Abstract
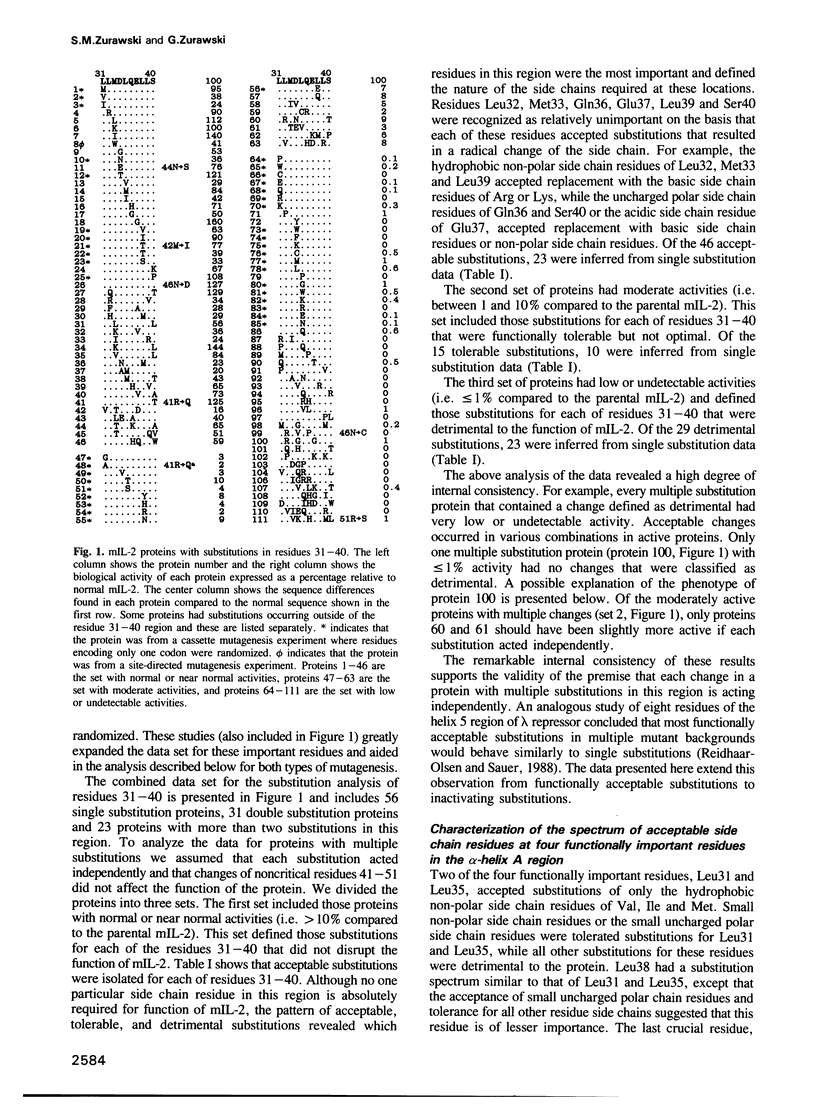
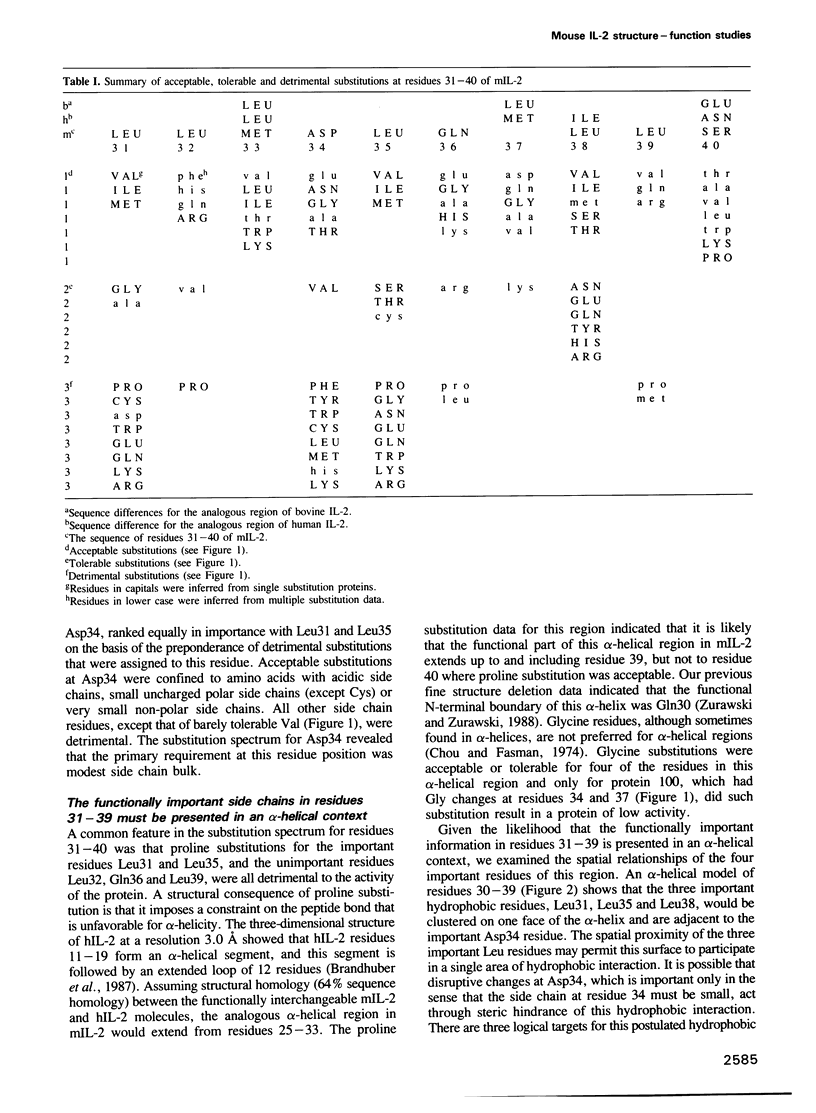
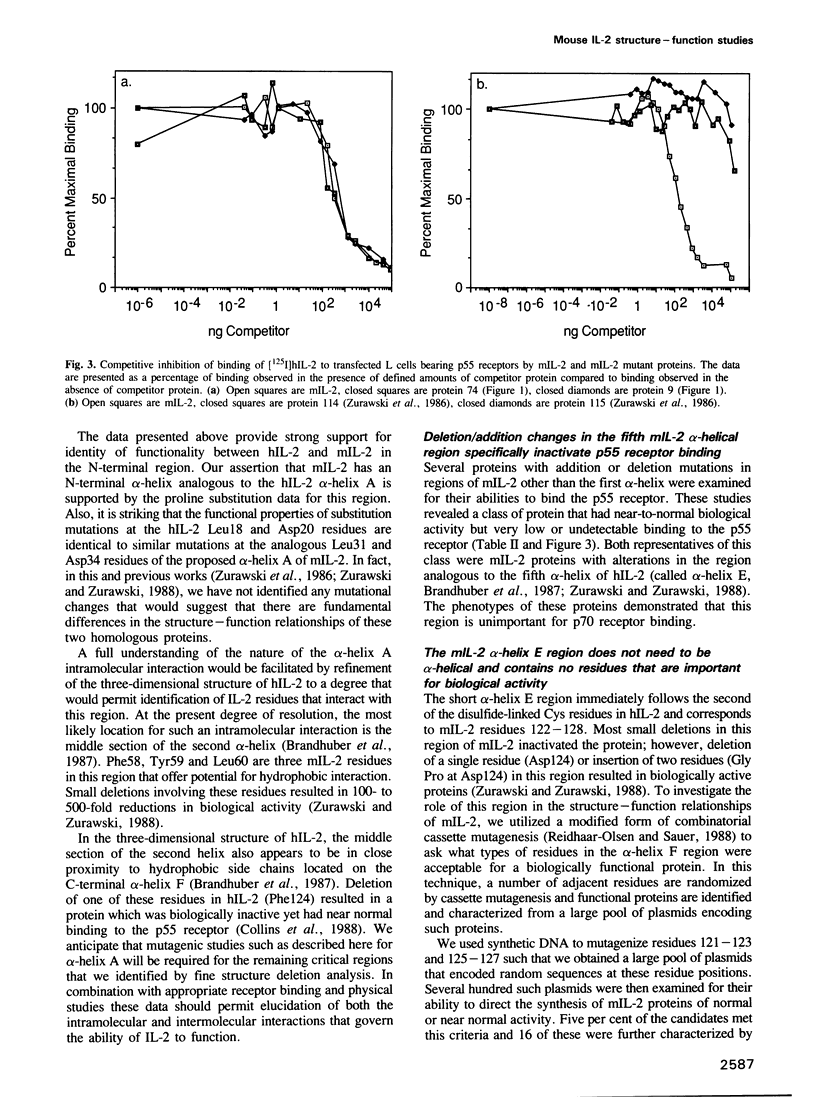
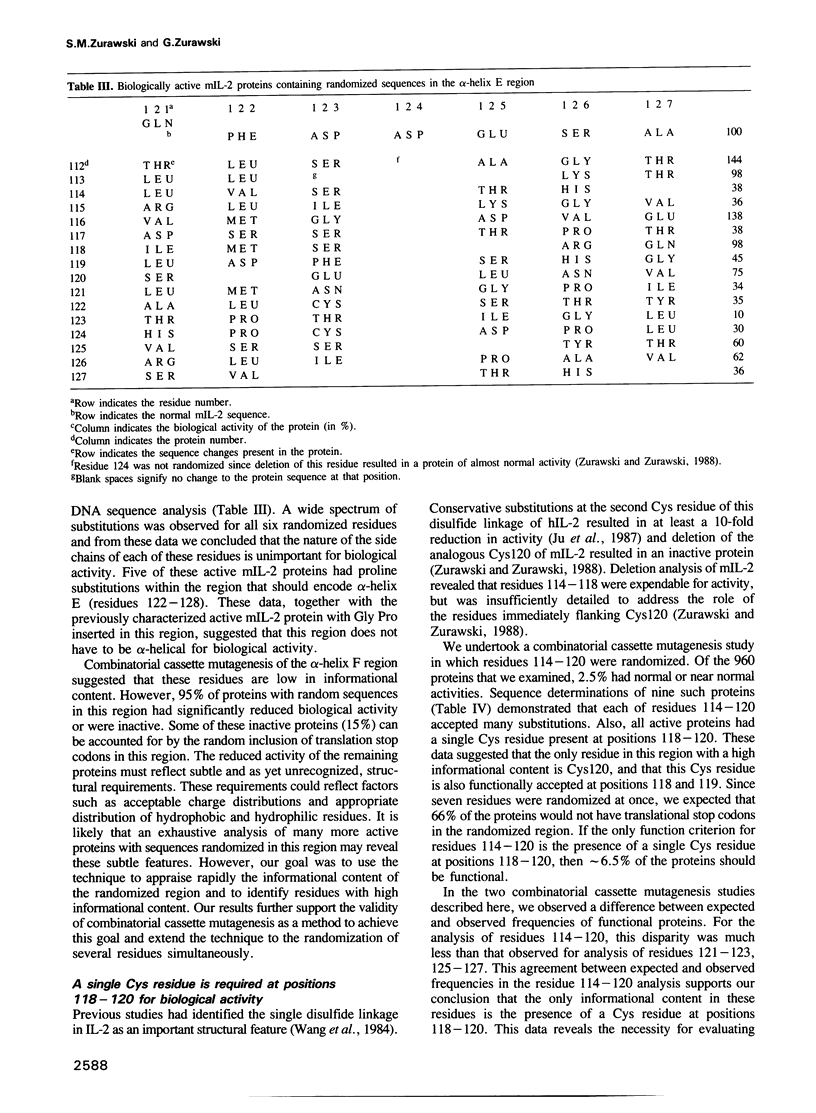
The function of two alpha-helical regions of mouse interleukin-2 were analyzed by saturation substitution analysis. The functional parts of the first alpha-helix (A) was defined as residues 31-39 by the observation that proline substitutions within this region inactivate the protein. Four residues within alpha-helix A, Leu31, Asp34, Leu35 and Leu38, were found to be crucial for biological activity. Structural modeling suggested that these four residues are clustered on one face of alpha-helix A. Residues 31 and 35 had to remain hydrophobic for the molecule to be functional. At residue 38 there was a preference for hydrophobic side chain residues, while at residue 34 some small side chain residues as well as acidic or amide side chain residues were functionally acceptable. Inactivating changes at residue 34 had no effect upon the ability of the protein to interact with the p55 receptor. Disruption of the fifth alpha-helix (E), which had little effect upon biological