Abstract
Bisphenol A (BPA) is an organic compound to which human populations are ubiquitously exposed. Epidemiological data suggest BPA exposure might be associated with higher rates of diabetes and reproductive anomalies. Health concerns also include transgenerational consequences, but these mechanisms are crudely defined. Similarly, little is known about synergistic interactions between BPA and other substances. Here we show that acute and chronic exposure to BPA causes genome-wide modulation of several functionally coherent genetic pathways in the fruit fly Drosophila melanogaster. In particular, BPA exposure causes massive downregulation of testis-specific genes and upregulation of ribosome-associated genes widely expressed across tissues. In addition, it causes the modulation of transposable elements that are specific to the ribosomal DNA loci, suggesting that nucleolar stress might contribute to BPA toxicity. The upregulation of ribosome-associated genes and the impairment of testis-specific gene expression are significantly enhanced upon BPA exposure with a high-sugar diet. Our results suggest that BPA and dietary sugar might functionally interact, with consequences to regulatory programs in both reproductive and somatic tissues.
Keywords: BPA, diabetes, nucleolus, ribosome, testis
EXPOSURE to environmental toxins is widespread. It is well documented, for instance, that human populations are commonly exposed to bisphenol A (BPA), bis(2-ethylhexyl) phthalate (DEHP), dioxins, and other organic molecules produced at industrial scales. Toxins might act by modulating epigenetic pathways to change the expression of genes and normal cellular homeostasis and could result in transgenerational consequences. The fast pace at which new compounds are invented and become ubiquitous in the environment poses a challenge to safety assessment. This challenge emerges not only from the large number of compounds to evaluate, but also from the high dimensionality of toxic responses across tissue types and developmental stages. This is further aggravated by the potential for unexpected synergism among toxins in mixtures. Sensitivity might also be strongly dependent on the specific epigenomic features and genetic makeup of an individual (Du et al. 2004).
Genomic analyses have uncovered levels of human genetic variation not surmised a few decades ago (e.g., Sudmant et al. 2010). Cost-effective epigenetic and physiological models are needed for (i) addressing tissue-specific responses to toxins, (ii) gaining insights into mechanisms of epigenetic toxicity, and (iii) evaluating the manifestation of these responses across naturally occurring genotypes. In particular, cost-effective models with massive publicly available genetic information (Zwarts et al. 2011; King et al. 2012a,b; Mackay et al. 2012) might allow for genome-wide evaluation of toxic responses and assessment of coexposure outcomes in conjunction with the evaluation of unique individual responses.
BPA is one of the most abundantly produced chemicals worldwide, reaching an annual volume >4 million tons (Burridge 2003). Multiple studies find consistently high levels of BPA in human subjects, presumably due to continued exposure (vom Saal et al. 2007). Indeed, BPA can be detected in the urine of >90% of the American population (Calafat et al. 2005). Exposures occur not only in isolation, but also in mixtures with other toxins. Layered on top of exposures to novel combinations of toxins is the observation that the human diet has changed substantially in the last few centuries; sugar in particular has increased dramatically in the western diet. Mouse studies suggest that BPA disrupts insulin production and glucose homeostasis (Alonso-Magdalena et al. 2006) and epidemiological studies (Lang et al. 2008; vom Saal and Myers 2008) found that increased BPA levels in human urine were associated with significantly increased risks of diabetes and heart disease. However, mechanisms for understanding BPA’s contribution to diabetes are not well defined and raise the question of whether the relationship between BPA and diabetes is causal. Furthermore, epidemiological data have been inconclusive (Magliano and Lyons 2013). The relationship between BPA and diabetes is further confounded because BPA is often co-ingested with high sugar. Ingestion of drinks and food packaged in vessels lined with BPA appears to be a major source of exposure. BPA and high sugar might individually have long-term effects on disease susceptibility, but might also display synergistic interactions with each other. Altogether, poorly defined mechanisms not only hamper the development of bottom–up causal models of toxic BPA action but also prevent the optimal identification of high-risk individuals.
We investigated BPA toxicity under typical and high-sugar diets, using a genetically tractable Drosophila melanogaster model. Here we report significant interactions between BPA and sugar intake. We observed genome-wide modulation in both somatic and germline tissues of flies raised on a typical diet or on a high-sugar diet, with and without coexposure to BPA. Mode of exposure resulted in distinct outcomes: short-term acute BPA exposure prominently affected the expression of gut-specific genes, while chronic exposure impaired the activity of genes expressed in testis. Interestingly, combined exposure to BPA and high sugar activated the transcription of several genes that code for ribosomal proteins. These results reveal novel pathways contributing to BPA toxicity and highlight potential synergism among substances that are ubiquitous and often co-ingested in the diet.
Materials and Methods
Drosophila strains and treatments
The y[1]; bw[1]; e[4]; ci[1] ey[R] strain was maintained in vials containing the same standard diet in use at the Bloomington Drosophila Stock Center at Indiana University without light malt extract (http://flystocks.bio.indiana.edu/Fly_Work/media-recipes/bloomfood.htm). The base recipe for the control diet contains 150 mM of sucrose. For the treatments, the standard diet was prepared, and after the food cooled down to 60°, 3.7 g/liter of powdered BPA and/or 102.7 g/liter of powdered sucrose and/or 0.8% (v/v) of DEHP were added to the food and homogenized with a mixer. Hence, the treatments contain 450 mM of sucrose (high sugar), 16 mM of BPA, and/or 2 mM of DEHP. The food was poured in vials and kept at room temperature overnight to dry. For acute exposure, four replicas per treatment were set up with 30 flies (2-day-old males) and exposed for 48 hr (supporting information, Figure S1). For chronic exposure, four replicas per treatment were set up with 15 virgin females and 10 males. Two genotypes were used for chronic exposure [Yohio and Ycongo (Lemos et al. 2008)]. Vials were kept and newly emerged adults, which developed in the diets containing the different compounds, were collected and aged for 2 days with continued exposure to the same rearing condition in which they developed (Figure S2). All flies were grown at 25°, with ∼65% of relative humidity, and a 14-hr light/10-hr dark cycle. All flies used for gene expression analyses were flash-frozen in liquid nitrogen and stored at −80°.
Gene expression analysis
An ∼18,000-feature array spotted primarily with PCR products designed for single exons was used. This gene set was supplemented with PCR products designed for Y-linked genes, Drosophila testis ESTs, and transposable elements (Lemos et al. 2008). Designs for microarray data collection are shown in Figure S3 and Figure S4 for acute and chronic exposures, respectively. Total RNA was extracted from whole flies, using TRIzol (Life Technologies). The synthesis of cDNA probes and hybridization conditions were carried out using 3DNA protocols and reagents (Genisphere). Microarray slides were scanned with an Axon 400B scanner (Axon Instruments) and the data extracted with GenePix Pro 6.0 software. We used stringent quality-control criteria to ensure reliability of foreground intensity reads for both Cy3 and Cy5 channels (Lemos et al. 2008). This procedure screens out poor-quality arrays. It ensures that the resulting data are minimally sensitive to the specifics of normalization. Foreground fluorescence of dye intensities was normalized by the Loess method in Bioconductor/Limma (Smyth and Speed 2003). We used the normexp method for background correction and the loess and Aquantile methods for within- and between-array normalization, respectively (Smyth and Speed 2003). The significance of variation in gene expression was assessed with the Bayesian analysis of gene expression levels (BAGEL) (Townsend and Hartl 2002). Gene expression estimates from duplicate spots were averaged previous to the computation of statistical significance of differential expression. Results were checked for consistency, using Linear Models in Limma. False discovery rates were empirically estimated by permutation of the data set. Specifically, we have used permutation of columns (i.e., slides) rather than rows (i.e., genes) because the former is more conservative. The microarray gene expression data reported here can be obtained at the Gene Expression Omnibus database under accession nos. GSE55655 and GSE55670. For quantitative PCR analyses, four biological replicates of each sample were used to make cDNA, using the QuantiTect Reverse-Transcription Kit (QIAGEN, Valencia, CA), and real-time qPCR analyses were carried out with the Fast Sybr Green Master Mix (Applied Biosystems, Foster City, CA), using 7900HT Fast Real-Time PCR (Applied Biosystems). The expression level of the R1 and R2 genes was analyzed with the software REST (Pfaffl et al. 2002).
