Abstract
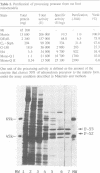
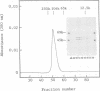
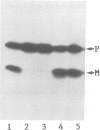
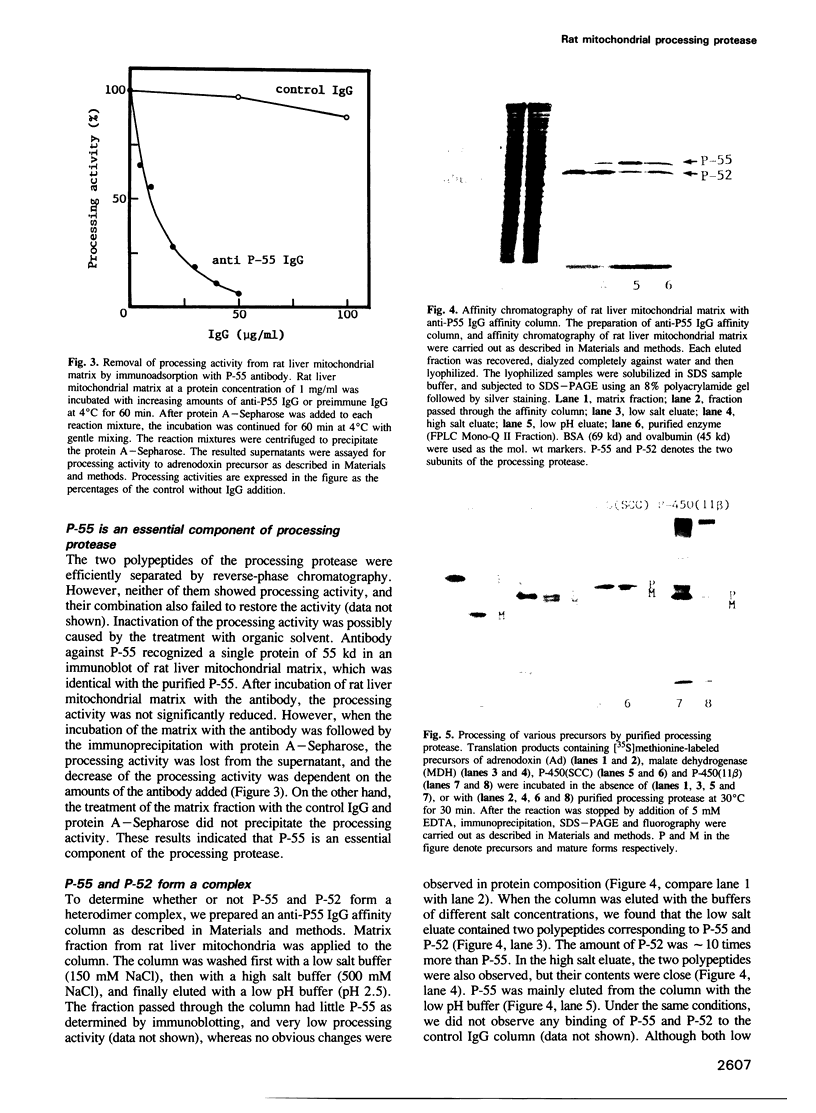
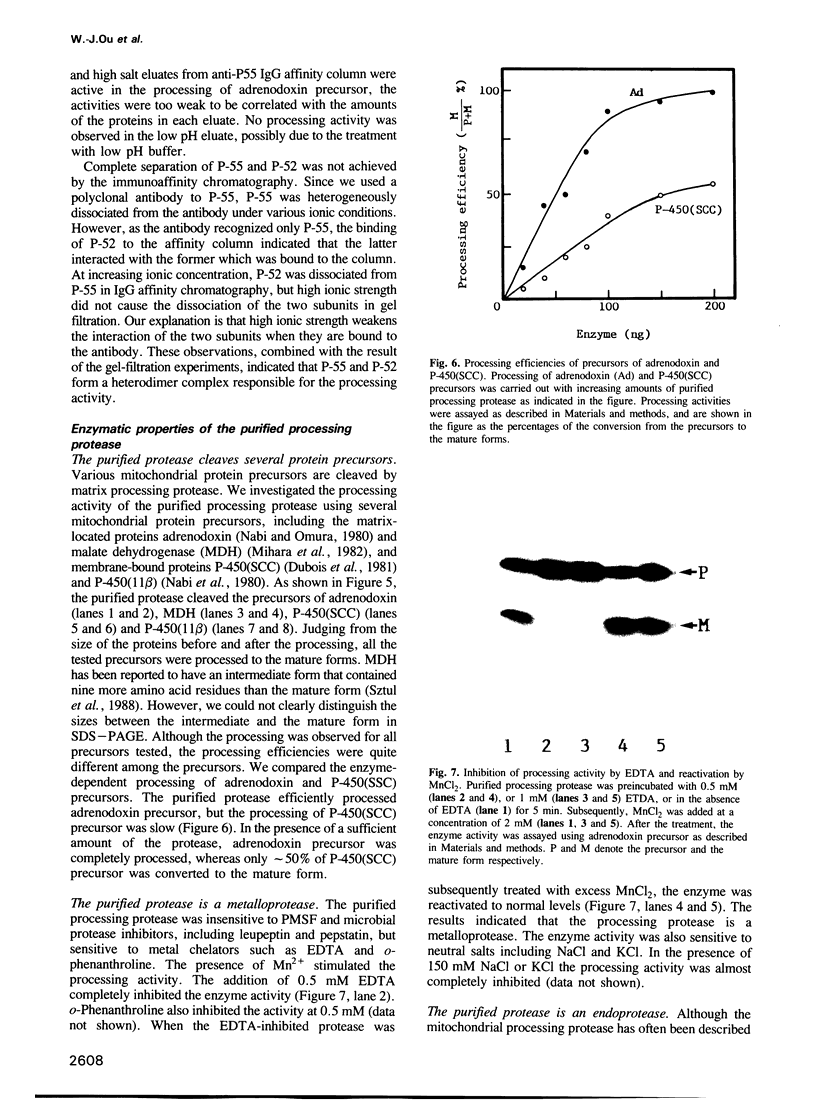
A processing protease has been purified from the matrix fraction of rat liver mitochondria. The purified protease contained two protein subunits of 55 kd (P-55) and 52 kd (P-52) as determined by SDS-PAGE. The processing protease was estimated to be 105 kd in gel filtration, indicating that the two protein subunits form a heterodimeric complex. At high ionic conditions, the two subunits dissociated. The purified processing protease cleaved several mitochondrial protein precursors destined to different mitochondrial compartments, including adrenodoxin, malate dehydrogenase, P-450(SCC) and P-450(11 beta), but the processing efficiencies were different each other. The endoprotease nature of the processing protease was confirmed with the purified enzyme using adrenodoxin precursor as the substrate; both the mature form and the extension peptide were detected after the processing. The processing activity of the protease was inhibited by metal chelators, and reactivated by Mn2+, indicating that the protease is a metalloprotease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böhni P. C., Daum G., Schatz G. Import of proteins into mitochondria. Partial purification of a matrix-located protease involved in cleavage of mitochondrial precursor polypeptides. J Biol Chem. 1983 Apr 25;258(8):4937–4943. [PubMed] [Google Scholar]
- Cerletti N., Böhni P. C., Suda K. Import of proteins into mitochondria. Isolated yeast mitochondria and a solubilized matrix protease correctly process cytochrome c oxidase subunit V precursor at the NH2 terminus. J Biol Chem. 1983 Apr 25;258(8):4944–4949. [PubMed] [Google Scholar]
- Conboy J. G., Fenton W. A., Rosenberg L. E. Processing of pre-ornithine transcarbamylase requires a zinc-dependent protease localized to the mitochondrial matrix. Biochem Biophys Res Commun. 1982 Mar 15;105(1):1–7. doi: 10.1016/s0006-291x(82)80002-8. [DOI] [PubMed] [Google Scholar]
- Daum G., Gasser S. M., Schatz G. Import of proteins into mitochondria. Energy-dependent, two-step processing of the intermembrane space enzyme cytochrome b2 by isolated yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13075–13080. [PubMed] [Google Scholar]
- DuBois R. N., Simpson E. R., Tuckey J., Lambeth J. D., Waterman M. R. Evidence for a higher molecular weight precursor of cholesterol side-chain-cleavage cytochrome P-450 and induction of mitochondrial and cytosolic proteins by corticotropin in adult bovine adrenal cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1028–1032. doi: 10.1073/pnas.78.2.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers M., Schatz G. Protein unfolding and the energetics of protein translocation across biological membranes. Cell. 1988 Feb 26;52(4):481–483. doi: 10.1016/0092-8674(88)90458-8. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Gilmore R., Blobel G. Purification of microsomal signal peptidase as a complex. Proc Natl Acad Sci U S A. 1986 Feb;83(3):581–585. doi: 10.1073/pnas.83.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya S., Okada M., Ito A., Aoyagi H., Kanmera T., Kato T., Sagara Y., Horiuchi T., Omura T. Synthetic partial extension peptides of P-450(SCC) and adrenodoxin precursors: effects on the import of mitochondrial enzyme precursors. J Biochem. 1987 Oct;102(4):821–832. doi: 10.1093/oxfordjournals.jbchem.a122121. [DOI] [PubMed] [Google Scholar]
