Abstract
Background
There is little information on the association of the APOEe4 allele and AD risk in African populations. In previous analyses from the Indianapolis-Ibadan dementia project, we have reported that APOE ε4 increased the risk for Alzheimer’s disease (AD) in African Americans but not in Yoruba. This study represents a replication of this earlier work using enriched cohorts and extending the analysis to include cognitive decline.
Methods
In this longitudinal study of two community dwelling cohorts of elderly Yoruba and African Americans, APOE genotyping was conducted from blood samples taken on or before 2001 (1,871 African Americans & 2,200 Yoruba). Mean follow up time was 8.5 years for African Americans and 8.8 years for Yoruba. The effects of heterozygosity or homozygosity of ε4 and of the possession of e4 on time to incident AD and on cognitive decline were determined using Cox’s proportional hazards regression and mixed effects models.
Results
After adjusting for covariates, one or two copies of the APOE ε4 allele were significant risk factors for incident AD (p < 0.0001) and cognitive decline in the African-American population (p < 0001). In the Yoruba, only homozygosity for APOE ε4 was a significant risk factor for AD (p = 0.0002) but not for cognitive decline (p = 0.2346), however, possession of an e4 allele was significant for both incident AD (p = 0.0489) and cognitive decline (p = 0.0425).
Conclusions
In this large longitudinal comparative study, APOE ε4 had a significant, but weaker, effect on incident AD and on cognitive decline in Yoruba than in African Americans. The reasons for these differences remain unclear.
Keywords: Alzheimer disease, cognitive impairment, APOE ε4, African Americans, Yoruba
Introduction
The APOE ε4 allele is a major risk factor for Alzheimer Disease (AD) and dementia in most populations (Farrer et al., 1997; Chuang et al., 2010; Ohara et al., 2011). Early studies with African American cohorts reported inconsistent results (Farrer et al., 1997; Sahota et al., 1997). However, a recent meta-analysis has reported that the ε4 allele is a risk factor for AD in African American populations with an odds ratio (OR) of 2.31 (Reitz et al., 2013).
Few studies have examined the role of APOE in populations with African ancestry in non-African countries other than the US and fewer still in African populations. One study reported the ε4 allele was significantly associated with AD in African Caribbean people but the magnitude of the association was small (Stewart et al., 2001) and a preliminary analysis of cross sectional data from Kenya suggested that ε4 was not a risk factor for AD (Chen et al., 2010).
The role of the APOE ε4 allele on cognitive decline has also been analyzed in samples from African American cohorts (Kuller et al., 1998; Knopman et al., 2009; Sawyer et al., 2009). Two studies have reported that ε4 is a risk factor for cognitive decline in African Americans with no discernible differences in effect size when compared with Caucasian populations (Knopman et al., 2009; Sawyer et al., 2009). There are no reports of the effects of ε4 on cognitive decline in African cohorts. As part of the Indianapolis-Ibadan dementia project, we have previously reported that possession of the ε4 allele was associated with a higher risk for AD for African Americans (Murrell et al., 2006) whereas ε4 was not associated with increased risk for AD in Yoruba (Gureje et al., 2006). However, the sample size was modest (n = 582 for Yoruba, n = 480 for African Americans) and the analyses included both prevalent and incident AD patients. With additional enrichment cohorts and longer follow-up, we now re-examine this outcome in both the Indianapolis and Ibadan sample, which includes a larger number of participants with incident AD. We also extend the analysis to test whether APOE ε4 is associated with cognitive decline in both cohorts.
Methods
Study participants
Since 1992, we have been conducting a comparative, community-based epidemiologic study of prevalence, incidence, and risk factors for AD in populations of African origin: elderly African Americans in Indianapolis, Indiana, and Yoruba in Ibadan, Nigeria. The elderly Yoruba were residents of the Idikan area and adjacent wards in the more ancient parts of the city of Ibadan. Residents of the wards are typically small traders and craftsmen. Food comes from the local markets that surround the wards and is mostly locally grown.
A detailed description of the construction of the original cohorts from 1992 has been previously reported (Hendrie et al., 2001). In 2001, new study participants of 70 years and over were recruited. Recruitment in Ibadan for both the baseline and enrichment samples involved a total population survey carried out by means of screening in a geographically defined area identifying participants 65 years and over at baseline and 70 years and over in 2001. In Indianapolis, the 65 years and older self-identified African American baseline cohort was constructed from a random sample based upon the 1990 census data with addresses being provided by the Indianapolis Water Company. For the enrichment sample, the project enrolled additional community dwelling participants randomly selected from Medicare records self-identified as African American and at least 70 years of age. The enriched sample included in Indianapolis, 1,893 participants who were added to the 749 survivors of the original cohort and in Ibadan, 1,939 participants were added to the 903 survivors. The surviving 1992 cohorts and the newly recruited 2001 cohorts were similar in basic demographics, age, and gender (Hendrie et al., 2013). The institutional review boards of Indiana University School of Medicine and University of Ibadan approved the study.