Metrics of tissue perturbation
Tissue specificity of differentially expressed (DE) genes was assessed using the FlyAtlas (Chintapalli et al. 2007; Robinson et al. 2013). We filtered the data to include only a nonredundant set of adult tissues: brain, eye, adult thoracicoabdominal ganglion (tag), adult salivary gland (sg), testis, accessory glands (acc), crop, midgut, tubule, hindgut, heart, and adult fat body. Genes with <200 expression counts were conservatively set to zero. We calculated a tissue prevalence index [TP(i)] for tissue i in a set of n tissues as:
TP(i) of 1 means that all genes in the set of differential expressed genes show evidence of expression in the focal tissue (Branco et al. 2013). The tissue salience ratio [TSr(i)] is calculated for a set of DE genes as the ratio between DE genes that display relative abundance in tissue i above and below the average expression measured in the whole organism:
The ratio allows us to evaluate the relative impact of a perturbation on genes that are highly expressed relative to genes that are lowly expressed in a particular tissue. Significance was calculated with standard Fisher’s exact tests on contingency tables with counts of genes displaying differential expression. Analyses of enrichment in gene ontology categories were performed with standard methods as previously implemented (Branco et al. 2013) and confirmed with the FlyMine platform (Lyne et al. 2007).
Results
Rapid expression responses and potentiation of BPA toxicity by coexposure with ubiquitous compounds
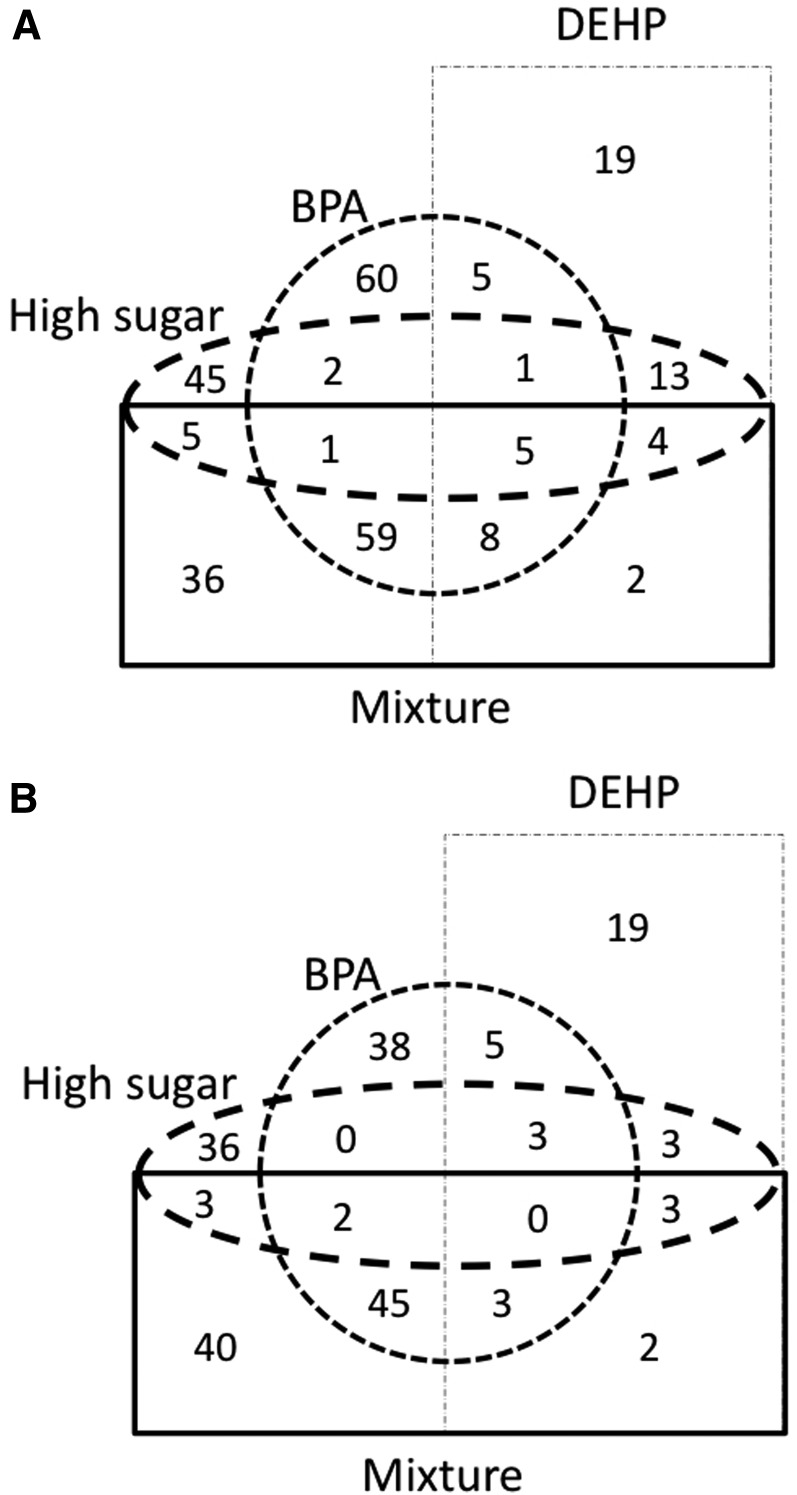
We investigated genome-wide expression responses of individuals treated with BPA (16 mM), high sugar (450 mM), and DEHP (2 mM) as well as their combination (BPA, high sugar, and DEHP) (Figure S1, Figure S3). The concentration of sucrose used for the high-sugar diet (450 mM) was three times the typical amount used in the base diet (150 mM), but below the concentration used in chronic high-sugar diets designed to induce insulin resistance and diabetic phenotypes in flies (1 M) (Baker and Thummel 2007; Musselman et al. 2011; Na et al. 2013). For each toxin, the amount used in the mixture (BPA, high sugar, and DEHP combined) was identical to that used in the single exposures. To evaluate the effects of a short-term exposure, flies were transferred from regular food to the exposure food for 48 hr (Figure S1). Hereafter, 48-hr exposure is called acute exposure. Acute exposure did not increase mortality rates of adults in any treatment, which suggests that the treatments were not overly toxic. Nevertheless, acute adult exposure elicited rapid expression responses (Figure 1, A and B). A high-sugar diet led to a mild modulation of expression with 126 genes differentially regulated [76 genes were upregulated and 50 genes were downregulated; Bayesian posterior probability (BPP) > 0.97]. DEHP showed modulation of comparable magnitude with 96 genes differentially expressed (58 genes were upregulated and 38 genes were downregulated; BPP > 0.97). BPA treatment elicited the highest response with 237 genes disrupted upon exposure (141 genes were upregulated and 96 genes were downregulated; BPP > 0.97). The joint exposure of all three compounds resulted in the differential gene regulation of the same magnitude as that of BPA alone, with 219 differentially expressed genes (120 genes were upregulated and 98 genes were downregulated; BPP > 0.97). A substantial fraction of the upregulated (30%) and downregulated (41%) genes were unique to the mixture (Figure 1, A and B).
Figure 1.