- Gasser S. M., Daum G., Schatz G. Import of proteins into mitochondria. Energy-dependent uptake of precursors by isolated mitochondria. J Biol Chem. 1982 Nov 10;257(21):13034–13041. [PubMed] [Google Scholar]
- Hartl F. U., Schmidt B., Wachter E., Weiss H., Neupert W. Transport into mitochondria and intramitochondrial sorting of the Fe/S protein of ubiquinol-cytochrome c reductase. Cell. 1986 Dec 26;47(6):939–951. doi: 10.1016/0092-8674(86)90809-3. [DOI] [PubMed] [Google Scholar]
- Hawlitschek G., Schneider H., Schmidt B., Tropschug M., Hartl F. U., Neupert W. Mitochondrial protein import: identification of processing peptidase and of PEP, a processing enhancing protein. Cell. 1988 Jun 3;53(5):795–806. doi: 10.1016/0092-8674(88)90096-7. [DOI] [PubMed] [Google Scholar]
- Hay R., Böhni P., Gasser S. How mitochondria import proteins. Biochim Biophys Acta. 1984 Jan 27;779(1):65–87. doi: 10.1016/0304-4157(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Hennig B., Neupert W. Assembly of cytochrome c. Apocytochrome c is bound to specific sites on mitochondria before its conversion to holocytochrome c. Eur J Biochem. 1981 Dec;121(1):203–212. doi: 10.1111/j.1432-1033.1981.tb06450.x. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Mellman I., Rosenberg L. E. A leader peptide is sufficient to direct mitochondrial import of a chimeric protein. EMBO J. 1985 May;4(5):1129–1135. doi: 10.1002/j.1460-2075.1985.tb03750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Schatz G. The cleavable prepiece of an imported mitochondrial protein is sufficient to direct cytosolic dihydrofolate reductase into the mitochondrial matrix. FEBS Lett. 1984 Dec 10;178(2):306–310. doi: 10.1016/0014-5793(84)80622-5. [DOI] [PubMed] [Google Scholar]
- Isaya G., Fenton W. A., Hendrick J. P., Furtak K., Kalousek F., Rosenberg L. E. Mitochondrial import and processing of mutant human ornithine transcarbamylase precursors in cultured cells. Mol Cell Biol. 1988 Dec;8(12):5150–5158. doi: 10.1128/mcb.8.12.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A., Ogishima T., Ou W., Omura T., Aoyagi H., Lee S., Mihara H., Izumiya N. Effects of synthetic model peptides resembling the extension peptides of mitochondrial enzyme precursors on import of the precursors into mitochondria. J Biochem. 1985 Dec;98(6):1571–1582. doi: 10.1093/oxfordjournals.jbchem.a135426. [DOI] [PubMed] [Google Scholar]
