and CD56 marker expression for kapha prakriti revealed moderate positive correlation of 0.23 and 0.16 respectively, as opposed to vata prakriti wherein a negative correlation (Pearson r = −0.20 and − 0.16 respectively) with P < 0.05 was observed. CD14 expression analysis with the percentage of individual prakriti compared to kapha prakriti showed a negative correlation (Pearson r = −0.19) with P 0.05. The Pearson correlation matrix is shown in Figure 2.
Figure 2.
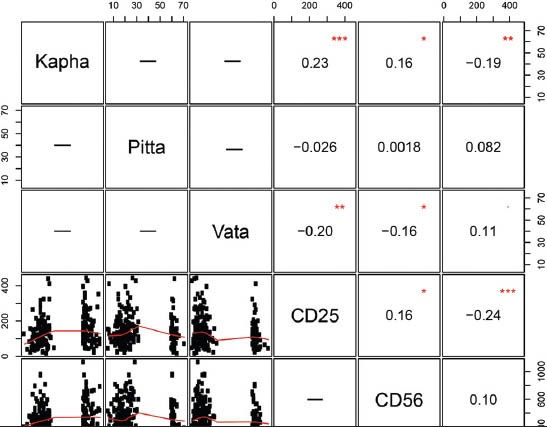
Pearson correlation matrix to find correlation between percentage of prakriti and cluster differentiation 14, CD25 and CD56 expression. Data represented as the percentage values of kapha, pitta and vata obtained from the Ayusoft against, the absolute count of CD marker of the population studied. The values inside the boxes are the Pearson's correlation r values and statistical significance is represented by * sign
DISCUSSION
In this study, we have attempted to evaluate the quantitative estimation of the dominant percentage of prakriti which could be further coupled to the traditional assessment of prakriti. In the absence of such data with respect to prakriti stratified Indian population, we have for the first time shown here the differences in CD marker expressions. This study may provide base line values for different CD markers within dominant prakriti and could be used for understanding prakriti based disease manifestation, etiology and improving the treatment modalities by an Ayurvedic practitioner.
Inter individual variations to elicit innate or acquired immune response is well-documented and it is attributed to the number of factors including the proportion of immune cells in circulation.[24,25,26,27,28] The preservation of health and control of disease manifestations are the primary objectives of Ayurveda and these in part, can be achieved by enhancing the immunity.[29,30,31] Ayurveda also suggests differences immune response in individuals which is based on their prakriti.[32,33] An increase in innate immunity was observed when herbal formulations were used for natural healing[17] and this has been attributed to increase in the expression of CD antigens. Our results show a significant difference in expression of CD14, CD25 and CD56 between the dominant prakritis in normal individuals.
The CD14 surface antigen is preferentially expressed by monocytes, macrophages, granulocytes (weak expression), B-cells, dendritic cells and hepatocytes and mediates the innate immune response to bacterial lipopolysaccharide. Ayurvedic principles[34] and anecdotal clinical experiences suggest that pitta prakriti has a higher tendency to develop hypersensitive skin responses, in particular, flares (red rashes). Accordingly, our study indicates that CD14 expression is higher in pitta dominant individuals, thereby suggesting a causal relationship. Consistent with this, Ferrero et al.,[35] and Merlin et al.,[36] have reported an increase in the hypersensitivity reaction with higher CD14 expression in in vivo mouse model.
The mammalian immune system protects the host from a broad range of pathogenic microorganism while avoiding excessive immune reactions that would be deleterious to the host. Interleukin 2-Rα (CD25) is a 55 kDa transmembrane polypeptide, expressed mainly by activated B-cell, T-cell and NK-cells. The deficiency of CD25 in humans results in recurrent infections and lymphocyte infiltration in multiple tissues.[37] The CD25 expression on B-cells appears to function both on innate and adaptive immune response mechanism[38] and in our study, we demonstrate a higher expression of CD25 in individuals with kapha prakriti. Ayurvedic literature also suggests that individuals with kapha prakriti show strong immune response as compared to vata prakriti.[32,33]
CD56 also known as neural cell adhesion molecule, a member of a large family of cell surface glycoprotein and mainly expressed by the NK-cells.[39] Depending on the context, they serve as a signaling receptors and help in cellular adhesion, migration, proliferation, apoptosis, differentiation, survival and cellular plasticity. NK-cells are the large granular, innate effector lymphocytes, which provide defense against microbial-infected or malignant cells through Fas or Perforin/granzyme pathway.[14,40] In our study, the kapha prakriti showed a higher level of expression of CD56 as compared to vata, which is concordance to the earlier report.[32,33]
As the selected samples were from healthy population, we did not observe any significant difference in expression of other CD markers which are mainly activated by T-cells and B-cells. From the perspective of prakriti, the differences between two single dosha prakritis will reveal only slight to moderate differences. For example, features such as dry skin of vata and soft skin of pitta may overlap, making distinction difficult. Prominent prakriti features, such as, premature graying of hair in pitta or extremely dry skin of vata, are immediately observable and assume decisive value. It is possible that the CD markers of only the few dominant prakriti features show detectable differences. Strong immunity in kapha prakriti, marked tendency to develop bright red hypersensitivity rashes in pitta prakriti and low immunity in vata relative to kapha are clear and decisive prakriti features in clinical Ayurveda. Identifying prakriti specific markers of specific diseases by unearthing and cataloguing prakriti specific genes in physiologically stable states may provide as a reference to facilitate genetic sub-grouping of human populations. The limitations of the present study is that the prakriti assessment carried out for (a) only healthy male individuals in the age group of 20-30 and (b) only few selected CD markers were tested and further studies are warranted for any definitive conclusions.
