Abstract
Functional roles of the cortical backward signal in long-term memory formation were studied in monkeys performing a visual pair-association task. Before the monkeys learned the task, the anterior commissure was transected, disconnecting the anterior temporal cortex of each hemisphere. After training with 12 pairs of pictures, single units were recorded from the inferotemporal cortex of the monkeys as the control. By injecting a grid of ibotenic acid, we unilaterally lesioned the entorhinal and perirhinal cortex, which provides massive direct and indirect backward projections ipsilaterally to the inferotemporal cortex. After the lesion, the monkeys fixated the cue stimulus normally, relearned the preoperatively learned set (set A), and learned a new set (set B) of paired associates. Then, single units were recorded from the same area as for the prelesion control. We found that (i) in spite of the lesion, the sampled neurons responded strongly and selectively to both the set A and set B patterns and (ii) the paired associates elicited significantly correlated responses in the control neurons before the lesion but not in the cells tested after the lesion, either for set A or set B stimuli. We conclude that the ability of inferotemporal neurons to represent association between picture pairs was lost after the lesion of entorhinal and perirhinal cortex, most likely through disruption of backward neural signals to the inferotemporal neurons, while the ability of the neurons to respond to a particular visual stimulus was left intact.
Full text
PDF

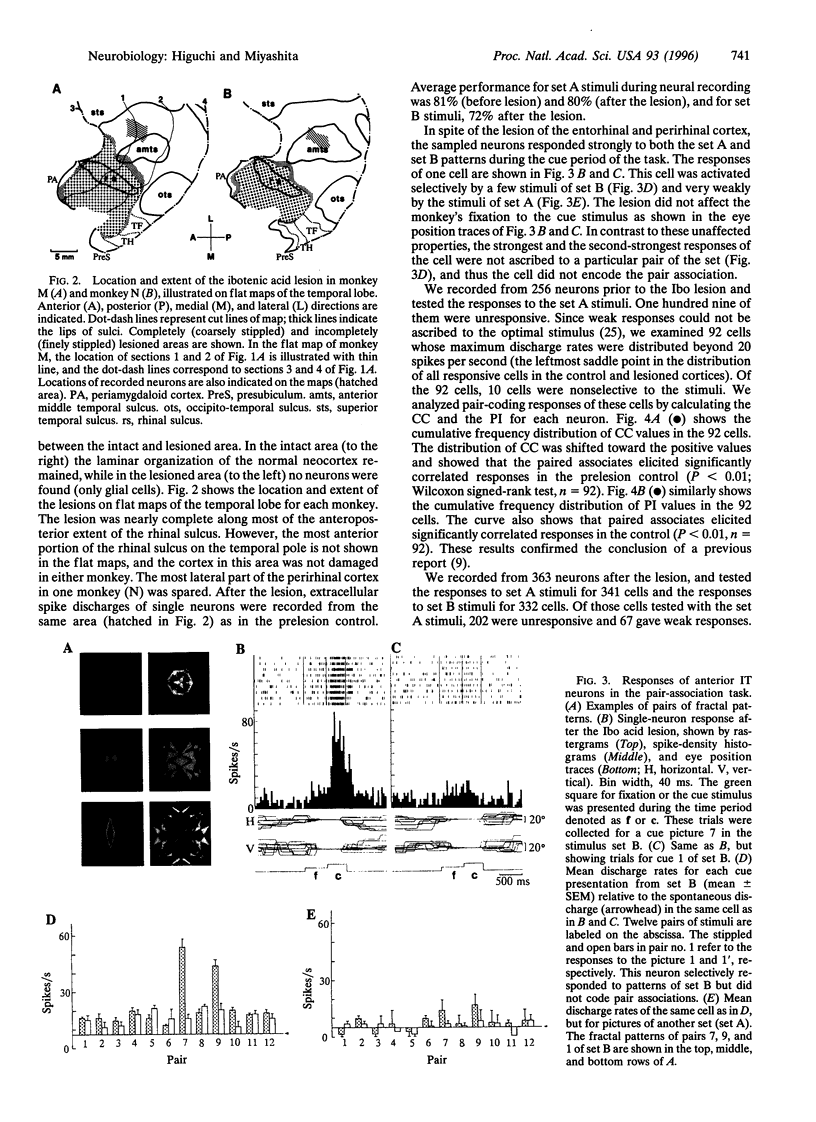
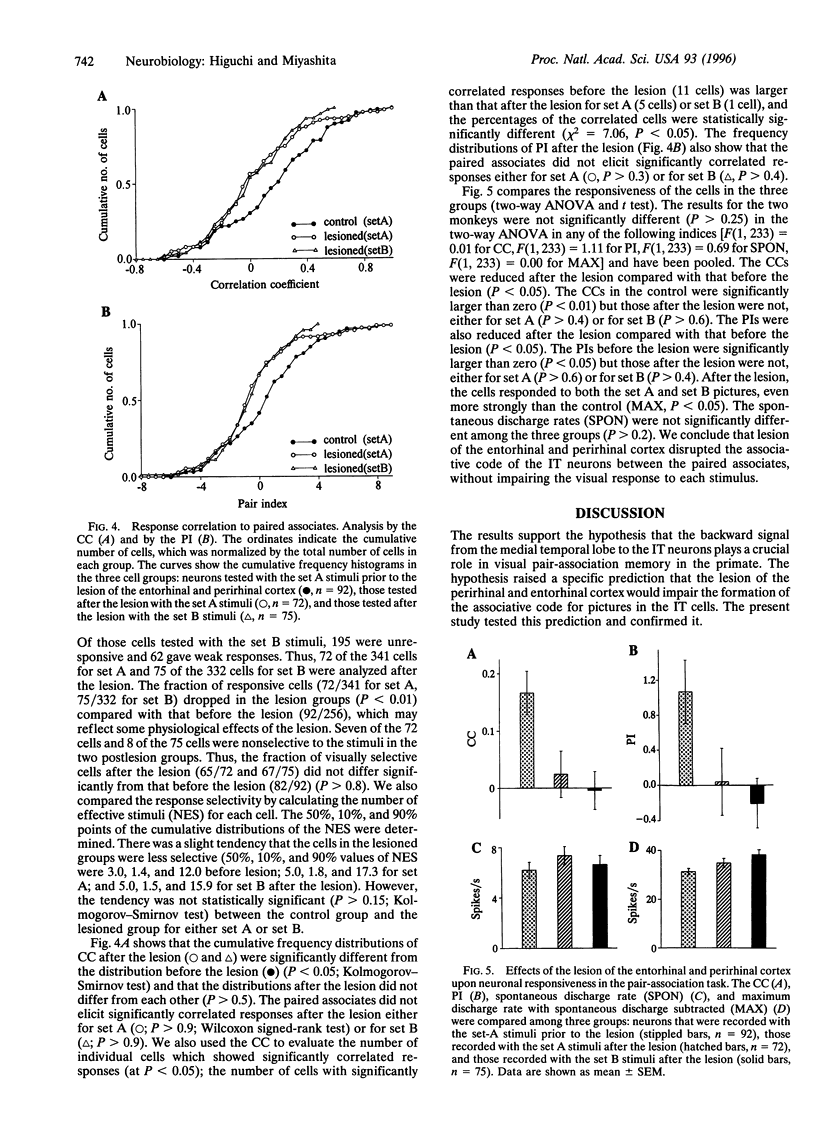

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Demeter S., Rosene D. L., Van Hoesen G. W. Fields of origin and pathways of the interhemispheric commissures in the temporal lobe of macaques. J Comp Neurol. 1990 Dec 1;302(1):29–53. doi: 10.1002/cne.903020104. [DOI] [PubMed] [Google Scholar]
- Desimone R., Albright T. D., Gross C. G., Bruce C. Stimulus-selective properties of inferior temporal neurons in the macaque. J Neurosci. 1984 Aug;4(8):2051–2062. doi: 10.1523/JNEUROSCI.04-08-02051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C. G., Bender D. B., Mishkin M. Contributions of the corpus callosum and the anterior commissure to visual activation of inferior temporal neurons. Brain Res. 1977 Aug 12;131(2):227–239. doi: 10.1016/0006-8993(77)90517-0. [DOI] [PubMed] [Google Scholar]