Acute exposures to BPA mixtures perturb gene expression in Drosophila. Upregulation (A) and downregulation (B) of gene expression in each acute treatment relative to the control diet (BPP > 0.97). The combined exposure (BPA, high sugar, and DEHP) reinforces individual effects and also results in the differential expression of a new set of genes.
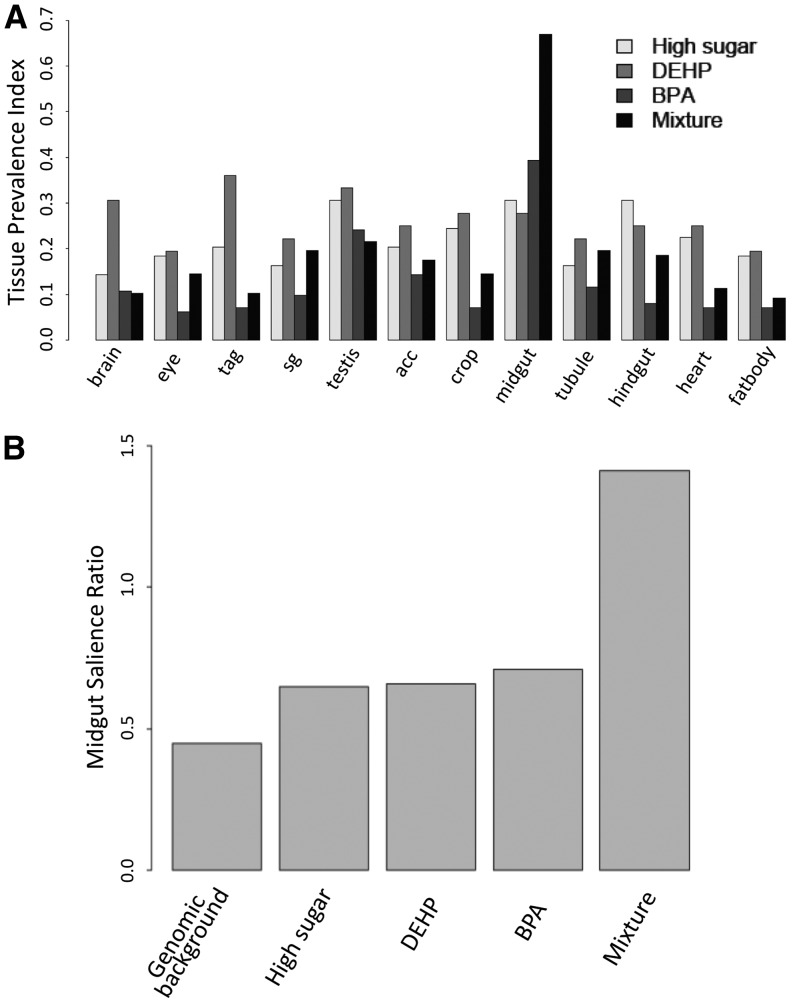
Acute exposure might elicit differential responses across organs due to variation in both distribution and concentration in different tissues. Specifically, nutrient absorption occurs primarily in the insect midgut (Ohlstein and Spradling 2006; Buchon et al. 2013), which might be expected to respond most rapidly to oral toxin intake. To address the issue we used two metrics, TP(i) and TSr(i), both of which combine our expression data sets with data available in the FlyAtlas database (Chintapalli et al. 2007; Robinson et al. 2013), a unique resource with tissue expression data collected across several dissected tissues. We observed that neither treatment with high sugar nor that with DEHP showed an overtly tissue-specific disruption in either sets of upregulated (Figure S5) or downregulated (Figure 2A) genes. On the other hand, genes knocked down upon BPA exposure were highly expressed in the midgut [Table S1; for BPP > 0.98, midgut salience ratio of 1.7 (BPA) vs. 0.45 (genomic background), 0.91 (high sugar), and 0.80 (DEHP), P < 2.2e-16, Fisher’s exact test]. Gene ontology analysis indicated that these genes are associated with proteolysis (GO:0006508; 19 genes, P < 3.03e-11) and lipid metabolism (GO:0044238; 4 genes, P < 0.015). Furthermore, analysis of the BPA-containing mixture indicated that the downregulation of midgut-expressed genes was substantially enhanced with coexposure to high sugar and DEHP (Figure 2A). Indeed, more than half of the genes knocked down with this mixture were expressed specifically in the midgut (Figure 2B, Table S1; for BPP > 0.98, midgut salience ratio of mixture is 6.22). This result suggests that toxic effects of BPA in the gut can be substantially heightened through interaction with other compounds in a mixture. TSr(i)’s reinforced the conclusion that BPA and the BPA-containing mixture differ significantly in their gene regulatory consequences and suggest synergistic interactions (Figure 2, A and B; Table S1). Gene ontology analysis of genes differentially expressed due to the mixture confirmed the previous results, indicating significant enrichments for genes involved in protein degradation (GO:0006508; 23 genes, P < 5.83e-14) and metabolic processes (GO:0008152; 36 genes, P < 3.12e-4). Altogether, we conclude that acute exposure in adults caused rapid transcription responses and that common exposure sources might have synergistic effects when combined with BPA, even when their individual ability to disturb gene expression is comparatively mild.
Figure 2.
Acute exposures to BPA mixtures perturb the expression of midgut-specific genes. (A) Tissue prevalence indexes [TP(i)] for the set of downregulated genes (BPP > 0.95) across 12 tissues (see Materials and Methods). Data indicate that the acute treatment perturbs gene expression in the midgut (P < 0.001; Fisher’s exact test for enrichment in the midgut). (B) Tissue salience ratio [TSr(i)] for the midgut indicates a significantly stronger gene knockdown with the mixture (P < 0.001, Fisher’s exact test).
