Abstract
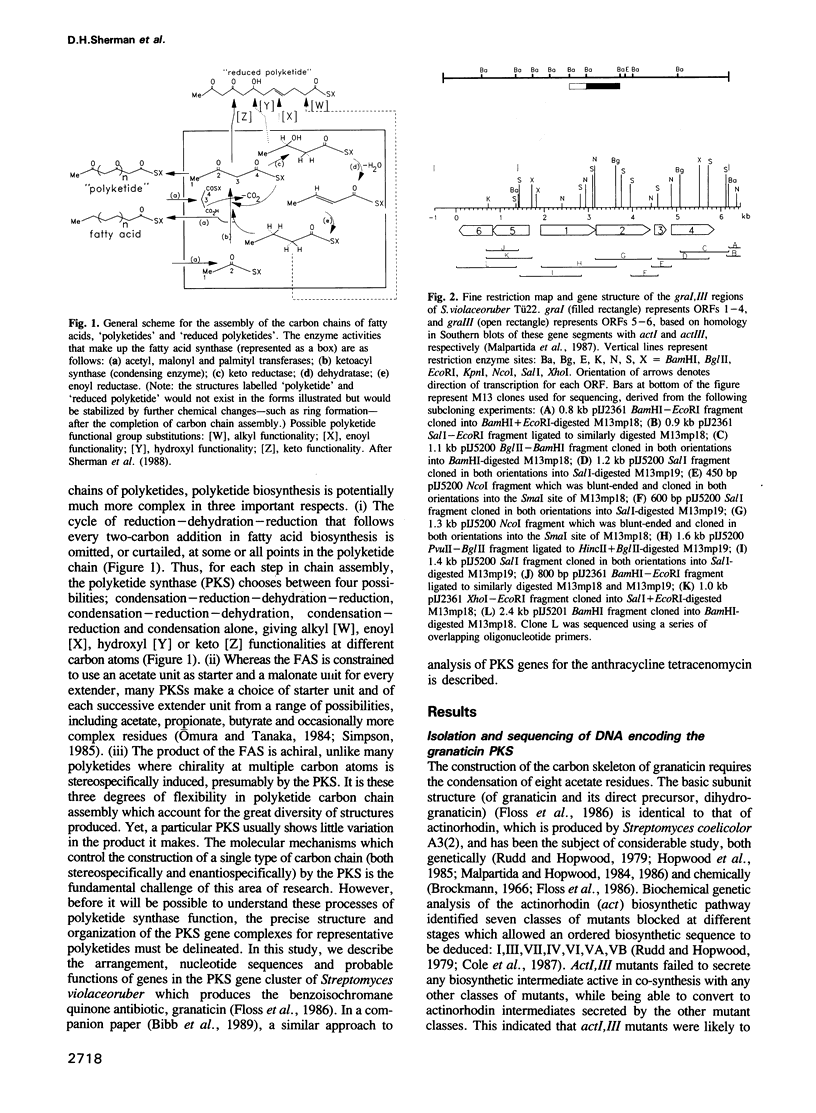
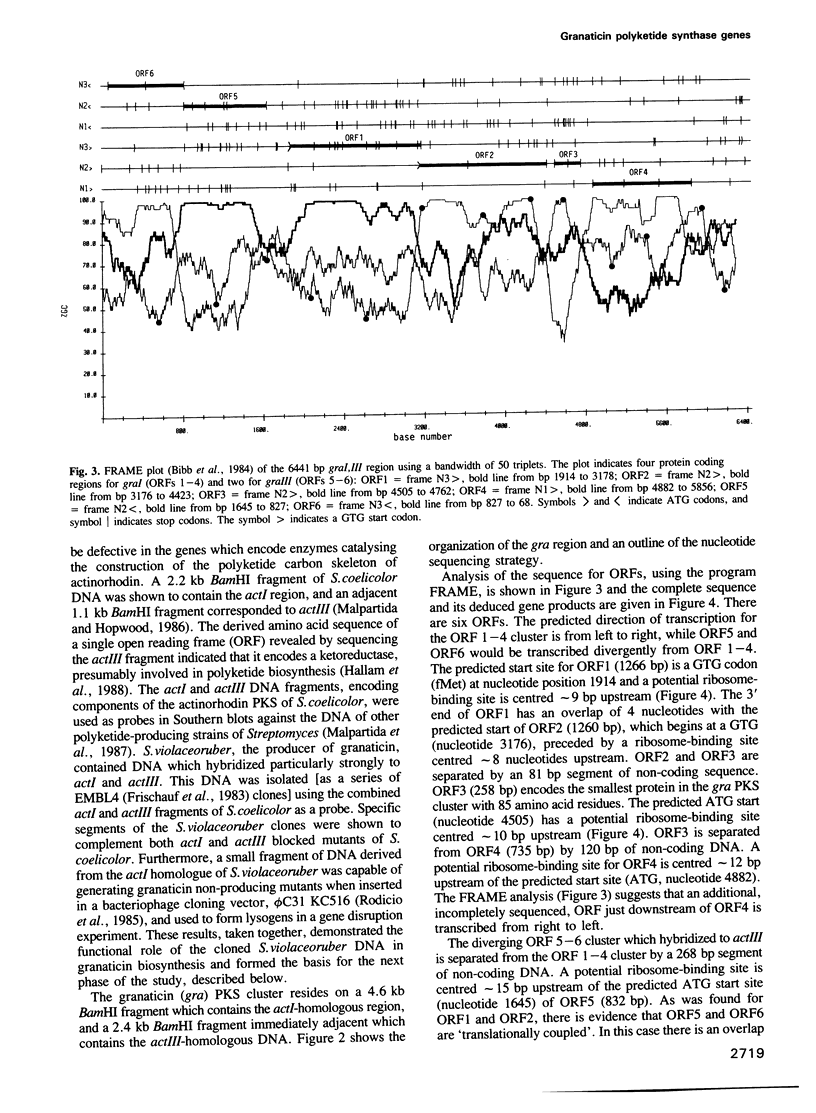
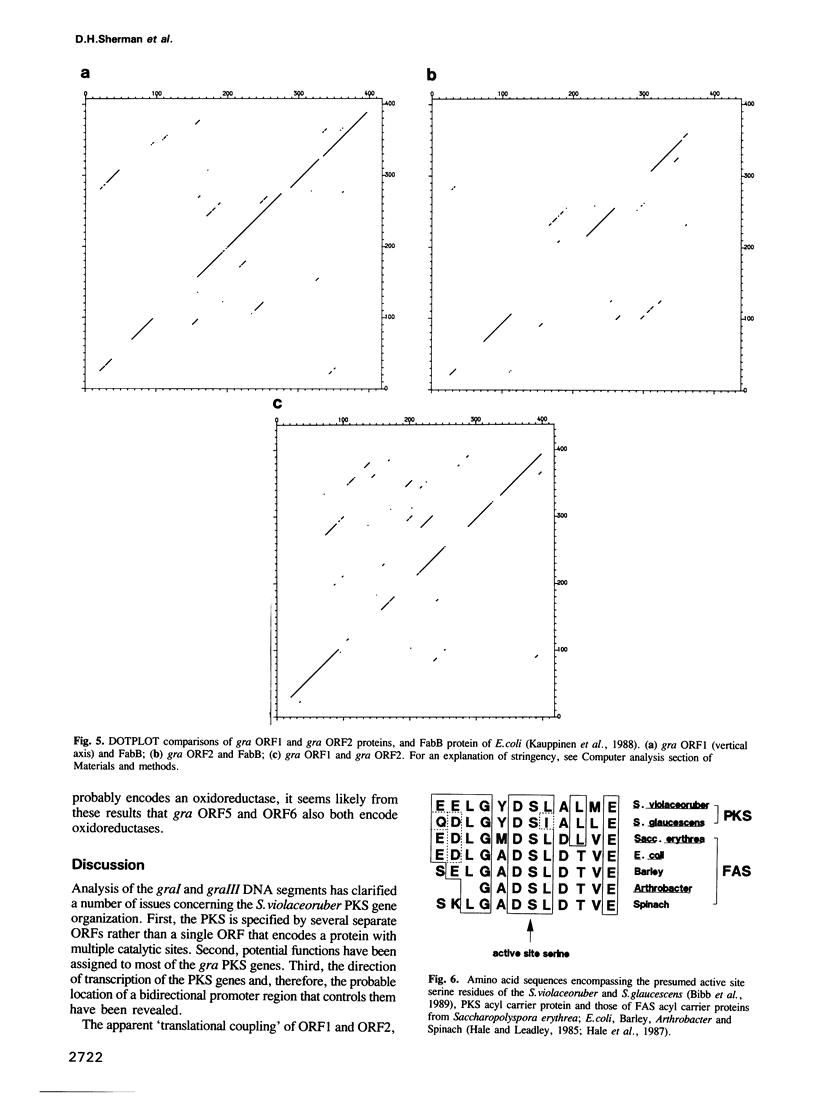
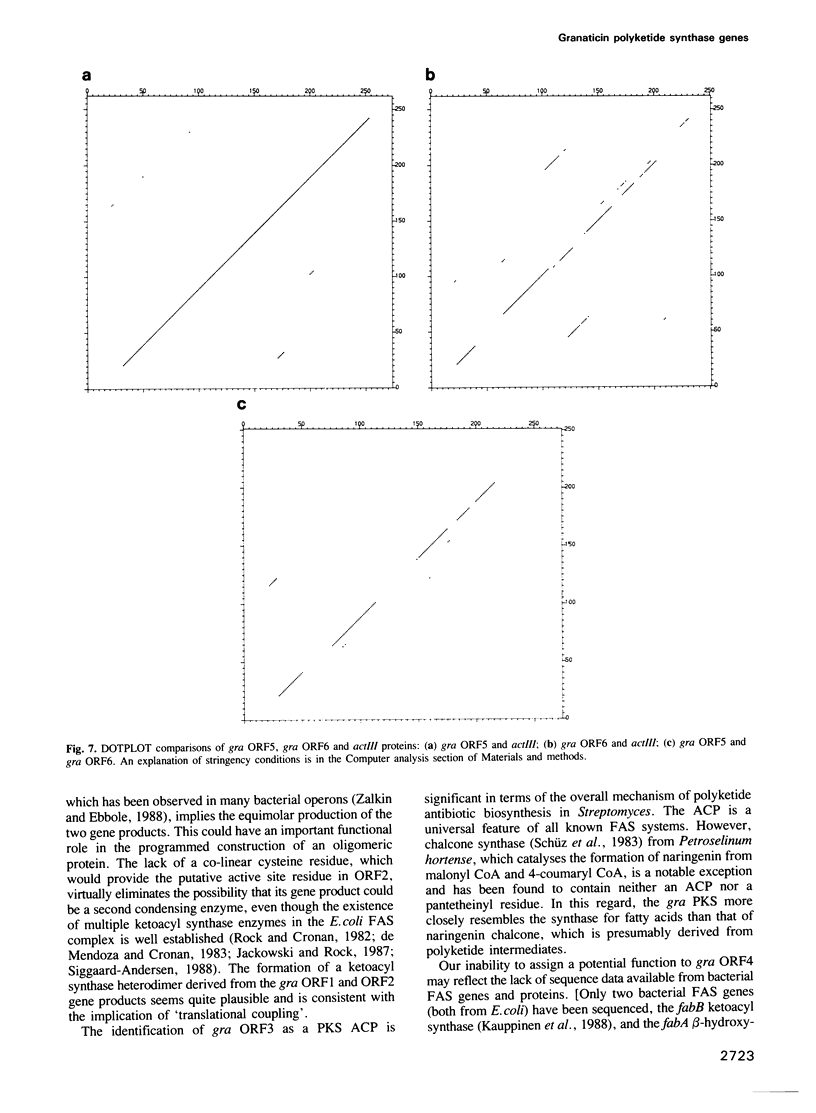
A 6.5 kb region of DNA from Streptomyces violaceoruber, which contains polyketide synthase (PKS) genes for production of the benzoisochromane quinone moiety of the antibiotic, granaticin, was cloned and sequenced. Of six open reading frames (ORFs) identified, four (ORFs 1-4) would be transcribed in one direction and two (ORFs 5 and 6) divergently from ORFs 1-4. ORF1 and ORF2, which show evidence for translation coupling, encode (deduced) gene products which strongly resemble each other and the Escherichia coli fatty acid ketoacyl synthase (condensing enzyme), FabB. We conclude that ORF1 (which contains a characteristic cysteine residue) functions as a condensing enzyme, possibly as part of a heterodimeric protein including the product of ORF2. The predicted ORF3 gene product strikingly resembles acyl carrier proteins (ACPs) of fatty acid synthase (FAS), particularly in the region of the active site motif, while the predicted ORF5 and ORF6 gene products resemble known oxidoreductases, suggesting that they function as reductive steps required during assembly