Abstract
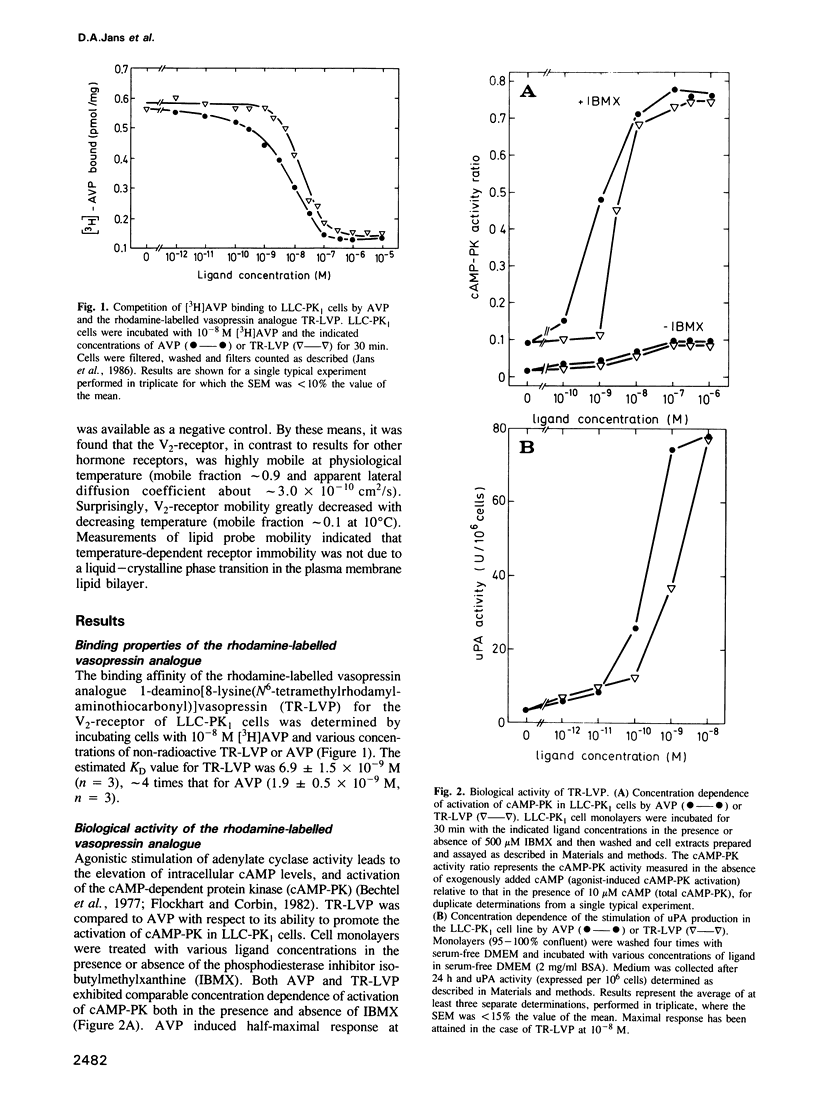
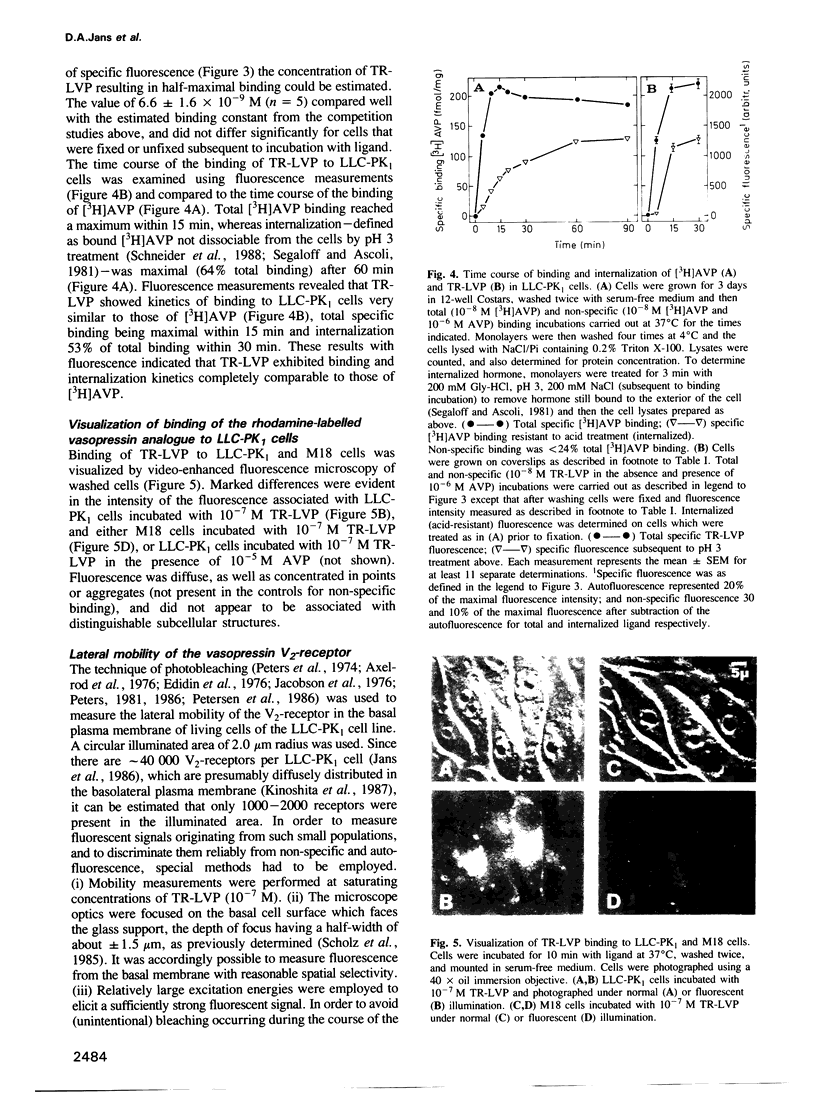
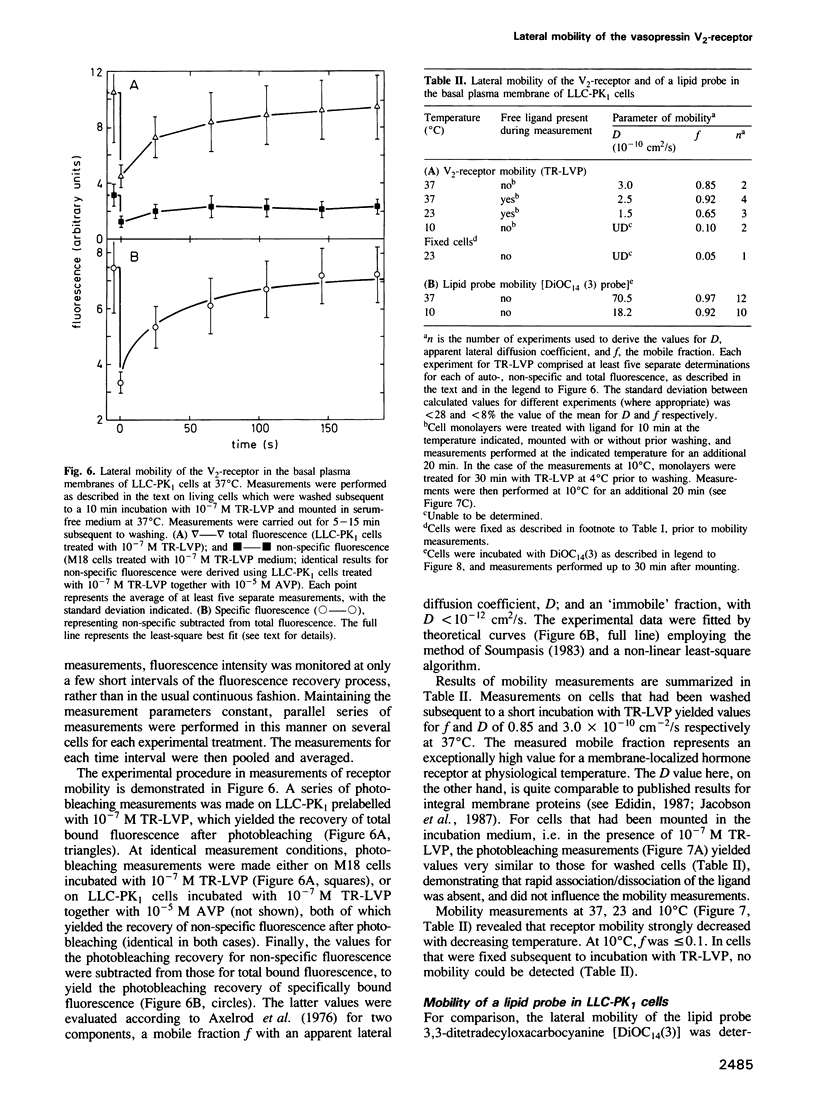
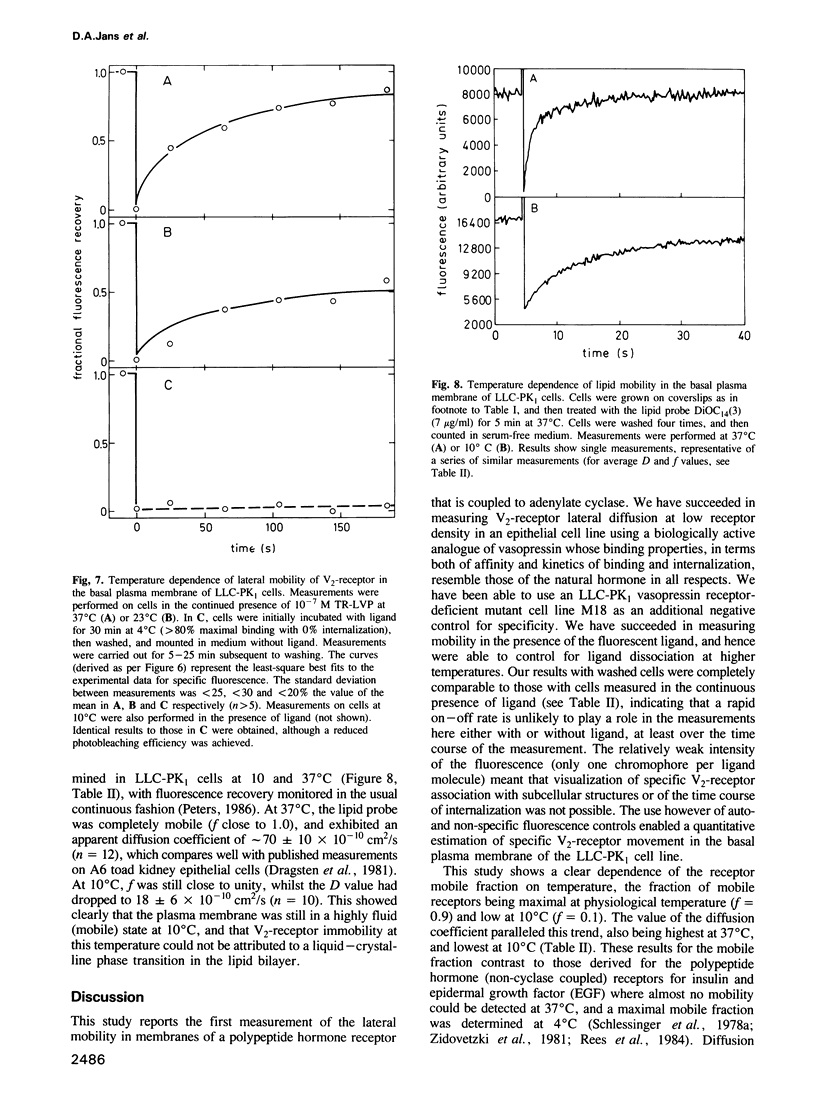
The lateral mobility of membrane-associated hormone receptors has been proposed to play an important role in signal transduction. Direct measurements, however, have shown that the receptors for insulin, epidermal growth factor and beta-adrenergic antagonists exhibit low mobility at physiological temperature. The present study, which represents the first report of lateral mobility of a polypeptide hormone receptor coupled to adenylate cyclase, yielded quite different results. The lateral mobility of the vasopressin renal-type (V2)-receptor was measured in the basal plasma membrane of cells of the LLC-PK1 porcine epithelial line, using the technique of fluorescence microphotolysis (photobleaching) and a rhodamine-labelled analogue of vasopressin. The analogue, 1-deamino[8-lysine(N6-tetramethylrhodamylaminothiocarbonyl)] vasopressin (TR-LVP) was synthesized and shown to have binding properties and biological activities very similar to those of Arg8-vasopressin (AVP). TR-LVP could be used to label specifically the V2-receptor of living LLC-PK1 cells, whereby LLC-PK1 cells incubated with TR-LVP in the presence of a 100-fold excess of AVP, or cells from the LLC-PK1 V2-receptor-deficient line M18 incubated with TR-LVP could be used as controls for non-specific binding. Using optical sectioning, specific receptor mobility could be measured both in the absence and presence of free TR-LVP. The V2-receptor was found to be largely mobile at 37 degrees C: the mobile fraction (f) was approximately 0.9, and the apparent lateral diffusion coefficient (D) approximately 3.0 X 10(-10) cm2/s. V2-receptor mobility greatly decreased with decreasing temperature: at 10 degrees C f was reduced to approximately 0.1.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel P. J., Beavo J. A., Krebs E. G. Purification and characterization of catalytic subunit of skeletal muscle adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Apr 25;252(8):2691–2697. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brandt D. R., Ross E. M. Catecholamine-stimulated GTPase cycle. Multiple sites of regulation by beta-adrenergic receptor and Mg2+ studied in reconstituted receptor-Gs vesicles. J Biol Chem. 1986 Feb 5;261(4):1656–1664. [PubMed] [Google Scholar]