Chronic exposure reveals sugar-mediated potentiation of BPA toxicity
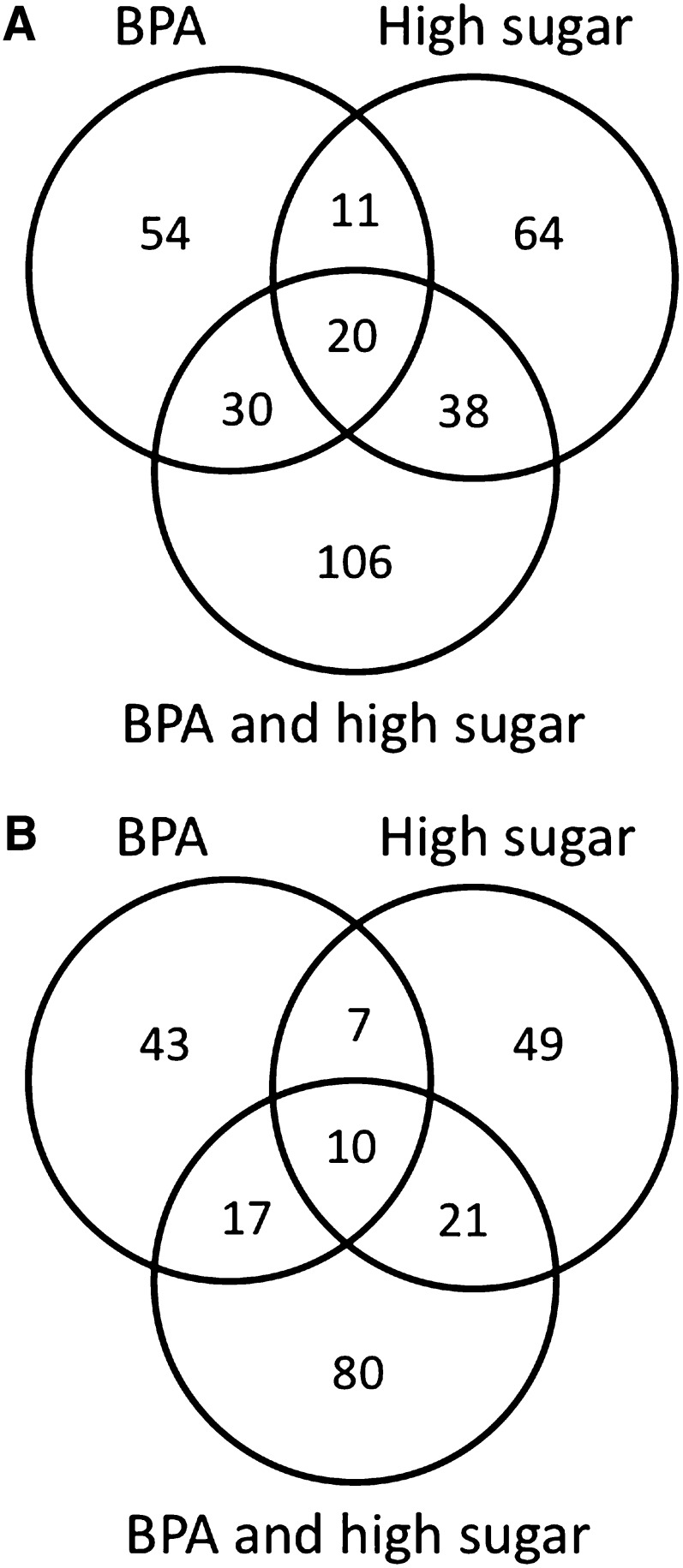
To further address the specific interaction between BPA and sugar, we investigated the consequences of chronic BPA exposure during development (Figure S2). Flies were raised in (i) BPA, (ii) high sugar, and (iii) the combination of BPA and high sugar. As before, we used flies developed in a BPA-free control food with typical amounts of sugar (150 mM) as a reference. Flies that developed from eggs to adults on these treatment diets were considered chronically exposed. Male flies that emerged from these treatments were collected and used for genome-wide gene expression analysis (Figure S4). Noteworthy, we observed that exposure to the mixture of BPA and high sugar led to an evident delay in development. Relative to controls, the treatments with BPA or high sugar revealed the differential expression of 192 and 220 genes (BPP > 0.97), respectively. Only 48 differentially expressed genes were shared between the two conditions. On the other hand, flies chronically exposed to the BPA/high-sugar mixture showed a larger and unique disruption of gene transcription (Figure 3, A and B): 322 genes were affected by the BPA/high-sugar combination. About 45% and 36% of the genes up- and downregulated in the BPA/high-sugar mixture were also observed in the single exposures (Figure 3, A and B). Finally, we observed significant agreement between fold-change estimates for the effect of the BPA/high-sugar mixture in the two genotypes assayed (Spearman’s rank correlation ρ = 0.41, P = 8.42e-05; genes with BPP > 0.95 between control and treatment diet in both data sets). Altogether, the data point to unique and shared expression responses to chronic BPA, high sugar, and their combination. As observed for acute exposure, the combined exposure suggests that BPA and sugar generate a distinct gene regulatory response. Interaction in the combined exposure might happen either through the independent disruption of functionally related pathways or through the enhancement of the disruption of a single pathway targeted by each individual component in the mixture.
Figure 3.
Chronic exposure to BPA perturbs gene expression in Drosophila. Upregulation (A) and downregulation (B) of gene expression in each acute treatment relative to the control diet (BPP > 0.97). The mixture (BPA and high sugar) reinforces individual effects and modulates the transcription of a new set of genes.
BPA exposure knocks down the expression of testis-expressed genes
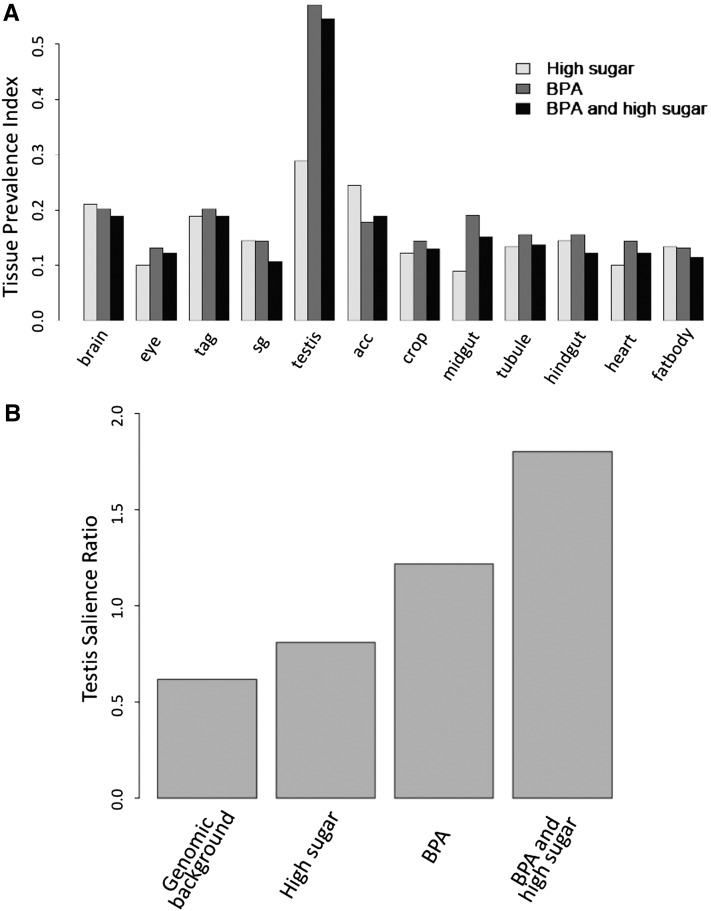
BPA has been implicated in the disruption of a variety of reproductive traits in humans and model organisms (Allard and Colaiacovo 2010; Lawson et al. 2011; Hunt et al. 2012). To address the issue in Drosophila, we investigated the consequence of the BPA/sugar interaction on gene expression levels in the testis. In agreement with expectations, chronic BPA exposure showed a statistically significant increase in the prevalence of testis-expressed genes in the set of candidates that were downregulated (Figure 4A; P < 0.001, Fisher’s exact test). Moreover, 24 of the 76 downregulated genes (BPP > 0.97) were expressed exclusively in the testis (32% testis-specific representation vs. 19% for the whole set of genes analyzed; P < 0.01, Fisher’s exact test). On the other hand, testis-specific gene expression was not disrupted upon development in a high-sugar diet without BPA (19 genes of 86; 22% testis-specific representation). Furthermore, and in agreement with our observations concerning interactions between BPA and sugar, the BPA-mediated impairment of testis-expressed genes was enhanced upon coexposure with a high-sugar diet. Accordingly, BPA co-exposure with high sugar caused the downregulation of 47 testis-specific genes, which accounts for 37% of all genes downregulated. This is a statistically significant enrichment in testis-specific genes (P < 0.001, Fisher’s exact test) and represents the disruption of nearly twice as many testis-specific genes as with BPA alone. Measurements of tissue salience further underscored the pattern. They showed that the set of genes that is downregulated with the BPA/high-sugar diet displays a substantial increase in the number of genes highly expressed in testis (Figure 4B and Table S2; P = 1.05e-13, Fisher’s exact test). These results are in agreement with the observation that BPA impairs germline tissues (Allard and Colaiacovo 2010; Lawson et al. 2011; Hunt et al. 2012) and suggest that excess sugar might contribute to these toxic effects.
Figure 4.
Chronic exposure to BPA perturbs the expression of testis-specific genes. (A) Tissue prevalence indexes [TP(i)] for the set of downregulated genes across 12 tissues (see Materials and Methods) indicate that the chronic treatment perturbs gene expression in the testis (P < 0.001; Fisher’s exact test for enrichment in the testis). (B) Tissue salience ratio [TSr(i)] for the testis indicates a significantly stronger testis gene knockdown with the mixture (P < 0.001, Fisher’s exact test).