Study design
A two-stage design was used at each evaluation with in-home cognitive and functional evaluations for all participants followed by a full diagnostic workup of selected participants based on the performance of the first stage cognitive tests (Hendrie et al., 2001). Baseline evaluation was in 1992 for the original cohorts and in 2001 for the enrichment cohorts with follow ups occurring in approximately two to three year intervals (1994, 1998, 2001, 2004, 2007, 2009, and only a screening conducted in 2011).
Cognitive assessment
The Community Screening Interview for Dementia (CSID) was used during the first stage in-home evaluation with a cognitive assessment of the study participant and an interview with a close relative evaluating the daily functioning of the participant. The CSID was developed by our group specifically for use in comparative epidemiological studies of dementia in culturally disparate populations (Hall et al., 2000). The cognitive assessment in CSID evaluates multiple cognitive domains (language, attention and calculation, memory, orientation, praxis, and comprehension and motor response). For the analysis on cognitive decline, we used a cognitive score that incorporated all cognitive items from the screening exam. CSID scores range from 0 to 80, with higher scores indicating better cognitive functioning. We also investigated the CSID memory sub-score which ranged from 0–29.
Clinical evaluation
Clinical evaluations included (1) a cognitive assessment adapted from the Consortium to Establish a Registry of Alzheimer’s Disease (CERAD)(Morris et al., 1989); (2) a standardized clinician examination that included neurologic and physical exams, and functional status review (the Clinician Home-based Interview to assess Function, CHIF) (Hendrie et al., 2006); and (3) a structured interview recording symptoms and functional status with a close relative adapted from the Cambridge Examination for Mental Disorders of the Elderly informant interview (CAMDEX) (Hendrie et al., 1988). Following the second stage of evaluation, participants were diagnosed as having normal cognitive function or dementia. Diagnosis was made in a consensus diagnostic conference of clinicians reviewing the cognitive test results, the clinician’s assessment, the informant interview, and available medical records. Dementia was diagnosed with both the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition (DSM-III-R)(American Psychiatric Association, 1987) and International Classification of Diseases, 10th Revision (ICD-10) (American Pychiatric Association Press, 1992) criteria. AD was diagnosed using criteria proposed by NINCDS/ADRDA (McKhann et al., 1984).
Other information
The design of this non-cognitive portion of the questionnaire was the result of extensive discussion between the Indianapolis/Ibadan investigators to identify questions which could be used harmoniously at both sites and involved a review of source documents such as CAMDEX (Hendrie et al., 1988) and CERAD (Morris et al., 1989). Demographic information including age, sex and education (years of education for African Americans, whether or not they attended school for the Yoruba) were available on all study participants. Information was also collected on whether the participant ever consumed alcohol or smoked regularly. In addition, medical conditions that may affect cognitive function were collected at each of the evaluation times. In particular, medical history of coronary heart disease (CHD), cancer, diabetes, heart attack, hypertension, Parkinson’s disease, stroke, and depression were collected from self or informant reports as affirmative answers to whether the participants had ever been diagnosed or treated for these diseases.
Blood samples
DNA was extracted from blood spots collected on filter paper and from fresh blood using standard procedures from participants at both sites who consented to the procedure at or before 2001. APOE genotypes were determined by Hhal digestion of amplified products (Hixson and Vernier, 1990). In the African Americans, 1919 (48.2%) out of 3,983 participants without dementia at baseline had APOE genotyped. In the Yoruba, 2,226 (51.1%) out of 4,353 participants without dementia at baseline had APOE genotyped.
Analysis
The primary study outcome was time from baseline to incident AD. Participants with prevalent dementia were excluded from the analyses. Those who were not diagnosed with dementia were censored on the last evaluation date. Since our follow-up evaluations were conducted at regularly scheduled short intervals (2 to 3 years), right censoring can be used instead of interval censoring (Leffondre et al., 2013). Time of follow-up was used as the outcome variable since previous studies have shown that Cox’s models using time of follow-up offer more robust results than chronological age in cohort studies (Chalise et al., 2011).
Participants were divided into three APOE genotype groups: ε4 homozygotes, ε4 heterozygotes, and non-carriers. A separate analysis of e4 carriers and non-carriers was also conducted. Also, t-tests and χ2 tests were used to compare continuous and categorical variables between those with incident AD and the cognitively normal. Univariate Cox proportional hazards regression models were used to identify demographic, life style, and illness variables significantly associated with incident AD at the α = 0.15 level within each site. These were included in multivariable models where forward and backwards selection modeling techniques were employed to identify a final parsimonious model with APOE genotype as the independent variable and covariates significant at the α = 0.05 level. Hazard ratios (HR), 95% confidence intervals (CI) and p-values are reported from the final models. Kaplan–Meier estimator and log-rank tests were used to estimate and compare the distributions of time to incident AD among the APOE genotype groups in each cohort.