- Buku A., Schwartz I. L., Gazis D., Ma C. L., Eggena P. Synthesis and biological activities of a fluorescent photoaffinity analog of vasopressin. Endocrinology. 1985 Jul;117(1):196–200. doi: 10.1210/endo-117-1-196. [DOI] [PubMed] [Google Scholar]
- Casey P. J., Gilman A. G. G protein involvement in receptor-effector coupling. J Biol Chem. 1988 Feb 25;263(6):2577–2580. [PubMed] [Google Scholar]
- Corbin J. D. Determination of the cAMP-dependent protein kinase activity ratio in intact tissues. Methods Enzymol. 1983;99:227–232. doi: 10.1016/0076-6879(83)99057-2. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- DeMeyts P., Bainco A. R., Roth J. Site-site interactions among insulin receptors. Characterization of the negative cooperativity. J Biol Chem. 1976 Apr 10;251(7):1877–1888. [PubMed] [Google Scholar]
- Derzko Z., Jacobson K. Comparative lateral diffusion of fluorescent lipid analogues in phospholipid multibilayers. Biochemistry. 1980 Dec 23;19(26):6050–6057. doi: 10.1021/bi00567a016. [DOI] [PubMed] [Google Scholar]
- Devis P. E., Grohol S. H., Taub M. Dibutyryl cyclic AMP resistant MDCK cells in serum free medium have reduced cyclic AMP dependent protein kinase activity and a diminished effect of PGE1 on differentiated function. J Cell Physiol. 1985 Oct;125(1):23–35. doi: 10.1002/jcp.1041250105. [DOI] [PubMed] [Google Scholar]
- Dousa T., Hechter O., Schwartz I. L., Walter R. Neurohypophyseal hormone-responsive adenylate cyclase from mammalian kidney. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1693–1697. doi: 10.1073/pnas.68.8.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragsten P. R., Blumenthal R., Handler J. S. Membrane asymmetry in epithelia: is the tight junction a barrier to diffusion in the plasma membrane? Nature. 1981 Dec 24;294(5843):718–722. doi: 10.1038/294718a0. [DOI] [PubMed] [Google Scholar]