Finally, data for acute BPA exposure suggested that the 48-hr exposure elicited similar, albeit much subtler, signatures of testis gene downregulation. Accordingly, we observed a slight but statistically significant increase in the testis salience ratios of genes downregulated with BPA exposure (Table S3; P < 0.05, Fisher’s exact test), which cannot be observed with either sugar or DEHP alone. Interestingly, acute exposure to the mixture led to a much diminished testis salience possibly because of the stronger biases toward downregulation of midgut genes (cf. Table S1 and Table S3). These observations suggest that BPA might be sufficient to negatively affect testis gene expression within 48 hr of adult exposure. However, acute BPA disruption in the testis might depend on absorption rates that are modified when mixtures affect gene expression in gut tissues.
Upregulation of genes associated with energy metabolism during chronic BPA exposure
Excess sugar intake is expected to cause upregulation of energy metabolism. In agreement with this expectation, we observed that flies raised on a high-sugar diet displayed upregulation of pathways associated with energy metabolism. This included highly significant enrichments in genes associated with oxidative phosphorylation (GO:0006119; P = 6e-07), ATP synthesis-coupled electron transport (GO:0042773; P = 2e-05), and having electron carrier activity (GO:0009055; P = 0.02). This pattern was corroborated with the Kyoto Encyclopedia of Genes and Genomes (KEGG) database annotation, which pointed to significant enrichment in oxidative phosphorylation (dme00190; P = 4e-08) and ubiquinone biosynthesis (dme130; P = 0.01). Furthermore, we observed 30 genes whose protein products are localized in the mitochondria (GO:0005739; P = 0.001), with 20 of those localized specifically to the mitochondrial inner membrane (GO:0005743; P = 5e-05). We observed that a number of genes responsive to high sugar encode proteins that are localized to the nucleus, although this is not a set displaying statistically significant enrichment. This set includes candidates such as P53, His4r, Trr, SRm160 (a splicing and mRNA-processing enzyme with a Piwi domain), Hrb87F (a ribonuclear protein), and RpS3 (ribosomal protein S3).
Gene upregulation due to BPA exposure alone also showed significant statistical enrichment for targets involved in energy metabolism, with candidates localizing to the mitochondrial electron transport chain (GO:0005746; P = 0.03). This observation was also supported by the KEGG metabolic pathway database, which identified eight candidates involved in oxidative phosphorylation (dme00190; P = 0.06) and included genes such as the cytochrome C oxidase subunit Vb. The tissue prevalence index did not single out any individual tissue in the sets of genes upregulated by BPA, high sugar, and the BPA/high sugar combination (Figure S6). Notwithstanding, the TSr(i) suggested biases in the upregulation of genes that are typically highly expressed in the midgut and hindgut (Table S4). Collectively these data suggest that energy metabolism and mitochondria-associated processes might be commonly disrupted by both excess sugar and BPA toxicity, with potentially widespread consequences for gene expression across many tissues.
BPA-mediated disruption of pathways associated with the ribosome
While intriguing, BPA-mediated upregulation of mitochondria-associated genes is a fraction of all genes upregulated upon chronic BPA exposure. Noteworthy, the set of all genes upregulated by BPA exposure (BPP > 0.97) also showed significant enrichments for candidates involved in translation (GO:0006412; P = 0.002), with 23 proteins localizing to the ribosomes (GO:0005840, P = 2e-09) and displaying enrichment for the large ribosomal subunit (15 genes, GO:0015934; P = 7e-07). Furthermore, the set of upregulated genes overlapped significantly with the set of genes observed in two studies designed specifically to identify ribosomal loci. Accordingly, we observed 22 genes in common (P = 9e-12) with Marygold et al. (2007) and 13 genes in common (P = 9e-10) with Alonso and Santarem (2006).
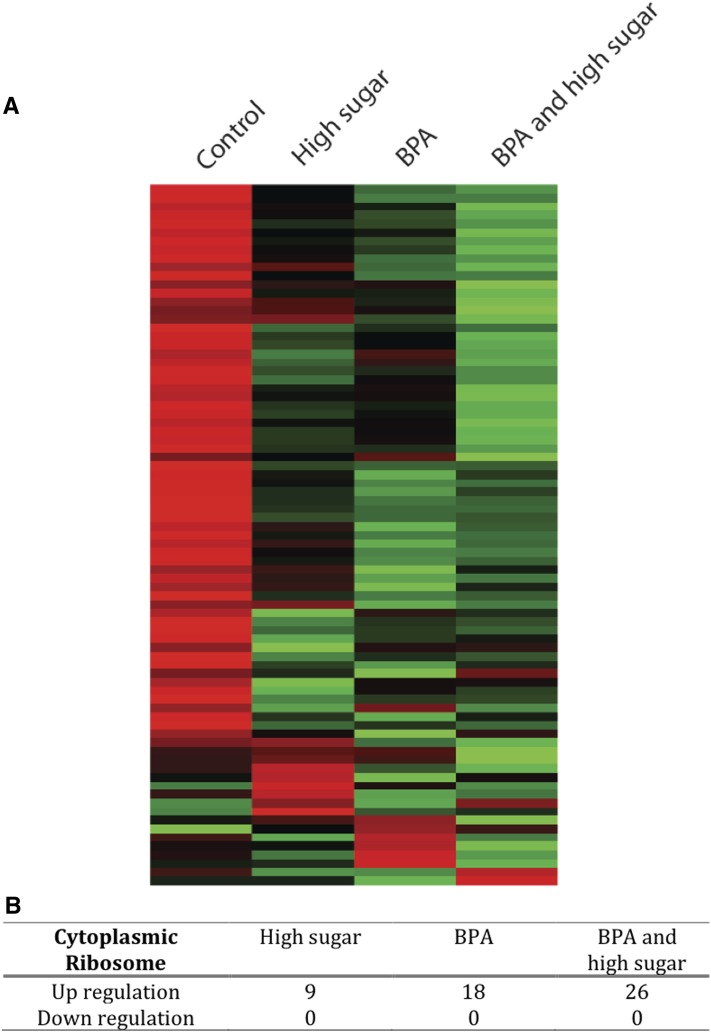
Furthermore, the addition of a high amount of dietary sugar appeared to increase BPA’s disruption by further straining ribosome-related pathways. This pattern was stronger for cytoplasmic ribosomes (Figure 5; P < 0.001, Fisher’s exact test), but disruption of a few mitochondrial ribosome genes was also verified (Figure S7). In the BPA/high-sugar treatment, we observed ribosomal genes that were not differentially expressed under BPA exposure with low sugar. We also observed larger fold changes in the common set of upregulated genes. Indeed, 77% of genes differentially expressed in the cytoplasmic ribosome were more induced in the BPA/high-sugar treatment than in the BPA treatment (P < 0.01; Figure 5). Specifically, BPA exposure under a high-sugar diet disrupted 32 genes associated with the ribonucleoprotein complex (GO:0030529; P = 6e-04), with 26 proteins localizing to the ribosomes (P = 2e-08) and displaying enrichment for components of both large (16 genes, GO:0015934; P = 4e-07) and small (10 genes, GO:0022627; P = 2e-07) ribosomal subunits. At a lenient BPP > 0.95 threshold, more than a third of the cytoplasmic ribosomal genes showed evidence of upregulation while none showed evidence of downregulation upon any combination of BPA/high sugar exposure (Figure 5, A and B). Collectively, the data indicate that a high-sugar diet might have unexpected and possibly synergistic modulation of BPA-mediated toxicity, even when its independent contribution to the disruption of specific pathways is mild.
Figure 5.