- Jensen R. E., Yaffe M. P. Import of proteins into yeast mitochondria: the nuclear MAS2 gene encodes a component of the processing protease that is homologous to the MAS1-encoded subunit. EMBO J. 1988 Dec 1;7(12):3863–3871. doi: 10.1002/j.1460-2075.1988.tb03272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochen A. L., Berhanu P. Chymotrypsin substrate analogues inhibit endocytosis of insulin and insulin receptors in adipocytes. J Cell Biol. 1986 Nov;103(5):1807–1816. doi: 10.1083/jcb.103.5.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalousek F., Hendrick J. P., Rosenberg L. E. Two mitochondrial matrix proteases act sequentially in the processing of mammalian matrix enzymes. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7536–7540. doi: 10.1073/pnas.85.20.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalousek F., Orsulak M. D., Rosenberg L. E. Newly processed ornithine transcarbamylase subunits are assembled to trimers in rat liver mitochondria. J Biol Chem. 1984 May 10;259(9):5392–5395. [PubMed] [Google Scholar]
- Kumamoto T., Ito A., Omura T. Characterization of a mitochondrial matrix protease catalyzing the processing of adrenodoxin precursor. J Biochem. 1986 Jul;100(1):247–254. doi: 10.1093/oxfordjournals.jbchem.a121700. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAda P. C., Douglas M. G. A neutral metallo endoprotease involved in the processing of an F1-ATPase subunit precursor in mitochondria. J Biol Chem. 1982 Mar 25;257(6):3177–3182. [PubMed] [Google Scholar]
- Mihara K., Omura T., Harano T., Brenner S., Fleischer S., Rajagopalan K. V., Blobel G. Rat liver L-glutamate dehydrogenase, malate dehydrogenase, D-beta-hydroxybutyrate dehydrogenase, and sulfite oxidase are each synthesized as larger precursors by cytoplasmic free polysomes. J Biol Chem. 1982 Apr 10;257(7):3355–3358. [PubMed] [Google Scholar]
- Miura S., Mori M., Amaya Y., Tatibana M. A mitochondrial protease that cleaves the precursor of ornithine carbamoyltransferase. Purification and properties. Eur J Biochem. 1982 Mar 1;122(3):641–647. [PubMed] [Google Scholar]
- Mollay C., Vilas U., Kreil G. Cleavage of honeybee prepromelittin by an endoprotease from rat liver microsomes: identification of intact signal peptide. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2260–2263. doi: 10.1073/pnas.79.7.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy D. I., Strittmatter W. J. Requirement for metalloendoprotease in exocytosis: evidence in mast cells and adrenal chromaffin cells. Cell. 1985 Mar;40(3):645–656. doi: 10.1016/0092-8674(85)90213-2. [DOI] [PubMed] [Google Scholar]
- Nabi N., Kominami S., Takemori S., Omura T. In vitro synthesis of mitochondrial cytochromes P-450(scc) and P-450(11-beta) and microsomal cytochrome P-450(C-21) by both free and bound polysomes isolated from bovine adrenal cortex. Biochem Biophys Res Commun. 1980 Nov 28;97(2):687–693. doi: 10.1016/0006-291x(80)90319-8. [DOI] [PubMed] [Google Scholar]
- Nabi N., Omura T. In vitro synthesis of adrenodoxin and adrenodoxin reductase: existence of a putative large precursor form of adrenodoxin. Biochem Biophys Res Commun. 1980 Nov 28;97(2):680–686. doi: 10.1016/0006-291x(80)90318-6. [DOI] [PubMed] [Google Scholar]
- Nelson N., Schatz G. Energy-dependent processing of cytoplasmically made precursors to mitochondrial proteins. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4365–4369. doi: 10.1073/pnas.76.9.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogishima T., Okada Y., Omura T. Import and processing of the precursor of cytochrome P-450(SCC) by bovine adrenal cortex mitochondria. J Biochem. 1985 Sep;98(3):781–791. doi: 10.1093/oxfordjournals.jbchem.a135335. [DOI] [PubMed] [Google Scholar]