CONCLUSION
In the present study, we have shown that significant differences in the expression of CD14, CD25 and CD56 subset with their constitution type or prakriti, which is in conformity with co-relations to textual references in Ayurveda.
ACKNOWLEDGMENTS
The authors are thankful to all the volunteers who have participated in this study. We thank Innovation in Science Pursuit for Inspired Research, for providing INSPIRE fellowship to HR. We gratefully acknowledge the office of the principal scientific advisor to the government of India (PSA-GOI) for financial support.
Footnotes
Source of Support: We gratefully acknowledge the office of the principal scientific advisor to the government of India for financial support,
Conflict of Interest: None declared.
REFERENCES
- 1.Banerjee M. Is pharmacogenomics a reality? Challenges and oppurtunities for India. Indian J Hum Genet. 2011;17(Suppl 1):S1–3. doi: 10.4103/0971-6866.80350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ginsburg GS, Willard HF. Genomic and personalized medicine: Foundations and applications. Transl Res. 2009;154:277–87. doi: 10.1016/j.trsl.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Hankey A. The scientific value of Ayurveda. J Altern Complement Med. 2005;11:221–5. doi: 10.1089/acm.2005.11.221. [DOI] [PubMed] [Google Scholar]
- 4.Jayasundar R. Ayurveda: A distinctive approach to health and disease. Curr Sci. 2010;98:908–14. [Google Scholar]
- 5.Valiathan MS. Chennai: Orient Longman; 2003. The Legacy of Caraka. [Google Scholar]
- 6.Acharya YT, editor. Varanasi: Chaukhambha Orientalia; 1997. Charak Samhitha, Vimanasthana 8/95. [Google Scholar]
- 7.Acharya YT, editor. Varanasi: Chaukhambha Sanskrit Sansthan; 2007. Sushrutha Samhita, Sharirasthana 4/65-76. [Google Scholar]
- 8.Hankey A. A test of the systems analysis underlying the scientific theory of Ayurveda's Tridosha. J Altern Complement Med. 2005;11:385–90. doi: 10.1089/acm.2005.11.385. [DOI] [PubMed] [Google Scholar]
- 9.Patwardhan B, Bodeker G. Ayurvedic genomics: Establishing a genetic basis for mind-body typologies. J Altern Complement Med. 2008;14:571–6. doi: 10.1089/acm.2007.0515. [DOI] [PubMed] [Google Scholar]
- 10.Patwardhan B, Warude D, Pushpangadan P, Bhatt N. Ayurveda and traditional Chinese medicine: A comparative overview. Evid Based Complement Alternat Med. 2005;2:465–73. doi: 10.1093/ecam/neh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caldecott T. Ayurveda the divine science of life. Recherche. 2006;67:02. [Google Scholar]
- 12.Hankey A. Ayurvedic physiology and etiology: Ayurvedo Amritanaam. The doshas and their functioning in terms of contemporary biology and physical chemistry. J Altern Complement Med. 2001;7:567–74. doi: 10.1089/10755530152639792. [DOI] [PubMed] [Google Scholar]
- 13.Valiathan MS. 1st ed. Hyderabad: Universities Press (India) Private Limited; 2009. The Legacy of Vagbhata. [Google Scholar]
- 14.Parkin J, Cohen B. An overview of the immune system. Lancet. 2001;357:1777–89. doi: 10.1016/S0140-6736(00)04904-7. [DOI] [PubMed] [Google Scholar]
- 15.Patwardhan B, Mashelkar RA. Traditional medicine-inspired approaches to drug discovery: Can Ayurveda show the way forward? Drug Discov Today. 2009;14:804–11. doi: 10.1016/j.drudis.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Gautam M, Diwanay SS, Gairola S, Shinde YS, Jadhav SS, Patwardhan BK. Immune response modulation to DPT vaccine by aqueous extract of Withania somnifera in experimental system. Int Immunopharmacol. 2004;4:841–9. doi: 10.1016/j.intimp.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Somarathna KI, Chandola HM, Ravishankar B, Pandya KN, Attanayake AM. A short-term intervention trial on HIV positive patients using a Sri Lankan classical rasayana drug-Ranahamsa Rasayanaya. Ayu. 2010;31:197–204. doi: 10.4103/0974-8520.72393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhushan P, Kalpana J, Arvind C. Classification of human population based on HLA gene polymorphism and the concept of Prakriti in Ayurveda. J Altern Complement Med. 2005;11:349–53. doi: 10.1089/acm.2005.11.349. [DOI] [PubMed] [Google Scholar]
- 19.Ghodke Y, Joshi K, Patwardhan B. Traditional medicine to modern pharmacogenomics: Ayurveda Prakriti Type and CYP2C19 gene polymorphism associated with the metabolic variability. Evid Based Complement Alternat Med 2011. 2011 doi: 10.1093/ecam/nep206. 249528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prasher B, Negi S, Aggarwal S, Mandal AK, Sethi TP, Deshmukh SR, et al. Whole genome expression and biochemical correlates of extreme constitutional types defined in Ayurveda. J Transl Med. 2008;6:48. doi: 10.1186/1479-5876-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.C-DAC software for Ayurveda, Ayusoft. 2010. [Last accessed on 2010 Nov 22]. Available from: http://www.ayusoft.cdac.in .