of the granaticin carbon skeleton. Comparison of the deduced ORF4 gene product with available protein databases failed to elucidate its potential function. The overall conclusion is that the granaticin-producing PKS would consist of at least six separate enzymes involved in carbon chain assembly, thus resembling a Type II, rather than a Type I, FAS.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibb M. J., Biró S., Motamedi H., Collins J. F., Hutchinson C. R. Analysis of the nucleotide sequence of the Streptomyces glaucescens tcmI genes provides key information about the enzymology of polyketide antibiotic biosynthesis. EMBO J. 1989 Sep;8(9):2727–2736. doi: 10.1002/j.1460-2075.1989.tb08414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Findlay P. R., Johnson M. W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene. 1984 Oct;30(1-3):157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- Chirala S. S., Kuziora M. A., Spector D. M., Wakil S. J. Complementation of mutations and nucleotide sequence of FAS1 gene encoding beta subunit of yeast fatty acid synthase. J Biol Chem. 1987 Mar 25;262(9):4231–4240. [PubMed] [Google Scholar]
- Cole S. P., Rudd B. A., Hopwood D. A., Chang C. J., Floss H. G. Biosynthesis of the antibiotic actinorhodin. Analysis of blocked mutants of Streptomyces coelicolor. J Antibiot (Tokyo) 1987 Mar;40(3):340–347. doi: 10.7164/antibiotics.40.340. [DOI] [PubMed] [Google Scholar]
- Collins J. F., Coulson A. F., Lyall A. The significance of protein sequence similarities. Comput Appl Biosci. 1988 Mar;4(1):67–71. doi: 10.1093/bioinformatics/4.1.67. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Li W. B., Coleman R., Narasimhan M., de Mendoza D., Schwab J. M. Derived amino acid sequence and identification of active site residues of Escherichia coli beta-hydroxydecanoyl thioester dehydrase. J Biol Chem. 1988 Apr 5;263(10):4641–4646. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Hale R. S., Jordan K. N., Leadlay P. F. A small, discrete acyl carrier protein is involved in de novo fatty acid biosynthesis in Streptomyces erythraeus. FEBS Lett. 1987 Nov 16;224(1):133–136. doi: 10.1016/0014-5793(87)80436-2. [DOI] [PubMed] [Google Scholar]
- Hale R. S., Leadlay P. F. Oligonucleotide probes for bacterial acylcarrier protein genes. Biochimie. 1985 Jul-Aug;67(7-8):835–839. doi: 10.1016/s0300-9084(85)80176-0. [DOI] [PubMed] [Google Scholar]