Chronic exposure to BPA and high sugar modulates the expression of genes encoding the protein components of the cytoplasmic ribosome. (A) Heat map shows global perturbation to the expression of 81 genes encoding components of the large and small subunits of the cytoplasmic ribosome. The scale color was normalized for each gene with green and red denoting high and low transcript abundance, respectively. (B) Up- and downregulation in the subset of genes differentially expressed (BPP > 0.95). The association is statistically significant (P < 0.0001, Fisher’s exact test). No statistically significant association was observed for genes encoding components of the mitochondrial ribosome (Figure S7).
Finally, further investigation of the acute exposure data sets revealed that the 48-hr treatments elicited similar, albeit much milder signatures of upregulation in ribosomal-related pathways. Accordingly, the set of upregulated genes in the acute exposure to the mixture (BPP > 0.95) showed significant enrichment in ribosome-related pathways, with 12 candidates that code for proteins belonging to the ribosomal subunits (GO:0044391; P = 0.002); 6 candidates from the small ribosomal subunit (RpS14a, RpS20, RpS21, RpS24, RpS26, and RpS27A; GO:0022627; P = 0.009), 5 candidates from the large ribosomal subunit (RpL4, RpL11, RpL24, RpL28, and RpL29), and 1 member of the mitochondrial ribosome complex (mRpL52).
Chronic exposure to BPA/high sugar shows signs of nucleolar stress
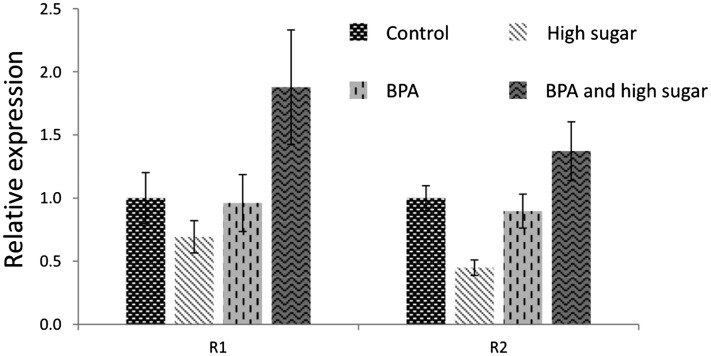
Expression of genes associated with ribosomes and the assembly of functional ribosomal subunits are tightly correlated with cellular activity and growth. Genes associated with ribosomes are highly expressed to ensure normal and continuous protein translation rates in the cell. These outstanding biosynthetic processes of transcription and translation emerge from the nucleolus, an intranuclear organelle responsible for assembling the ribosomal proteins translated in the cytoplasm and the locally transcribed ribosomal RNA (rRNA) to generate functional ribosomal subunits. Cell activity is partially regulated by the nucleolus, and consequences of its dysfunction are revealed in biological processes such as senescence, cell cycle regulation, and stress sensing (Boisvert et al. 2007; Nemeth and Langst 2011). Our gene expression data showed that BPA disrupts the expression of protein-coding genes necessary for ribosome synthesis, which suggests that the nucleolus might be affected. In Drosophila, the transposable elements R1 and R2 are specialized to insert into and reside exclusively in the 28s rDNA subunit. We addressed the expression pattern of R1 and R2 using quantitative PCR. Our results revealed that BPA alone does not affect the expression of R1 and R2, whereas sucrose slightly reduces the transcription levels of both transposon elements (Figure 6). On the other hand, the combined exposure to BPA and high sugar significantly increased the expression of R1 and R2, with a more than twofold change in the case of R1 [Figure 6; P < 0.05 as calculated with the REST software (Pfaffl et al. 2002)]. These observations corroborate genome-wide expression data, which showed that chronic exposure to the mixture of BPA and high sugar led to the disruption of genes associated with ribosomes. Altogether, the data indicated that BPA toxicity might be mediated through ribosomal pathways and that the disruption is enhanced when BPA is combined with a high-sugar diet.
Figure 6.
The combination of BPA and high sugar activates the expression of R1 and R2 elements. These transposon elements are located specifically in the rDNA array. Quantitative PCR shows that high sugar slightly decreased the expression of R1 and R2 while BPA alone had no effect on R1 and R2 transcription. However, flies chronically exposed to a diet containing BPA and high sugar showed induction of R1 and R2 expression (P < 0.01). Bars represent 95% confidence intervals for the mean.
Discussion
We investigated the extent of acute and chronic BPA toxicity and its interaction with a high-sugar diet. We observed that BPA exposure knocked down the expression of testis-specific genes and found that coexposure to a high-sugar diet significantly aggravated the impact of BPA. Surprisingly, BPA exposure substantially disrupted elements of the translational machinery, with significant disruptions to the expression of genes that code for both the small and large components of the ribosome. This signature was significantly more pronounced when BPA was combined with a high-sugar diet. The patterns were robust and observed under acute short-term exposures through oral ingestion as well as under chronic long-term exposure through development. Furthermore, BPA treatments impaired the expression of catabolic genes that are highly expressed in the midgut and might be independent of modulation of hormonal pathways. The analyses also showed that gene expression responses to BPA in the midgut were again enhanced by combination with a high-sugar diet. This suggests that these compounds might interact and act synergistically to perturb the physiology of gut tissues. Finally, our observations point to the disproportionate impact of BPA in the germline. Collectively, the data suggest that ribosome-mediated processes might modulate the sensitivity and consequences of BPA ingestion.
DNA methylation-independent pathways of BPA toxicity
Several studies using animal models and humans have indicated that BPA exposure might produce a broad range of adverse effects, including cancer (Weber Lozada and Keri 2011; Pupo et al. 2012), disruption of meiosis and other reproductive anomalies (Allard and Colaiacovo 2010; Hunt et al. 2012), impairment of genes that control brain development (Itoh et al. 2012), and behavior (Kundakovic et al. 2013). In some cases, the phenotypes are accompanied by disrupted expression of DNA methyltransferase 1 and 3A, two enzymes responsible for the incorporation of methyl marks on the DNA (Doherty et al. 2010; Itoh et al. 2012; Kundakovic et al. 2013). Alternative pathways for BPA modulation of the epigenome have been postulated; indeed, despite evidence showing that BPA might affect DNA methylation, it is unclear whether this is a direct outcome of BPA action or a correlated change in the epigenome. Drosophila is an especially useful model system in this regard. This is because (i) the organism is amenable for high-throughput genome biology and large-scale screens, (ii) whole-body analysis might be an ideal first step in physiological dissection of tissue-specific outcomes, and (iii) DNA methylation is normally either absent or occurring at extremely low levels (Lyko et al. 2000; Phalke et al. 2009; Gou et al. 2010; Schaefer and Lyko 2010). The latter allows DNA methylation confounders to be excluded and might provide insights into alternative pathways of epigenetic disruption triggered by BPA exposure. In Drosophila, nucleolar status might be probed through the expression of two transposable elements (R1 and R2) that localize exclusively to the rDNA array. These specialized elements recognize DNA sequences that are specific to the 28s rDNA. The R1 and R2 elements do not occur in any other locus of the Drosophila genome. To address the hypothesis that a BPA/high-sugar diet might affect nucleolar attributes, we assayed R1 and R2 expression upon treatment with BPA, sugar, and the combined exposure. We observed a significant induction of the R1 and R2 retrotransposons in flies exposed to the mixture containing BPA and high sugar. In contrast, the high-sugar diet caused downregulation of the expression of these elements, whereas BPA alone only mildly induced transcription of the R1 and R2 elements. These results raise the prospect that nucleolar stress might be relevant in BPA-mediated toxicity.