Mixed effects models on repeated CSID scores over time were used to determine the effect of APOE genotype on cognitive decline for each site. An unstructured variance-covariance matrix was specified. Interactions between APOE and time since baseline were included in each model, where significant interaction would indicate differences in slope, i.e. change over time, by APOE status. Covariates identified in the Cox’s model on incident AD were also included in the mixed effects models on cognitive decline.
To determine whether our results were subject to selection bias due to the lack of APOE genotypes, we compared demographic variables between participants included in the analyses and those without APOE genotypes using t-tests and χ2 tests.
Results
There were 1,919 African Americans and 2,226 Yoruba without dementia at baseline who had APOE genotyped. We excluded 48 African Americans and 26 Yoruba participants who had incident non-AD dementia from the analysis. Incident AD was diagnosed in 182 (9.7%) African Americans and 1,689 (90.3%) participants were determined to have normal cognition. Incident AD was diagnosed in 173 (7.9%) Yoruba participants and 2,027 (92.1%) were determined to have normal cognition. Median follow-up was 8.5 years for the African Americans and 8.8 years for the Yoruba.
Table 1 compares the baseline characteristics of the participants with incident AD with the participants with normal cognition within each cohort. For the African American cohort, the participants with incident AD were significantly more likely to possess at least one copy of the APOE ε4 allele (p < 0.0001), be older (p = 0.0002), be less educated (p < 0.0001), and be significantly more likely to come from the original cohort (p < 0.0001). They were also significantly less likely to report having had diabetes (p = 0.0089) and using alcohol (p = 0.0049).
Table 1.
Baseline characteristics of African-American and Yoruba participants with incident AD and normal cognition
| VARIABLE NAME | AFRICAN AMERICANS
|
YORUBA
|
||||
|---|---|---|---|---|---|---|
| NORMAL COGNITION (n = 1689) | INCIDENT AD (n = 182) | P-VALUE | NORMAL COGNITION (n = 2027) | INCIDENT AD (n = 173) | P-VALUE | |
| Number of APOE ε4 alleles, n (%) | <.0001 | 0.0537 | ||||
| 0 | 1128 (66.8%) | 88 (48.4%) | . | 1250 (61.7%) | 95 (54.9%) | . |
| 1 | 503 (29.8%) | 80 (44.0%) | . | 685 (33.8%) | 64 (37.0%) | . |
| 2 | 58 (3.4%) | 14 (7.7%) | . | 92 (4.5%) | 14 (8.1%) | . |
| Age at baseline, mean±sd | 75.52 ± 5.85 | 77.47 ± 6.69 | 0.0002 | 72.99 ± 6.26 | 75.72 ± 8.79 | <.0001 |
| Years of education (Indianapolis) mean±sd /Attended School (Ibadan) n (%) | 11.02 ± 2.78 | 9.53 ± 3.37 | <.0001 | 308 (15.2%) | 14 (8.1%) | 0.0112 |
| Female gender, n (%) | 1132 (67.0%) | 131 (72.0%) | 0.1750 | 1307 (64.5%) | 142 (82.1%) | <.0001 |
| From the 1992 Cohort, n (%) | 520 (30.8%) | 125 (68.7%) | <.0001 | 1109(54.7%) | 138 (79.8%) | <.0001 |
| Angina, n (%) | 193 (11.5%) | 28 (15.4%) | 0.1241 | 156 (7.7%) | 12 (6.9%) | 0.7180 |