- Ohashi A., Gibson J., Gregor I., Schatz G. Import of proteins into mitochondria. The precursor of cytochrome c1 is processed in two steps, one of them heme-dependent. J Biol Chem. 1982 Nov 10;257(21):13042–13047. [PubMed] [Google Scholar]
- Okamura T., John M. E., Zuber M. X., Simpson E. R., Waterman M. R. Molecular cloning and amino acid sequence of the precursor form of bovine adrenodoxin: evidence for a previously unidentified COOH-terminal peptide. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5705–5709. doi: 10.1073/pnas.82.17.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono H., Ito A. Transport of the precursor for sulfite oxidase into intermembrane space of liver mitochondria: binding of the precursor to outer mitochondrial membrane. J Biochem. 1984 Feb;95(2):353–358. doi: 10.1093/oxfordjournals.jbchem.a134615. [DOI] [PubMed] [Google Scholar]
- Ono H., Ito A. Transport of the precursor for sulfite oxidase into intermembrane space of liver mitochondria: characterization of import and processing activities. J Biochem. 1984 Feb;95(2):345–352. doi: 10.1093/oxfordjournals.jbchem.a134614. [DOI] [PubMed] [Google Scholar]
- Ou W. J., Ito A., Morohashi K., Fujii-Kuriyama Y., Omura T. Processing-independent in vitro translocation of cytochrome P-450(SCC) precursor across mitochondrial membranes. J Biochem. 1986 Nov;100(5):1287–1296. doi: 10.1093/oxfordjournals.jbchem.a121835. [DOI] [PubMed] [Google Scholar]
- Ou W. J., Ito A., Umeda M., Inoue K., Omura T. Specific binding of mitochondrial protein precursors to liposomes containing cardiolipin. J Biochem. 1988 Apr;103(4):589–595. doi: 10.1093/oxfordjournals.jbchem.a122312. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pollock R. A., Hartl F. U., Cheng M. Y., Ostermann J., Horwich A., Neupert W. The processing peptidase of yeast mitochondria: the two co-operating components MPP and PEP are structurally related. EMBO J. 1988 Nov;7(11):3493–3500. doi: 10.1002/j.1460-2075.1988.tb03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roise D., Horvath S. J., Tomich J. M., Richards J. H., Schatz G. A chemically synthesized pre-sequence of an imported mitochondrial protein can form an amphiphilic helix and perturb natural and artificial phospholipid bilayers. EMBO J. 1986 Jun;5(6):1327–1334. doi: 10.1002/j.1460-2075.1986.tb04363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara Y., Ito A., Omura T. Partial purification of a metalloprotease catalyzing the processing of adrenodoxin precursor in bovine adrenal cortex mitochondria. J Biochem. 1984 Dec;96(6):1743–1752. doi: 10.1093/oxfordjournals.jbchem.a135007. [DOI] [PubMed] [Google Scholar]
- Schleyer M., Schmidt B., Neupert W. Requirement of a membrane potential for the posttranslational transfer of proteins into mitochondria. Eur J Biochem. 1982 Jun 15;125(1):109–116. doi: 10.1111/j.1432-1033.1982.tb06657.x. [DOI] [PubMed] [Google Scholar]