- Hess D. T., Merker B. H. Technical modifications of Gallyas' silver stain for myelin. J Neurosci Methods. 1983 May;8(1):95–97. doi: 10.1016/0165-0270(83)90055-9. [DOI] [PubMed] [Google Scholar]
- Miyashita Y., Chang H. S. Neuronal correlate of pictorial short-term memory in the primate temporal cortex. Nature. 1988 Jan 7;331(6151):68–70. doi: 10.1038/331068a0. [DOI] [PubMed] [Google Scholar]
- Miyashita Y., Date A., Okuno H. Configurational encoding of complex visual forms by single neurons of monkey temporal cortex. Neuropsychologia. 1993 Oct;31(10):1119–1131. doi: 10.1016/0028-3932(93)90036-y. [DOI] [PubMed] [Google Scholar]
- Miyashita Y., Higuchi S., Sakai K., Masui N. Generation of fractal patterns for probing the visual memory. Neurosci Res. 1991 Oct;12(1):307–311. doi: 10.1016/0168-0102(91)90121-e. [DOI] [PubMed] [Google Scholar]
- Miyashita Y. Inferior temporal cortex: where visual perception meets memory. Annu Rev Neurosci. 1993;16:245–263. doi: 10.1146/annurev.ne.16.030193.001333. [DOI] [PubMed] [Google Scholar]
- Miyashita Y. Neuronal correlate of visual associative long-term memory in the primate temporal cortex. Nature. 1988 Oct 27;335(6193):817–820. doi: 10.1038/335817a0. [DOI] [PubMed] [Google Scholar]
- Miyashita Y., Rolls E. T., Cahusac P. M., Niki H., Feigenbaum J. D. Activity of hippocampal formation neurons in the monkey related to a conditional spatial response task. J Neurophysiol. 1989 Mar;61(3):669–678. doi: 10.1152/jn.1989.61.3.669. [DOI] [PubMed] [Google Scholar]
- Murray E. A., Gaffan D., Mishkin M. Neural substrates of visual stimulus-stimulus association in rhesus monkeys. J Neurosci. 1993 Oct;13(10):4549–4561. doi: 10.1523/JNEUROSCI.13-10-04549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome W. T., Wurtz R. H., Dürsteler M. R., Mikami A. Punctate chemical lesions of striate cortex in the macaque monkey: effect on visually guided saccades. Exp Brain Res. 1985;58(2):392–399. doi: 10.1007/BF00235320. [DOI] [PubMed] [Google Scholar]
- Pandya D. N., Karol E. A., Lele P. P. The distribution of the anterior commissure in the squirrel monkey. Brain Res. 1973 Jan 15;49(1):177–180. doi: 10.1016/0006-8993(73)90409-5. [DOI] [PubMed] [Google Scholar]
- Petrides M. Deficits on conditional associative-learning tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1985;23(5):601–614. doi: 10.1016/0028-3932(85)90062-4. [DOI] [PubMed] [Google Scholar]