| Anxiety, n (%) | 244 (14.5%) | 35 (19.3%) | 0.0854 | 31 (1.5%) | 1 (0.6%) | 0.3158 |
| Depression, n (%) | 165 (9.8%) | 15 (8.3%) | 0.5195 | 286 (14.1%) | 27 (15.6%) | 0.5884 |
| Family history of dementia, n (%) | 233 (14.0%) | 33 (18.2%) | 0.1239 | 48 (2.4%) | 3 (1.7%) | 0.5919 |
| Diabetes, n (%) | 467 (27.7%) | 34 (18.7%) | 0.0089 | 47 (2.3%) | 2 (1.2%) | 0.3199 |
| Head Injury, n (%) | 153 (9.1%) | 10 (5.5%) | 0.1006 | 64 (3.2%) | 3 (1.7%) | 0.2957 |
| Heart Attack, n (%) | 227 (13.6%) | 21 (11.5%) | 0.4430 | 292 (14.4%) | 27 (15.6%) | 0.6666 |
| Hypertension, n (%) | 1220 (73.0%) | 124 (68.1%) | 0.1654 | 469 (23.2%) | 33 (19.2%) | 0.2283 |
| Parkinsons Disease, n (%) | 11 (0.7%) | 0 (0.0%) | 0.2743 | 89 (4.4%) | 6 (3.5%) | 0.5667 |
| Stroke, n (%) | 232 (13.8%) | 18 (9.9%) | 0.1505 | 30 (1.5%) | 1 (0.6%) | 0.3337 |
| Alcohol Use, n (%) | 615 (38.5%) | 49 (27.7%) | 0.0049 | 670 (33.4%) | 30 (17.6%) | <.0001 |
| Smoking History, n (%) | 997 (59.2%) | 95 (52.5%) | 0.0813 | 629 (31.0%) | 49 (28.3%) | 0.4591 |
| Cancer, n (%) | 253 (15.1%) | 23 (12.6%) | 0.3726 | 18 (0.9%) | 3 (1.7%) | 0.2719 |
| Heart Problems, n (%) | 509 (30.2%) | 44 (24.2%) | 0.0931 | 383 (18.9%) | 3 (19.7%) | 0.8070 |
For the Yoruba cohort, the participants with incident AD were marginally more likely to possess at least one copy of APOE ε4 (p = 0.0537), significantly more likely to be older (p < 0.0001), have no education (p = 0.0112), be female (p < 0.0001), and be from the original cohort (p < 0.0001). They were also significantly less likely to report alcohol use (p < 0.0001).
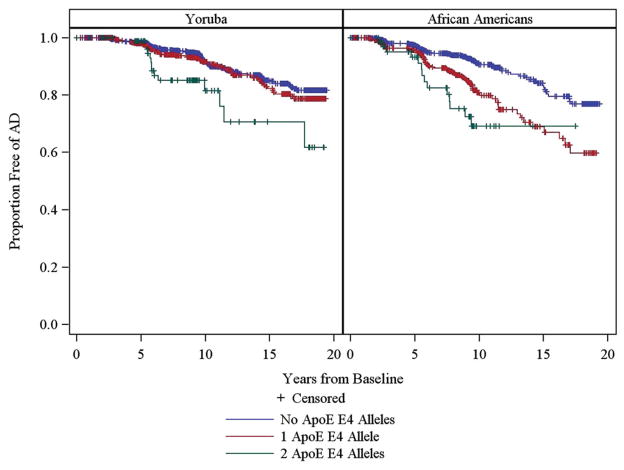
Figure 1 shows the Kaplan-Meier estimates of the survival function on time to AD for each cohort based upon the APOE genotypes. Log-rank tests found a significant difference between the three APOE groups in both the African Americans (p < 0.0001) and in the Yoruba (p = 0.0046).
Figure 1.
Kaplan-Meier survival estimates for time to incident AD.
Table 2 shows the results from the final Cox proportional hazards regression model on incident AD for both sites based upon number of APOE ε4 alleles. In the African Americans, after adjusting for years of education, baseline age, and cohort status, both APOE ε4 homozygotes and heterozygotes had significant increased risk of AD compared to non-carriers (p < 0.0001). In the Yoruba, those participants homozygous for the ε4 allele had significantly increased risk for AD compared to non-carriers after adjusting for age, gender, and history of smoking (HR = 2.95, p = 0.0002). Notably, there was a non-significant increase in risk for AD among those heterozygous for the ε4 allele as compared with those who did not have an ε4 allele (p = 0.2362). When APOE ε4 carrier status was used in the Cox models to compare the presence (1 or 2 copies) vs. absence of the APOE ε4 allele, the presence of the ε4 allele significantly increased the risk for AD for the African Americans (HR 2.47; 95% CI 1.84–3.32; p < 0.0001) and also for the Yoruba (HR 1.35; 95% CI 1.00–1.83; p = 0.0489).
Table 2.