- Edidin M., Zagyansky Y., Lardner T. J. Measurement of membrane protein lateral diffusion in single cells. Science. 1976 Feb 6;191(4226):466–468. doi: 10.1126/science.1246629. [DOI] [PubMed] [Google Scholar]
- Fahrenholz F., Boer R., Crause P., Fritzsch G., Grzonka Z. Interactions of vasopressin agonists and antagonists with membrane receptors. Eur J Pharmacol. 1984 Apr 13;100(1):47–58. doi: 10.1016/0014-2999(84)90314-5. [DOI] [PubMed] [Google Scholar]
- Fahrenholz F., Boer R., Crause P., Tóth M. V. Photoaffinity labelling of the renal V2 vasopressin receptor. Identification and enrichment of a vasopressin-binding subunit. Eur J Biochem. 1985 Nov 4;152(3):589–595. doi: 10.1111/j.1432-1033.1985.tb09236.x. [DOI] [PubMed] [Google Scholar]
- Flockhart D. A., Corbin J. D. Regulatory mechanisms in the control of protein kinases. CRC Crit Rev Biochem. 1982 Feb;12(2):133–186. doi: 10.3109/10409238209108705. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Burg M. B. Effect of vasopressin and cyclic AMP on permeability of isolated collecting tubules. Am J Physiol. 1966 Jul;211(1):255–259. doi: 10.1152/ajplegacy.1966.211.1.255. [DOI] [PubMed] [Google Scholar]
- Hargreaves W. R., Deamer D. W. Liposomes from ionic, single-chain amphiphiles. Biochemistry. 1978 Sep 5;17(18):3759–3768. doi: 10.1021/bi00611a014. [DOI] [PubMed] [Google Scholar]
- Henis Y. I., Hekman M., Elson E. L., Helmreich E. J. Lateral motion of beta receptors in membranes of cultured liver cells. Proc Natl Acad Sci U S A. 1982 May;79(9):2907–2911. doi: 10.1073/pnas.79.9.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R. N., Cherry W. R., Weaver G. W. The origin and characteristics of a pig kidney cell strain, LLC-PK. In Vitro. 1976 Oct;12(10):670–677. doi: 10.1007/BF02797469. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Ishihara A., Inman R. Lateral diffusion of proteins in membranes. Annu Rev Physiol. 1987;49:163–175. doi: 10.1146/annurev.ph.49.030187.001115. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Wu E., Poste G. Measurement of the translational mobility of concanavalin A in glycerol-saline solutions and on the cell surface by fluorescence recovery after photobleaching. Biochim Biophys Acta. 1976 Apr 16;433(1):215–222. doi: 10.1016/0005-2736(76)90189-9. [DOI] [PubMed] [Google Scholar]