Widespread consequences of disrupting the ribosomal machinery
Maintenance of chromosome integrity during cellular division is critical for cell viability. Distinct types of microtubules organize spindle position to establish accurate chromosome segregation and maintain cell orientation. Defects in spindle formation might result in cytokinesis errors, chromosome missegregation, and genome instability (Nousiainen et al. 2006). Remarkably, recent studies showed that ribosomal proteins such as RpS3, RpS3a, RpS4, RpL11, and RpL12 are associated with the mitotic spindle (Sauer et al. 2005; Torres et al. 2011). These proteins are typically studied as part of ribosomes, but post-translational modifications, including phosphorylation, dephosphorylation, and sumoylation, might modulate these proteins to activities outside of their main protein translation functions (Kim et al. 2005, 2006, 2009; Jang et al. 2011). For instance, studies on the ribosomal protein S3 (RpS3) suggest that it is associated with spindle formation (Jang et al. 2012a,b); RpS3-depleted cells displayed abnormal spindle shape, defects in chromosome movement, and mitotic arrest during metaphase.
Among genes upregulated by coexposure of BPA and high sugar, several suggest cross talk between the cytoskeleton and spindle machinery. Specifically, 33 genes were associated with cytoskeleton organization (GO:0007010; P = 1.32e-4). This set contains 15 ribosomal proteins, but also includes additional candidates that contributed to enrichment in the categories of “Cytoskeleton Organization” and “Mitotic Spindle Elongation” (GO:00000022; P = 2.07e-10). These observations were corroborated by the Reactome database, which pointed to enrichment in “Eukaryotic Translation Elongation” (24 candidates, P = 1.24e-15), “Eukaryotic Translation Initiation” (27 candidates, P = 3.43e-15), and “Processing of Capped Intron-Containing pre-mRNA” (32 candidates, P = 3.73e-12). Genes belonging to these categories and perturbed by BPA include the tumor suppressor gene Pten, β-tubulin at 56D, tsunagi, actin-related protein 2/3 complex subunit 4, eukaryotic translation elongation factor 2b, eukaryotic initiation factor 5B, stubarista/ribosomal protein SA, and small ribonucleoprotein particle protein SmD2.
The effects of BPA exposure on the impairment of chromosome synapsis and double-strand DNA break repair have previously been observed during Caenorhabditis elegans oogenesis (Allard and Colaiacovo 2010), and similar observations were noted in rhesus monkey females (Hunt et al. 2012). Hunt et al. (2012) further observed abnormal centromere associations, although the mechanisms have remained unclear. One interpretation is that the spindle apparatus is directly disrupted by BPA toxicity. However, the possibility of cross talk between bona fide pathways involved in spindle formation and ribosomal protein function provides an intriguing alternate route of disruption. For instance, a proteomic analysis of the human spindle identified 50 ribosomal proteins, a surprisingly high number that suggests that these proteins might interact with the spindle machinery (Sauer et al. 2005). One hypothesis is that microtubules and proteins of the spindle not only play a role in generating force for chromosome segregation and organization, but also serve as tracks for the intracellular transport of the ribosomes (Sauer et al. 2005). On the other hand, the enrichment of ribosomal proteins in the spindle might reflect a fundamental connection with the nucleolus. Intriguingly, the candidates identified by Sauer et al. (2005) include nucleolin and fibrillarin, two proteins that specifically target the nucleolus and are required for its formation (Ochs et al. 1985; Ma et al. 2007), as well as several other proteins that also localize to this organelle (e.g., nucleolar RNA helicases and small nucleolar ribonucleoproteins). Detailed investigation suggested that some proteins might play dual roles, forming associations with the microtubules during mitosis as well as localizing to the nucleolus during interphase (Zatsepina et al. 1999). Altogether, these observations further raise the possibility that nucleolar stress might be one consequence of combined BPA/sugar toxicity.
Collectively, our observations point to synergistic toxic interactions between BPA and dietary sugar and indicate significant perturbations in the expression of ribosome-associated proteins and nucleolar stress. Our analyses show that the interaction between BPA and sugar results in heightened disruption of ribosome-associated genes and heightened activation of nucleolus-specific transposable elements R1 and R2. The data suggest DNA methylation-independent mechanisms through which BPA exerts its toxic effects and raise the question of how persistent the insult might be upon its removal and between generations.
Supplementary Material
Acknowledgments
We thank John Gibbons, Katherine Silkaitis, and Shoukai Yu for helpful discussions and valuable comments on the manuscript. We thank Daniel L. Hartl for his generous support and sharing of probes for gene expression analyses. This work was partially supported by a Milton Award from Harvard University. B.L. acknowledges support from an Ellison Medical Foundation New Scholar in Aging Award and a Smith Family Award for Biomedical Research.
Footnotes
Communicating editor: J. A. Birchler
Literature Cited
- Allard P., Colaiacovo M. P., 2010. Bisphenol A impairs the double-strand break repair machinery in the germline and causes chromosome abnormalities. Proc. Natl. Acad. Sci. USA 107: 20405–20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso J., Santaren J. F., 2006. Characterization of the Drosophila melanogaster ribosomal proteome. J Proteome Res 5: 2025–2032. [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P., Morimoto S., Ripoll C., Fuentes E., Nadal A., 2006. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ. Health Perspect. 114: 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K. D., Thummel C. S., 2007. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab. 6: 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert F. M., van Koningsbruggen S., Navascues J., Lamond A. I., 2007. The multifunctional nucleolus. Nat. Rev. Mol. Cell Biol. 8: 574–585. [DOI] [PubMed] [Google Scholar]
- Branco A. T., Hartl D. L., Lemos B., 2013. Chromatin-associated proteins HP1 and Mod(mdg4) modify Y-linked regulatory variation in the Drosophila testis. Genetics 194: 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N., Broderick N. A., Lemaitre B., 2013. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat. Rev. Microbiol. 11: 615–626. [DOI] [PubMed] [Google Scholar]
- Burridge E., 2003. Bisphenol A: product profile. Eur Chem News 17: 14–20. [Google Scholar]
- Calafat A. M., Kuklenyik Z., Reidy J. A., Caudill S. P., Ekong J., et al. , 2005. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ. Health Perspect. 113: 391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli V. R., Wang J., Dow J. A., 2007. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39: 715–720. [DOI] [PubMed] [Google Scholar]
- Doherty L. F., Bromer J. G., Zhou Y., Aldad T. S., Taylor H. S., 2010. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm. Cancer 1: 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Bayir H., Lai Y., Zhang X., Kochanek P. M., et al. , 2004. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J. Biol. Chem. 279: 38563–38570. [DOI] [PubMed] [Google Scholar]
- Gou D., Rubalcava M., Sauer S., Mora-Bermudez F., Erdjument-Bromage H., et al. , 2010. SETDB1 is involved in postembryonic DNA methylation and gene silencing in Drosophila. PLoS ONE 5: e10581. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hunt P. A., Lawson C., Gieske M., Murdoch B., Smith H., et al. , 2012. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc. Natl. Acad. Sci. USA 109: 17525–17530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K., Yaoi T., Fushiki S., 2012. Bisphenol A, an endocrine-disrupting chemical, and brain development. Neuropathology 32: 447–457. [DOI] [PubMed] [Google Scholar]
