Abstract
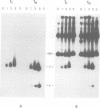
The replication of the single-stranded (ss) DNA plasmid pC194 by the rolling circle mechanism was investigated using chimeric plasmids that possess two pC194 replication origins. One of the origins was intact, whereas the other was either intact or mutated. The origins were activated by inducing synthesis of the pC194 replication protein, under the control of lambda phage pL promoter. Initiation of pC194 replication at one origin and termination at the other generated circular ssDNA molecules smaller than the parental chimeric plasmid. From the nature and the amount of ssDNA circles, the activity of an origin could be assessed. Our results show that (i) the signal for initiation of pC194 replication is more stringent than that for termination; (ii) the sequence and structure of the origin are important for its activity and (iii) successful termination of one replication cycle is not followed by reinitiation of another. This last observation differentiates a ssDNA plasmid (pC194) from a ssDNA phage (phi X174).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas P. D., Jansz H. S. Single-stranded DNA phage origins. Curr Top Microbiol Immunol. 1988;136:31–70. doi: 10.1007/978-3-642-73115-0_3. [DOI] [PubMed] [Google Scholar]
- Bergemann A. D., Whitley J. C., Finch L. R. Homology of mycoplasma plasmid pADB201 and staphylococcal plasmid pE194. J Bacteriol. 1989 Jan;171(1):593–595. doi: 10.1128/jb.171.1.593-595.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. R., Reinberg D., Schmidt-Glenewinkel T., Roth M., Zipursky S. L., Hurwitz J. DNA structures required for phi X174 A-protein-directed initiation and termination of DNA replication. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):701–715. doi: 10.1101/sqb.1983.047.01.081. [DOI] [PubMed] [Google Scholar]
- Brown D. R., Roth M. J., Reinberg D., Hurwitz J. Analysis of bacteriophage phi X174 gene A protein-mediated termination and reinitiation of phi X DNA synthesis. I. Characterization of the termination and reinitiation reactions. J Biol Chem. 1984 Aug 25;259(16):10545–10555. [PubMed] [Google Scholar]
- Dagert M., Jones I., Goze A., Romac S., Niaudet B., Ehrlich S. D. Replication functions of pC194 are necessary for efficient plasmid transduction by M13 phage. EMBO J. 1984 Jan;3(1):81–86. doi: 10.1002/j.1460-2075.1984.tb01764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto G. P., Zinder N. D. Reduction of the minimal sequence for initiation of DNA synthesis by qualitative or quantitative changes of an initiator protein. Nature. 1984 Sep 20;311(5983):279–280. doi: 10.1038/311279a0. [DOI] [PubMed] [Google Scholar]
- Goetz G. S., Hurwitz J. Studies on the role of the phi X174 gene A protein in phi X174 viral strand synthesis. III. Replication of DNA containing two viral replication origins. J Biol Chem. 1988 Nov 5;263(31):16443–16451. [PubMed] [Google Scholar]
- Goetz G. S., Schmidt-Glenewinkel T., Hu M. H., Belgado N., Hurwitz J. Studies on the role of the phi X174 gene A protein in phi X viral strand synthesis. II. Effects of DNA replication of mutations in the 30-nucleotide icosahedral bacteriophage origin. J Biol Chem. 1988 Nov 5;263(31):16433–16442. [PubMed] [Google Scholar]
- Goze A., Ehrlich S. D. Replication of plasmids from Staphylococcus aureus in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7333–7337. doi: 10.1073/pnas.77.12.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros M. F., te Riele H., Ehrlich S. D. Rolling circle replication of single-stranded DNA plasmid pC194. EMBO J. 1987 Dec 1;6(12):3863–3869. doi: 10.1002/j.1460-2075.1987.tb02724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss A. D., Ross H. F., Novick R. P. Functional analysis of a palindromic sequence required for normal replication of several staphylococcal plasmids. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2165–2169. doi: 10.1073/pnas.84.8.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss A., Ehrlich S. D. The family of highly interrelated single-stranded deoxyribonucleic acid plasmids. Microbiol Rev. 1989 Jun;53(2):231–241. doi: 10.1128/mr.53.2.231-241.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanescu S., Projan S. J. Replication termination for staphylococcal plasmids: plasmids pT181 and pC221 cross-react in the termination process. J Bacteriol. 1988 Aug;170(8):3427–3434. doi: 10.1128/jb.170.8.3427-3434.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. A., Murray R. W., Koepsel R. R. Mechanism of plasmid pT181 DNA replication. Biochim Biophys Acta. 1988 Dec 20;951(2-3):375–381. doi: 10.1016/0167-4781(88)90109-1. [DOI] [PubMed] [Google Scholar]
- Koepsel R. R., Murray R. W., Rosenblum W. D., Khan S. A. The replication initiator protein of plasmid pT181 has sequence-specific endonuclease and topoisomerase-like activities. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6845–6849. doi: 10.1073/pnas.82.20.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel B., Ehrlich S. D. Illegitimate recombination occurs between the replication origin of the plasmid pC194 and a progressing replication fork. EMBO J. 1986 Dec 20;5(13):3691–3696. doi: 10.1002/j.1460-2075.1986.tb04701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton N. P., Oultram J. D., Brehm J. K., Atkinson T. The replication proteins of plasmids pE194 and pLS1 have N-terminal homology. Nucleic Acids Res. 1988 Apr 11;16(7):3101–3101. doi: 10.1093/nar/16.7.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Adler G. K., Projan S. J., Carleton S., Highlander S. K., Gruss A., Khan S. A., Iordanescu S. Control of pT181 replication I. The pT181 copy control function acts by inhibiting the synthesis of a replication protein. EMBO J. 1984 Oct;3(10):2399–2405. doi: 10.1002/j.1460-2075.1984.tb02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbons L. P., van der Marel G. A., van Boom J. H., Altona C. Hairpin and duplex formation of the DNA octamer d(m5C-G-m5C-G-T-G-m5C-G) in solution. An NMR study. Nucleic Acids Res. 1986 May 27;14(10):4187–4196. doi: 10.1093/nar/14.10.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigac J., Vujaklija D., Toman Z., Gamulin V., Schrempf H. Structural instability of a bifunctional plasmid pZG1 and single-stranded DNA formation in Streptomyces. Plasmid. 1988 May;19(3):222–230. doi: 10.1016/0147-619x(88)90040-6. [DOI] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Sioud M., Baldacci G., Forterre P., de Recondo A. M. Novobiocin induces accumulation of a single strand of plasmid pGRB-1 in the archaebacterium Halobacterium GRB. Nucleic Acids Res. 1988 Aug 25;16(16):7833–7842. doi: 10.1093/nar/16.16.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Solar G. H., Puyet A., Espinosa M. Initiation signals for the conversion of single stranded to double stranded DNA forms in the streptococcal plasmid pLS1. Nucleic Acids Res. 1987 Jul 24;15(14):5561–5580. doi: 10.1093/nar/15.14.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Riele H., Michel B., Ehrlich S. D. Are single-stranded circles intermediates in plasmid DNA replication? EMBO J. 1986 Mar;5(3):631–637. doi: 10.1002/j.1460-2075.1986.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Riele H., Michel B., Ehrlich S. D. Single-stranded plasmid DNA in Bacillus subtilis and Staphylococcus aureus. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2541–2545. doi: 10.1073/pnas.83.8.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mansfeld A. D., van Teeffelen H. A., Baas P. D., Jansz H. S. Two juxtaposed tyrosyl-OH groups participate in phi X174 gene A protein catalysed cleavage and ligation of DNA. Nucleic Acids Res. 1986 May 27;14(10):4229–4238. doi: 10.1093/nar/14.10.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]