- Rolls E. T., Baylis G. C. Size and contrast have only small effects on the responses to faces of neurons in the cortex of the superior temporal sulcus of the monkey. Exp Brain Res. 1986;65(1):38–48. doi: 10.1007/BF00243828. [DOI] [PubMed] [Google Scholar]
- Sakai K., Miyashita Y. Neural organization for the long-term memory of paired associates. Nature. 1991 Nov 14;354(6349):152–155. doi: 10.1038/354152a0. [DOI] [PubMed] [Google Scholar]
- Sakai K., Naya Y., Miyashita Y. Neuronal tuning and associative mechanisms in form representation. Learn Mem. 1994 Jul-Aug;1(2):83–105. [PubMed] [Google Scholar]
- Shiwa T. Corticocortical projections to the monkey temporal lobe with particular reference to the visual processing pathways. Arch Ital Biol. 1987 Apr;125(2):139–154. [PubMed] [Google Scholar]
- Squire L. R., Zola-Morgan S. The medial temporal lobe memory system. Science. 1991 Sep 20;253(5026):1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Suzuki W. A., Amaral D. G. Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. J Neurosci. 1994 Mar;14(3 Pt 2):1856–1877. doi: 10.1523/JNEUROSCI.14-03-01856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D. C., Anderson C. H., Felleman D. J. Information processing in the primate visual system: an integrated systems perspective. Science. 1992 Jan 24;255(5043):419–423. doi: 10.1126/science.1734518. [DOI] [PubMed] [Google Scholar]
- Van Essen D. C., Maunsell J. H. Two-dimensional maps of the cerebral cortex. J Comp Neurol. 1980 May 15;191(2):255–281. doi: 10.1002/cne.901910208. [DOI] [PubMed] [Google Scholar]
- Webster M. J., Ungerleider L. G., Bachevalier J. Connections of inferior temporal areas TE and TEO with medial temporal-lobe structures in infant and adult monkeys. J Neurosci. 1991 Apr;11(4):1095–1116. doi: 10.1523/JNEUROSCI.11-04-01095.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S. M. Comparison of the cortical degeneration in the visual regions of the temporal lobe of the monkey following section of the anterior commissure and the splenium. J Comp Neurol. 1973 Mar 15;148(2):167–175. doi: 10.1002/cne.901480204. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S. M., Squire L. R. The primate hippocampal formation: evidence for a time-limited role in memory storage. Science. 1990 Oct 12;250(4978):288–290. doi: 10.1126/science.2218534. [DOI] [PubMed] [Google Scholar]