activity, resulted in an inability of the protein to interact with the p55 receptor. Mutagenesis of the alpha-helix E region demonstrated that alpha-helicity and the nature of the side chain residues in this region were unimportant for biological activity. The region immediately proximal to alpha-helix E was important only for the single intramolecular disulfide linkage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandhuber B. J., Boone T., Kenney W. C., McKay D. B. Three-dimensional structure of interleukin-2. Science. 1987 Dec 18;238(4834):1707–1709. doi: 10.1126/science.3500515. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Collins L., Tsien W. H., Seals C., Hakimi J., Weber D., Bailon P., Hoskings J., Greene W. C., Toome V., Ju G. Identification of specific residues of human interleukin 2 that affect binding to the 70-kDa subunit (p70) of the interleukin 2 receptor. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7709–7713. doi: 10.1073/pnas.85.20.7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G., Collins L., Kaffka K. L., Tsien W. H., Chizzonite R., Crowl R., Bhatt R., Kilian P. L. Structure-function analysis of human interleukin-2. Identification of amino acid residues required for biological activity. J Biol Chem. 1987 Apr 25;262(12):5723–5731. [PubMed] [Google Scholar]
- Miller J., Malek T. R., Leonard W. J., Greene W. C., Shevach E. M., Germain R. N. Nucleotide sequence and expression of a mouse interleukin 2 receptor cDNA. J Immunol. 1985 Jun;134(6):4212–4217. [PubMed] [Google Scholar]
- O'Garra A., Umland S., De France T., Christiansen J. 'B-cell factors' are pleiotropic. Immunol Today. 1988 Feb;9(2):45–54. doi: 10.1016/0167-5699(88)91259-5. [DOI] [PubMed] [Google Scholar]
- Reidhaar-Olson J. F., Sauer R. T. Combinatorial cassette mutagenesis as a probe of the informational content of protein sequences. Science. 1988 Jul 1;241(4861):53–57. doi: 10.1126/science.3388019. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Tsui L. C., Breitman M. L., Siminovitch L., Buchwald M. Persistence of freely replicating SV40 recombinant molecules carrying a selectable marker in permissive simian cells. Cell. 1982 Sep;30(2):499–508. doi: 10.1016/0092-8674(82)90247-1. [DOI] [PubMed] [Google Scholar]
- Wang A., Lu S. D., Mark D. F. Site-specific mutagenesis of the human interleukin-2 gene: structure-function analysis of the cysteine residues. Science. 1984 Jun 29;224(4656):1431–1433. doi: 10.1126/science.6427925. [DOI] [PubMed] [Google Scholar]
- Yokota T., Coffman R. L., Hagiwara H., Rennick D. M., Takebe Y., Yokota K., Gemmell L., Shrader B., Yang G., Meyerson P. Isolation and characterization of lymphokine cDNA clones encoding mouse and human IgA-enhancing factor and eosinophil colony-stimulating factor activities: relationship to interleukin 5. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7388–7392. doi: 10.1073/pnas.84.21.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski S. M., Mosmann T. R., Benedik M., Zurawski G. Alterations in the amino-terminal third of mouse interleukin 2: effects on biological activity and immunoreactivity. J Immunol. 1986 Nov 15;137(10):3354–3360. [PubMed] [Google Scholar]
- Zurawski S. M., Zurawski G. Identification of three critical regions within mouse interleukin 2 by fine structural deletion analysis. EMBO J. 1988 Apr;7(4):1061–1069. doi: 10.1002/j.1460-2075.1988.tb02914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