Results from final Cox’s proportional hazards models on incident AD risk for the African American and Yoruba cohorts
| AFRICAN AMERICANS
|
YORUBA
|
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-VALUE | HR | 95% CI | P-VALUE | |
| Number of APOE ε4 alleles | <0.0001 | 0.0009 | ||||
| 2 vs. 0 | 4.12 | 2.33–7.28 | <0.0001 | 2.95 | 1.67–5.19 | 0.0002 |
| 1 vs. 0 | 2.31 | 1.70–3.14 | <0.0001 | 1.21 | 0.88–1.67 | 0.2362 |
| Age a baseline | 1.13 | 1.11–1.16 | <0.0001 | 1.10 | 1.08–1.12 | <0.0001 |
| Years of education | 0.690 | 0.85–0.94 | <0.0001 | N.I. | N.I. | N.I. |
| Original cohort | 2.55 | 1.79–3.61 | <0.0001 | N.I. | N.I. | N.I. |
| Female gender | N.I. | N.I. | N.I. | 3.12 | 2.03–4.80 | <0.0001 |
| History of smoking | N.I. | N.I. | N.I. | 1.60 | 1.10–2.31 | 0.0134 |
N.I.: not included in final model
Table 3 shows the results from the mixed effects models on cognitive decline for the two cohorts for the number of APOE ε4 alleles after adjusting for the covariates used in the Cox models. For the African Americans, there was a significant interaction between time since baseline and the APOE groups (p < 0.0001) indicating significantly more decline for both the heterozygote and homozygote groups than non-carriers (p < 0.001 for both). For the Yoruba, the interaction was not significant (p>0.05), indicating that the APOE ε4 allele does not affect cognitive decline using this model. When APOE ε4 carrier status was used in the model for cognitive decline, ε4 carriers had significantly increased risk for cognitive decline in both the African Americans (p = 0.0001) and in the Yoruba (p = 0.0425).
Table 3.
Results from mixed effects models on cognitive decline* for both cohorts
| AFRICAN AMERICANS
|
YORUBA
|
|||||
|---|---|---|---|---|---|---|
| PARAMETER ESTIMATE | STANDARD ERROR | P-VALUE | PARAMETER ESTIMATE | STANDARD ERROR | P-VALUE | |
| Number of APOE ε4 alleles | 0.3469 | 0.9849 | ||||
| 2 vs. 0 | −0.90 | 0.82 | 0.2727 | −0.11 | 0.64 | 0.6221 |
| 1 vs. 0 | −0.37 | 0.34 | 0.2739 | −0.02 | 0.28 | 0.9142 |
| Age at baseline | −0.35 | 0.03 | <0.0001 | −0.30 | 0.02 | <0.0001 |
| Years of education | 1.01 | 0.05 | <0.0001 | N.I. | N.I. | N.I. |
| Original cohort | −1.42 | 0.32 | <0.0001 | N.I. | N.I. | N.I. |
| Years Since Baseline | −0.75 | 0.03 | <0.0001 | −0.58 | 0.03 | <0.0001 |
| Female gender | N.I. | N.I. | N.I. | −6.95 | 0.29 | <0.0001 |
| History of smoking | N.I. | N.I. | N.I. | −1.79 | 0.30 | <0.0001 |
| Interaction – Number of APOE ε4 alleles and Years Since Baseline | <0.0001 | 0.1152 | ||||
| 2 vs. 0 | −0.48 | 0.15 | 0.0009 | −0.13 | 0.11 | 0.2346 |
| 1 vs. 0 | −0.18 | 0.06 | 0.0007 | −0.08 | 0.04 | 0.1152 |
N.I.: not included in final model
Cognitive decline was based upon cognitive scores from the Community Screening Interview for Dementia
When the memory subdomain was analyzed separately for cognitive decline using the number of e4 allele model, the results were similar to those for global cognitive decline (African Americans: β(ε4 homozygous) = −0.26, p = 0.0004; β(ε4 heterozygous) = −0.07, p = 0.009; Yoruba: β(ε4 homozygous) = −0.11 p = 0.054, β(ε4 heterozygous) = −0.02 p = 0.25).
African American participants who did not have APOE genotyped were significantly less educated (p < 0.0001) and more likely to be male (p = 0.0057). In the Yoruba those without an APOE genotype were significantly older (p < 0.0001) than those who were genotyped and included in this analysis. In both cohorts, participants without genotyping had slightly lower cognitive scores at baseline than those who were genotyped (p < 0.0001 for both).
Discussion
In this analysis, the APOE ε4 allele is a significant risk factor for incident AD in the African American cohort (homozygotes-HR: 4.12, CI: 2.33–7.28; heterozygotes-HR: 2.31, CI: 1.70–3.14, presence of an e4 allele, HR 2.47; 95% CI 1.84–3.32; p < 0.0001). These results are consistent with our previously published results using smaller samples and with the results of the recently published Alzheimer Disease Genetic Consortium (ADGC) study which examined African American cohorts (Reitz et al., 2013). These data indicate a dose dependent effect of the APOE ε4 allele on AD risk in African Americans.
In contrast to our previous findings with Yoruba where we found no relationship with ε4 and AD (Gureje et al., 2006), in this analysis we did detect a significant association for incident AD with the presence of an APOE e4 allele (HR: 1.35 95%; CI:1.00–1.83). We also found a significant association with homozygosity but not heterozygosity (homozygotes HR: 2.95, CI: 1.67–5.19; heterozygotes-HR: 1.21, CI: 0.88–1.67). There are no other studies of the effect of ε4 on incident AD in African populations with which to compare.