- 22.Mallone R, Mannering SI, Brooks-Worrell BM, Durinovic-Belló I, Cilio CM, Wong FS, et al. Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T cell responses: Position statement of the T-Cell Workshop Committee of the Immunology of Diabetes Society. Clin Exp Immunol. 2011;163:33–49. doi: 10.1111/j.1365-2249.2010.04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geneva: World Health Organization; 2010. [Latest Accessed on August 2013]. World Health Organization. WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy; p. 1. Introduction. Available from: http://www.ncbi.nlm.nih.gov/books/NBK138675 . [PubMed] [Google Scholar]
- 24.Saxena RK, Choudhry V, Nath I, Das SN, Paranjape RS, Babu G, et al. Normal ranges of some selected lymphocytesub-populations in peripheral blood of normal healthy Indians. Curr Sci. 2004;86:969–75. [Google Scholar]
- 25.Das BR, Bhanushali AA, Khadapkar R, Jeswani KD, Bhavsar M, Dasgupta A. Reference ranges for lymphocyte subsets in adults from western India: Influence of sex, age and method of enumeration. Indian J Med Sci. 2008;62:397–406. [PubMed] [Google Scholar]
- 26.Thakar MR, Abraham PR, Arora S, Balakrishnan P, Bandyopadhyay B, Joshi AA, et al. Establishment of reference CD4+T cell values for adult Indian population. AIDS Res Ther. 2011;8:35. doi: 10.1186/1742-6405-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calder PC, Kew S. The immune system: A target for functional foods? Br J Nutr. 2002;88(Suppl 2):S165–77. doi: 10.1079/BJN2002682. [DOI] [PubMed] [Google Scholar]
- 28.Calder PC. Immunological parameters: What do they mean? J Nutr. 2007;137:773S–80. doi: 10.1093/jn/137.3.773S. [DOI] [PubMed] [Google Scholar]
- 29.Chatterjee B, Pancholi J. Prakriti-based medicine: A step towards personalized medicine. Ayu. 2011;32:141–6. doi: 10.4103/0974-8520.92539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi K, Ghodke Y, Shintre P. Traditional medicine and genomics. J Ayurveda Integr Med. 2010;1:26–32. doi: 10.4103/0975-9476.59824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tripathi NS. Concept of formation of “Prakriti” in ayurveda. Indian J Res. 2011;5:1–5. [Google Scholar]
- 32.Priyavrat S, editor. Varanasi: Chaukhambha Orientalia; 2010. Charaka Samhita, Su.28/7. [Google Scholar]
- 33.Priyavrat S, editor. Varanasi: Chaukhambha Orientalia; 2010. Charaka Samhita, Vim.8/96-98. [Google Scholar]
- 34.Lochan K, editor. New Delhi: Chaukhamba Publications; 2008. Ashtanga Hrudaya Suthrasthana 11/26. [Google Scholar]
- 35.Ferrero E, Jiao D, Tsuberi BZ, Tesio L, Rong GW, Haziot A, et al. Transgenic mice expressing human CD14 are hypersensitive to lipopolysaccharide. Proc Natl Acad Sci U S A. 1993;90:2380–4. doi: 10.1073/pnas.90.6.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merlin T, Woelky-Bruggmann R, Fearns C, Freudenberg M, Landmann R. Expression and role of CD14 in mice sensitized to lipopolysaccharide by Propionibacterium acnes. Eur J Immunol. 2002;32:761–72. doi: 10.1002/1521-4141(200203)32:3<761::AID-IMMU761>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 37.Roifman CM. Human IL-2 receptor alpha chain deficiency. Pediatr Res. 2000;48:6–11. doi: 10.1203/00006450-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Brisslert M, Bokarewa M, Larsson P, Wing K, Collins LV, Tarkowski A. Phenotypic and functional characterization of human CD25+B cells. Immunology. 2006;117:548–57. doi: 10.1111/j.1365-2567.2006.02331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gattenlöhner S, Stühmer T, Leich E, Reinhard M, Etschmann B, Völker HU, et al. Specific detection of CD56 (NCAM) isoforms for the identification of aggressive malignant neoplasms with progressive development. Am J Pathol. 2009;174:1160–71. doi: 10.2353/ajpath.2009.080647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SE, Yagita H, et al. Activation of NK cell cytotoxicity. Mol Immunol. 2005;42:501–10. doi: 10.1016/j.molimm.2004.07.034. [DOI] [PubMed] [Google Scholar]