- Jans D. A., Gajdas E. L., Dierks-Ventling C., Hemmings B. A., Fahrenholz F. Long-term stimulation of cAMP production in LLC-PK1 pig kidney epithelial cells by salmon calcitonin or a photoactivatable analogue of vasopressin. Biochim Biophys Acta. 1987 Oct 1;930(3):392–400. doi: 10.1016/0167-4889(87)90012-7. [DOI] [PubMed] [Google Scholar]
- Jans D. A., Resink T. J., Hemmings B. A. Complementation between LLC-PK1 mutants affected in polypeptide hormone-receptor function. Eur J Biochem. 1987 Feb 2;162(3):571–576. doi: 10.1111/j.1432-1033.1987.tb10677.x. [DOI] [PubMed] [Google Scholar]
- Jans D. A., Resink T. J., Hemmings B. A. Dependence of urokinase-type-plasminogen-activator induction on cyclic AMP-dependent protein kinase activation in LLC-PK1 cells. Biochem J. 1987 Apr 15;243(2):413–418. doi: 10.1042/bj2430413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans D. A., Resink T. J., Wilson L. E., Reich E., Hemmings B. A. Isolation of a mutant LLC-PK1 cell line defective in hormonal responsiveness. A pleiotropic lesion in receptor function. Eur J Biochem. 1986 Oct 15;160(2):407–412. doi: 10.1111/j.1432-1033.1986.tb09986.x. [DOI] [PubMed] [Google Scholar]
- Kahn C. R. Membrane receptors for hormones and neurotransmitters. J Cell Biol. 1976 Aug;70(2 Pt 1):261–286. doi: 10.1083/jcb.70.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely S. L., Corbin J. D., Park C. R. Regulation of adenosine 3:5-monophosphate-dependent protein kinase. J Biol Chem. 1975 Jul 10;250(13):4832–4840. [PubMed] [Google Scholar]
- Kinoshita Y., Fukase M., Kubota T., Fujita T. Basolaterally-localized hormone responsiveness of reconstructed monolayer from cultured kidney cells. Horm Metab Res. 1987 Aug;19(8):393–394. doi: 10.1055/s-2007-1011834. [DOI] [PubMed] [Google Scholar]
- Lynch C. J., Morbach L., Blackmore P. F., Exton J. H. Alpha-subunits of Ns are released from the plasma membrane following cholera toxin activation. FEBS Lett. 1986 May 12;200(2):333–336. doi: 10.1016/0014-5793(86)81163-2. [DOI] [PubMed] [Google Scholar]
- Nagamine Y., Sudol M., Reich E. Hormonal regulation of plasminogen activator mRNA production in porcine kidney cells. Cell. 1983 Apr;32(4):1181–1190. doi: 10.1016/0092-8674(83)90301-x. [DOI] [PubMed] [Google Scholar]