Despite the significant association between APOE ε4 homozygosity and AD in the Yoruba, the association between ε4 and AD remains weaker for Yoruba than for African Americans. There is no overlap in confidence limits between the 2 cohorts in the analysis of the effects on AD of the presence of the e4 alelle It is possible that these differences in ε4 effect reflect genetic differences between the two cohorts. The greatest genetic diversity occurs in Sub-Saharan African populations, including Nigeria, where it has been estimated that the genetic variation exceeds that found amongst European populations (Tishkoff et al., 2009). As a result, there may be unique variants segregating in the Nigerian population that may affect protein function and gene expression that, in turn, interact with environmental factors such as diet. Many of these variants are population specific and some are predicted to affect protein function (Tennessen et al., 2012), modify chromatin accessibility, transcription factor binding (Degner et al., 2012) and DNA methylation levels resulting in gene expression differences (Bell et al., 2011). Patterns of genetic variation are shaped by demographic forces such as migration events, fluctuation in population size and by evolutionary forces such as natural selection and mutation. A classic example of this is the single base mutation in the FY gene (DuffyO) that in the homozygous state renders the individual resistant to malaria. It has been suggested that APOE has a meat adaptive function and evolved with an increase in consumption of animal tissue. The gene is thought to confer resistance to hypercholesterolemia and infections (Finch and Sapolsky, 1999; Finch and Stanford 2004) Another theory is that the ε4 allele would be advantageous under seasonal periods of starvation, due to its elevating effect of cholesterol which would otherwise be too low but detrimental in areas where animal fats were readily available (Corbo and Scacchi, 1999). APOE ε4 may be advantageous in infections with pathogens requiring host lipid for survival. The high affinity ε4 has for some lipids actually hindered their uptake by the pathogen (Martin, 1999). Thus, the ε4 allele may be advantageous to the Yoruba where infectious disease stills plays an important role in health. On the other hand, under certain environmental conditions such as a high fat diet and low physical activity, individuals who carry an ε4 allele will have higher risk of mortality and disease due to higher cholesterol levels.
In addition, the strong association observed in the African American cohort may be a result of admixture. Admixture refers to the mixed ancestry, both African and Caucasian, which is typically observed among individuals who are African American. It is hypothesized that the effect of the APOE ε4 allele in African American populations may be due to its Caucasian ancestry. Evaluation of this hypothesis, as well as the potential effect of unique African variants modifying the effect of the APOE e4 allele, can be best evaluated using whole genome sequencing data in these two populations.
There are many environmental and life style differences between the Yoruba and the African Americans (Hendrie et al., 2004). For example the Yoruba diet has been described as being low in fat and low calorie (Hendrie et al., 2004). Probably, as a consequence, the levels of cholesterol and triglycerides are significantly lower in the Yoruba than in the African Americans (Deeg et al., 2008).
The role of cholesterol in the etiology of AD, however, remains controversial (Reitz et al., 2013).
In a previous study we reported very similar significant interactions between cholesterol levels, APOE e4 and AD risk for both Yoruba and African Americans (Evans et al., 2000; Hall et al., 2006). However, in our study increasing levels of cholesterol increased the risk for AD, but only in those participants who did not possess an e4 allele. The risk associated with e4 remained lower in Yoruba than in African Americans regardless of cholesterol levels suggesting that the e4 link to lipid metabolism maybe due to differences in genes associated with lipid metabolism rather than cholesterol levels per se.
It is noteworthy that the second major genetic variant affecting AD risk in African Americans is ABCA7, which mediates the biogenesis of high density lipoproteins (Reitz et al., 2013). The authors conclude that, together with the confirmation of the effect of e4, their findings suggest that lipid metabolism is a prominent pathway for Late Onset Alzheimer Disease in African Americans. The status of ABCA7 and AD risk in African populations remain untested however.
In our analysis of cognitive decline as an outcome we find a similar pattern as with incident AD, with the Indianapolis cohort showing a significant effect for the possession of both a single and double copy of an ε4 allele while the Yoruba cohort showed a significant effect for the presence of an e4 allele (p = 0.0425) but no significant effect for either homozygosity or heterozygosity.
There is some evidence that the ε4 effect on cognitive decline varies according to the cognitive domain measured. Consistent with previous reports, in our study, the association with APOEe4 appears to be driven primarily by the scores in the memory subdomain (Unverzagt et al., 2011). Our African American findings are also similar to previous studies that included African American samples (Knopman et al., 2009; Sawyer et al., 2009). As far as we can determine this represents the first analysis of the effects of ε4 in cognitive decline in an African population.