- Schmidt B., Wachter E., Sebald W., Neupert W. Processing peptidase of Neurospora mitochondria. Two-step cleavage of imported ATPase subunit 9. Eur J Biochem. 1984 Nov 2;144(3):581–588. doi: 10.1111/j.1432-1033.1984.tb08505.x. [DOI] [PubMed] [Google Scholar]
- Strous G. J., van Kerkhof P., Dekker J., Schwartz A. L. Metalloendoprotease inhibitors block protein synthesis, intracellular transport, and endocytosis in hepatoma cells. J Biol Chem. 1988 Dec 5;263(34):18197–18204. [PubMed] [Google Scholar]
- Suzuki T., Ichihara S., Mizushima S. Purification and characterization of a signal peptide, a product of protein secretion across the cytoplasmic membrane of Escherichia coli. J Biochem. 1988 Mar;103(3):470–473. doi: 10.1093/oxfordjournals.jbchem.a122294. [DOI] [PubMed] [Google Scholar]
- Sztul E. S., Chu T. W., Strauss A. W., Rosenberg L. E. Import of the malate dehydrogenase precursor by mitochondria. Cleavage within leader peptide by matrix protease leads to formation of intermediate-sized form. J Biol Chem. 1988 Aug 25;263(24):12085–12091. [PubMed] [Google Scholar]
- Teintze M., Slaughter M., Weiss H., Neupert W. Biogenesis of mitochondrial ubiquinol:cytochrome c reductase (cytochrome bc1 complex). Precursor proteins and their transfer into mitochondria. J Biol Chem. 1982 Sep 10;257(17):10364–10371. [PubMed] [Google Scholar]
- Witte C., Jensen R. E., Yaffe M. P., Schatz G. MAS1, a gene essential for yeast mitochondrial assembly, encodes a subunit of the mitochondrial processing protease. EMBO J. 1988 May;7(5):1439–1447. doi: 10.1002/j.1460-2075.1988.tb02961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe M. P., Ohta S., Schatz G. A yeast mutant temperature-sensitive for mitochondrial assembly is deficient in a mitochondrial protease activity that cleaves imported precursor polypeptides. EMBO J. 1985 Aug;4(8):2069–2074. doi: 10.1002/j.1460-2075.1985.tb03893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe M. P., Schatz G. Two nuclear mutations that block mitochondrial protein import in yeast. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4819–4823. doi: 10.1073/pnas.81.15.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Jensen R. E., Yaffe M. P., Oppliger W., Schatz G. Import of proteins into yeast mitochondria: the purified matrix processing protease contains two subunits which are encoded by the nuclear MAS1 and MAS2 genes. EMBO J. 1988 Dec 1;7(12):3857–3862. doi: 10.1002/j.1460-2075.1988.tb03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwizinski C., Neupert W. Precursor proteins are transported into mitochondria in the absence of proteolytic cleavage of the additional sequences. J Biol Chem. 1983 Nov 10;258(21):13340–13346. [PubMed] [Google Scholar]
- Zwizinski C., Schleyer M., Neupert W. Transfer of proteins into mitochondria. Precursor to the ADP/ATP carrier binds to receptor sites on isolated mitochondria. J Biol Chem. 1983 Apr 10;258(7):4071–4074. [PubMed] [Google Scholar]
- Zwizinski C., Wickner W. Purification and characterization of leader (signal) peptidase from Escherichia coli. J Biol Chem. 1980 Aug 25;255(16):7973–7977. [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]