- Orly J., Schramm M. Coupling of catecholamine receptor from one cell with adenylate cyclase from another cell by cell fusion. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4410–4414. doi: 10.1073/pnas.73.12.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. Fluorescence microphotolysis to measure nucleocytoplasmic transport and intracellular mobility. Biochim Biophys Acta. 1986 Dec 22;864(3-4):305–359. doi: 10.1016/0304-4157(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Peters R. Lateral mobility of proteins and lipids in the red cell membrane and the activation of adenylate cyclase by beta-adrenergic receptors. FEBS Lett. 1988 Jul 4;234(1):1–7. doi: 10.1016/0014-5793(88)81290-0. [DOI] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Peters R. Translational diffusion in the plasma membrane of single cells as studied by fluorescence microphotolysis. Cell Biol Int Rep. 1981 Aug;5(8):733–760. doi: 10.1016/0309-1651(81)90231-9. [DOI] [PubMed] [Google Scholar]
- Ransnäs L. A., Insel P. A. Subunit dissociation is the mechanism for hormonal activation of the Gs protein in native membranes. J Biol Chem. 1988 Nov 25;263(33):17239–17242. [PubMed] [Google Scholar]
- Rees A. R., Gregoriou M., Johnson P., Garland P. B. High affinity epidermal growth factor receptors on the surface of A431 cells have restricted lateral diffusion. EMBO J. 1984 Aug;3(8):1843–1847. doi: 10.1002/j.1460-2075.1984.tb02057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Axelrod D., Koppel D. E., Webb W. W., Elson E. L. Lateral transport of a lipid probe and labeled proteins on a cell membrane. Science. 1977 Jan 21;195(4275):307–309. doi: 10.1126/science.556653. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Cuatrecasas P., Willingham M. C., Pastan I. Quantitative determination of the lateral diffusion coefficients of the hormone-receptor complexes of insulin and epidermal growth factor on the plasma membrane of cultured fibroblasts. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5353–5357. doi: 10.1073/pnas.75.11.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. Signal transduction by allosteric receptor oligomerization. Trends Biochem Sci. 1988 Nov;13(11):443–447. doi: 10.1016/0968-0004(88)90219-8. [DOI] [PubMed] [Google Scholar]