Strengths
The 19 year longitudinal Indianapolis Ibadan Project is the largest and longest comparative study of dementia that includes African and African American cohorts. Results from the study represent the most comprehensive analysis of the effects of ε4 on incident AD and cognitive decline currently reported with African populations.
Weaknesses
APOE analyses were conducted in approximately half of the total sample. Participants in Ibadan who did not have APOE genotyping were significantly older while those in Indianapolis were significantly less educated than those with APOE genotyping. It is possible that the non-geno-typed samples contained more AD patients and therefore this analysis may underestimate the effects of APOE e4.
The Yoruba sample came from a circumscribed geographical area. Caution should be used to generalize these findings to the entire Nigerian population.
The study used self or informant reports for identifying co morbidities thus raising the possibility that these were under reported particularly in the Yoruba population. These, together with the population biases described above, may account for some surprising co morbidity findings such as African Americans with incident AD being less likely to have diabetes.
No information on individual diet or medication was included in this study
The CSID is a relatively crude instrument to measure cognitive decline.
In summary, the ε4 allele of APOE was strongly related to increased risk for incident AD and cognitive decline in African Americans while a weaker but significant effect for incident AD was only found for ε4 homozygosity and for the presence of an e4 allele for cognitive decline in Yoruba.
Acknowledgments
No additional acknowledgments.
Footnotes
Description of authors’ roles
Hugh C. Hendrie designed and implemented the study, analyzed the data, interpreted the results, and wrote the paper. Kathleen A. Lane analyzed the data, interpreted the results, and wrote the paper. Christianna Purnell wrote and reviewed the paper. Sujuan Gao designed the study, analyzed the data, interpreted the results, and reviewed the paper. Olusegun Baiyewu, Ann Hake, Adesola Ogunniyi, Oye Gureje, Frederick W. Unverzagt, Jill Murrell, and Kathleen Hall designed and implemented the study, interpreted the results, and reviewed the paper. Christopher M. Callahan, Andrew J. Saykin, and Tatiana Foroud interpreted the results and reviewed the paper.
Conflict of interest
This research was supported by NIH grant RO1 AG09956 and NIH grant P30 AG10133.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Assocation; 1987. revised. [Google Scholar]
- American Pychiatric Association Press. The International Statistical Classification of Diseases and Related Health Problems: 1 and 2. 1992. ICD-10. [Google Scholar]
- Bell JT, et al. DNA methylation patterns associate with genetic and gene expression variation in HapMap cell lines. Genome Biology. 2011;12:R10. doi: 10.1186/gb-2011-12-1-r10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalise P, Chicken E, McGee D. Baseline age effect on parameter estimates in Cox models. Journal of Statistical Computation and Simulation. 2011;82:1767–1774. [Google Scholar]
- Chen CH, et al. A comparative study to screen dementia and APOE genotypes in an ageing East African population. Neurobiol Aging. 2010;31:732–740. doi: 10.1016/j.neurobiolaging.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang YF, et al. Association between APOE epsilon4 allele and vascular dementia: the Cache County study. Dementia and Geriatric Cognitive Disorders. 2010;29:248–253. doi: 10.1159/000285166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo RM, Scacchi R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a ‘thrifty’ allele? Annals of Human Genetics. 1999;63:301–310. doi: 10.1046/j.1469-1809.1999.6340301.x. [DOI] [PubMed] [Google Scholar]
- Deeg M, et al. A comparison of cardiovascular disease risk factor biomarkers in African Americans and Yoruba Nigerians. Ethnicity & Disease. 2008;18:427–433. [PMC free article] [PubMed] [Google Scholar]
- Degner JF, et al. DNase I sensitivity QTLs are a jamor determinant of human expression variation. Nature. 2012;482:390–394. doi: 10.1038/nature10808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, et al. Serum cholesterol, APOE genotype, and the risk of Alzheimer’s disease: a population-based study of African Americans. Neurology. 2000;54:240–242. doi: 10.1212/wnl.54.1.240. [DOI] [PubMed] [Google Scholar]
- Farrer LA, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Finch CE, Sapolsky RM. The evolution of Alzheimer disease, the reproductive schedule, and APOE isoforms. Neurobiology of Aging. 1999;20:407–428. doi: 10.1016/s0197-4580(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Finch CE, Stanford CB. Meat-adaptive genes and the evolution of slower aging in humans. Quarterly Review of Biology. 2004;63:301–310. doi: 10.1086/381662. [DOI] [PubMed] [Google Scholar]