- Jang C. Y., Shin H. S., Kim H. D., Kim J. W., Choi S. Y., et al. , 2011. Ribosomal protein S3 is stabilized by sumoylation. Biochem. Biophys. Res. Commun. 414: 523–527. [DOI] [PubMed] [Google Scholar]
- Jang C. Y., Kim H. D., Kim J., 2012a Ribosomal protein S3 interacts with TRADD to induce apoptosis through caspase dependent JNK activation. Biochem. Biophys. Res. Commun. 421: 474–478. [DOI] [PubMed] [Google Scholar]
- Jang C. Y., Kim H. D., Zhang X., Chang J. S., Kim J., 2012b Ribosomal protein S3 localizes on the mitotic spindle and functions as a microtubule associated protein in mitosis. Biochem. Biophys. Res. Commun. 429: 57–62. [DOI] [PubMed] [Google Scholar]
- Kim H. D., Lee J. Y., Kim J., 2005. Erk phosphorylates threonine 42 residue of ribosomal protein S3. Biochem. Biophys. Res. Commun. 333: 110–115. [DOI] [PubMed] [Google Scholar]
- Kim T. S., Jang C. Y., Kim H. D., Lee J. Y., Ahn B. Y., et al. , 2006. Interaction of Hsp90 with ribosomal proteins protects from ubiquitination and proteasome-dependent degradation. Mol. Biol. Cell 17: 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T. S., Kim H. D., Shin H. S., Kim J., 2009. Phosphorylation status of nuclear ribosomal protein S3 is reciprocally regulated by protein kinase C{delta} and protein phosphatase 2A. J. Biol. Chem. 284: 21201–21208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King E. G., Macdonald S. J., Long A. D., 2012a Properties and power of the Drosophila Synthetic Population Resource for the routine dissection of complex traits. Genetics 191: 935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King E. G., Merkes C. M., McNeil C. L., Hoofer S. R., Sen S., et al. , 2012b Genetic dissection of a model complex trait using the Drosophila Synthetic Population Resource. Genome Res. 22: 1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M., Gudsnuk K., Franks B., Madrid J., Miller R. L., et al. , 2013. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc. Natl. Acad. Sci. USA 110: 9956–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang I. A., Galloway T. S., Scarlett A., Henley W. E., Depledge M., et al. , 2008. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 300: 1303–1310. [DOI] [PubMed] [Google Scholar]
- Lawson C., Gieske M., Murdoch B., Ye P., Li Y., et al. , 2011. Gene expression in the fetal mouse ovary is altered by exposure to low doses of bisphenol A. Biol. Reprod. 84: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos B., Araripe L. O., Hartl D. L., 2008. Polymorphic Y chromosomes harbor cryptic variation with manifold functional consequences. Science 319: 91–93. [DOI] [PubMed] [Google Scholar]
- Lyko F., Whittaker A. J., Orr-Weaver T. L., Jaenisch R., 2000. The putative Drosophila methyltransferase gene dDnmt2 is contained in a transposon-like element and is expressed specifically in ovaries. Mech. Dev. 95: 215–217. [DOI] [PubMed] [Google Scholar]
- Lyne R., Smith R., Rutherford K., Wakeling M., Varley A., et al. , 2007. FlyMine: an integrated database for Drosophila and Anopheles genomics. Genome Biol. 8: R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N., Matsunaga S., Takata H., Ono-Maniwa R., Uchiyama S., et al. , 2007. Nucleolin functions in nucleolus formation and chromosome congression. J. Cell Sci. 120: 2091–2105. [DOI] [PubMed] [Google Scholar]
- Mackay T. F., Richards S., Stone E. A., Barbadilla A., Ayroles J. F., et al. , 2012. The Drosophila melanogaster Genetic Reference Panel. Nature 482: 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliano D. J., Lyons J. G., 2013. Bisphenol A and diabetes, insulin resistance, cardiovascular disease and obesity: controversy in a (plastic) cup? J. Clin. Endocrinol. Metab. 98: 502–504. [DOI] [PubMed] [Google Scholar]
- Marygold S. J., Roote J., Reuter G., Lambertsson A., Ashburner M., et al. , 2007. The ribosomal protein genes and Minute loci of Drosophila melanogaster. Genome Biol 8: R216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman L. P., Fink J. L., Narzinski K., Ramachandran P. V., Hathiramani S. S., et al. , 2011. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis. Model. Mech. 4: 842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na J., Musselman L. P., Pendse J., Baranski T. J., Bodmer R., et al. , 2013. A Drosophila model of high sugar diet-induced cardiomyopathy. PLoS Genet. 9: e1003175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth A., Langst G., 2011. Genome organization in and around the nucleolus. Trends Genet. 27: 149–156. [DOI] [PubMed] [Google Scholar]
- Nousiainen M., Sillje H. H., Sauer G., Nigg E. A., Korner R., 2006. Phosphoproteome analysis of the human mitotic spindle. Proc. Natl. Acad. Sci. USA 103: 5391–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs R. L., Lischwe M. A., Spohn W. H., Busch H., 1985. Fibrillarin: a new protein of the nucleolus identified by autoimmune sera. Biol. Cell 54: 123–133. [DOI] [PubMed] [Google Scholar]
- Ohlstein B., Spradling A., 2006. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439: 470–474. [DOI] [PubMed] [Google Scholar]
- Pfaffl M. W., Horgan G. W., Dempfle L., 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30: e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phalke S., Nickel O., Walluscheck D., Hortig F., Onorati M. C., et al. , 2009. Retrotransposon silencing and telomere integrity in somatic cells of Drosophila depends on the cytosine-5 methyltransferase DNMT2. Nat. Genet. 41: 696–702. [DOI] [PubMed] [Google Scholar]
- Pupo M., Pisano A., Lappano R., Santolla M. F., De Francesco E. M., et al. , 2012. Bisphenol A induces gene expression changes and proliferative effects through GPER in breast cancer cells and cancer-associated fibroblasts. Environ. Health Perspect. 120: 1177–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. W., Herzyk P., Dow J. A., Leader D. P., 2013. FlyAtlas: database of gene expression in the tissues of Drosophila melanogaster. Nucleic Acids Res. 41: D744–D750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer G., Korner R., Hanisch A., Ries A., Nigg E. A., et al. , 2005. Proteome analysis of the human mitotic spindle. Mol. Cell. Proteomics 4: 35–43. [DOI] [PubMed] [Google Scholar]
- Schaefer M., Lyko F., 2010. Lack of evidence for DNA methylation of Invader4 retroelements in Drosophila and implications for Dnmt2-mediated epigenetic regulation. Nat. Genet. 42: 920–921, author reply 921. [DOI] [PubMed] [Google Scholar]
- Smyth G. K., Speed T., 2003. Normalization of cDNA microarray data. Methods 31: 265–273. [DOI] [PubMed] [Google Scholar]
- Sudmant P. H., Kitzman J. O., Antonacci F., Alkan C., Malig M., et al. , 2010. Diversity of human copy number variation and multicopy genes. Science 330: 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres J. Z., Summers M. K., Peterson D., Brauer M. J., Lee J., et al. , 2011. The STARD9/Kif16a kinesin associates with mitotic microtubules and regulates spindle pole assembly. Cell 147: 1309–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J. P., Hartl D. L., 2002. Bayesian analysis of gene expression levels: statistical quantification of relative mRNA level across multiple strains or treatments. Genome Biol. 3: RESEARCH0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal F. S., Myers J. P., 2008. Bisphenol A and risk of metabolic disorders. JAMA 300: 1353–1355. [DOI] [PubMed] [Google Scholar]
- vom Saal F. S., Akingbemi B. T., Belcher S. M., Birnbaum L. S., Crain D. A., et al. , 2007. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol. 24: 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber Lozada K., Keri R. A., 2011. Bisphenol A increases mammary cancer risk in two distinct mouse models of breast cancer. Biol. Reprod. 85: 490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatsepina O. V., Rousselet A., Chan P. K., Olson M. O., Jordan E. G., et al. , 1999. The nucleolar phosphoprotein B23 redistributes in part to the spindle poles during mitosis. J. Cell Sci. 112(Pt 4): 455–466. [DOI] [PubMed] [Google Scholar]
- Zwarts L., Magwire M. M., Carbone M. A., Versteven M., Herteleer L., et al. , 2011. Complex genetic architecture of Drosophila aggressive behavior. Proc. Natl. Acad. Sci. USA 108: 17070–17075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.