- Schneider H. G., Raue F., Zink A., Koppold A., Ziegler R. Down-regulation of calcitonin receptors in T47D cells by internalization of calcitonin-receptor complexes. Mol Cell Endocrinol. 1988 Jul;58(1):9–15. doi: 10.1016/0303-7207(88)90048-2. [DOI] [PubMed] [Google Scholar]
- Scholz M., Schulten K., Peters R. Single-cell flux measurement by continuous fluorescence microphotolysis. Eur Biophys J. 1985;13(1):37–44. doi: 10.1007/BF00266308. [DOI] [PubMed] [Google Scholar]
- Scott J. D., Glaccum M. B., Fischer E. H., Krebs E. G. Primary-structure requirements for inhibition by the heat-stable inhibitor of the cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1613–1616. doi: 10.1073/pnas.83.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segaloff D. L., Ascoli M. Removal of the surface-bound human choriogonadotropin results in the cessation of hormonal responses in cultured Leydig tumor cells. J Biol Chem. 1981 Nov 25;256(22):11420–11423. [PubMed] [Google Scholar]
- Skorecki K. L., Verkman A. S., Ausiello D. A. Vasopressin receptor-adenylate cyclase interactions. Studies in an intact cultured renal epithelial cell line (LLC-PK1). Miner Electrolyte Metab. 1986;12(1):64–70. [PubMed] [Google Scholar]
- Soderling T. R., Corbin J. D., Park C. R. Techniques for the study of protein kinase activation in intact cells. Methods Enzymol. 1974;38:358–367. doi: 10.1016/0076-6879(74)38052-4. [DOI] [PubMed] [Google Scholar]
- Soumpasis D. M. Theoretical analysis of fluorescence photobleaching recovery experiments. Biophys J. 1983 Jan;41(1):95–97. doi: 10.1016/S0006-3495(83)84410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidovetzki R., Yarden Y., Schlessinger J., Jovin T. M. Rotational diffusion of epidermal growth factor complexed to cell surface receptors reflects rapid microaggregation and endocytosis of occupied receptors. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6981–6985. doi: 10.1073/pnas.78.11.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]