- Gureje O, et al. APOE epsilon4 is not associated with Alzheimer’s disease in elderly Nigerians. Annals of Human Genetics. 2006;59:182–185. doi: 10.1002/ana.20694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KS, Gao S, Emsley CL, Ogunniyi AO, Morgan O, Hendrie HC. Community screening interview for dementia (CSI ‘D’); performance in five disparate study sites. International Journal of Geriatric Psychiatry. 2000;15:521–531. doi: 10.1002/1099-1166(200006)15:6<521::aid-gps182>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Hall K, et al. Cholesterol, APOE genotype, and Alzheimer disease: an epidemiologic study of Nigerian Yoruba. Neurology. 2006;66:223–227. doi: 10.1212/01.wnl.0000194507.39504.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrie HC, et al. The CAMDEX: a standardized instrument for the diagnosis of mental disorder in the elderly: a replication with a US sample. Journal of the American Geriatrics Society. 1988;36:402–408. doi: 10.1111/j.1532-5415.1988.tb02378.x. [DOI] [PubMed] [Google Scholar]
- Hendrie HC, et al. Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. JAMA. 2001;285:739–747. doi: 10.1001/jama.285.6.739. [DOI] [PubMed] [Google Scholar]
- Hendrie H, et al. Alzheimer’s disease, genes, and environment: the value of international studies. Canadian Journal of Psychiatry. 2004;49:92–99. doi: 10.1177/070674370404900203. [DOI] [PubMed] [Google Scholar]
- Hendrie HC, et al. The development of a semi-structured home interview (CHIF) to directly assess function in cognitively impaired elderly people in two cultures. International Psychogeriatrics. 2006;18:653–666. doi: 10.1017/S104161020500308X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrie H, et al. Homocysteine levels and dementia risk in Yoruba and African Americans. International Psychogeriatrics. 2013;25:1859–1866. doi: 10.1017/S1041610213001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. Journal of Lipid Research. 1990;31:545–548. [PubMed] [Google Scholar]
- Knopman DS, Mosley TH, Catellier DJ, Coker LH. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimers Dementia. 2009;5:207–214. doi: 10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]
- Kuller LH, et al. Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke. 1998;29:388–398. doi: 10.1161/01.str.29.2.388. [DOI] [PubMed] [Google Scholar]
- Leffondre K, Touraine C, Helmer C, Joly P. Internal-censored time-to-event and competing risk with death: is the illness-death model more accurate than the Cox model? International Journal of Epidemiology. 2013;42:1177–1186. doi: 10.1093/ije/dyt126. [DOI] [PubMed] [Google Scholar]
- Martin GM. APOE alleles and lipophylic pathogens. Neurobiol Aging. 1999;20:441–443. doi: 10.1016/s0197-4580(99)00078-0. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris JC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Murrell JR, et al. Association of apolipoprotein E genotype and Alzheimer disease in African Americans. Archives of Neurology. 2006;63:431–434. doi: 10.1001/archneur.63.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara T, et al. Apolipoprotein genotype for prediction of Alzheimer’s disease in older Japanese: the Hisayama Study. Journal of the American Geriatrics Society. 2011;59:1074–1079. doi: 10.1111/j.1532-5415.2011.03405.x. [DOI] [PubMed] [Google Scholar]
- Reitz C, et al. Variants in the ATP-binding cassette transporter (ABCA7), apolipoprotein E 4, and the risk of late-onset Alzheimer disease in African Americans. JAMA. 2013;309:1483–1492. doi: 10.1001/jama.2013.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahota A, et al. Apolipoprotein E-associated risk for Alzheimer’s disease in the African-American population is genotype dependent. Annals of Neurology. 1997;42:659–661. doi: 10.1002/ana.410420418. [DOI] [PubMed] [Google Scholar]
- Sawyer K, Sachs-Ericsson N, Preacher KJ, Blazer DG. Racial differences in the influence of the APOE epsilon 4 allele on cognitive decline in a sample of community-dwelling older adults. Gerontology. 2009;55:32–40. doi: 10.1159/000137666. [DOI] [PubMed] [Google Scholar]
- Stewart R, Russ C, Richards M, Brayne C, Lovestone S, Mann A. Apolipoprotein E genotype, vascular risk and early cognitive impairment in an African Caribbean population. Dementia and Geriatric Cognitive Disorders. 2001;12:251–256. doi: 10.1159/000051267. [DOI] [PubMed] [Google Scholar]
- Tennessen JA, et al. Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science. 2012;337:64–69. doi: 10.1126/science.1219240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishkoff SA, et al. The genetic risk structure and history of Africans and African Americans. Science. 2009;324:1035–1044. doi: 10.1126/science.1172257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unverzagt FW, et al. Incidence and risk factors for cognitive impairment no dementia and mild cognitive impairment in African Americans. Alzheimer Disease and Associated Disorders. 2011;25:4–10. doi: 10.1097/WAD.0b013e3181f1c8b1. [DOI] [PMC free article] [PubMed] [Google Scholar]