- Hallam S. E., Malpartida F., Hopwood D. A. Nucleotide sequence, transcription and deduced function of a gene involved in polyketide antibiotic synthesis in Streptomyces coelicolor. Gene. 1988 Dec 30;74(2):305–320. doi: 10.1016/0378-1119(88)90165-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Malpartida F., Kieser H. M., Ikeda H., Duncan J., Fujii I., Rudd B. A., Floss H. G., Omura S. Production of 'hybrid' antibiotics by genetic engineering. Nature. 1985 Apr 18;314(6012):642–644. doi: 10.1038/314642a0. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Acetoacetyl-acyl carrier protein synthase, a potential regulator of fatty acid biosynthesis in bacteria. J Biol Chem. 1987 Jun 5;262(16):7927–7931. [PubMed] [Google Scholar]
- Kauppinen S., Siggaard-Andersen M., von Wettstein-Knowles P. beta-Ketoacyl-ACP synthase I of Escherichia coli: nucleotide sequence of the fabB gene and identification of the cerulenin binding residue. Carlsberg Res Commun. 1988;53(6):357–370. doi: 10.1007/BF02983311. [DOI] [PubMed] [Google Scholar]
- Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984 Jul;12(1):19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- Lydiate D. J., Malpartida F., Hopwood D. A. The Streptomyces plasmid SCP2*: its functional analysis and development into useful cloning vectors. Gene. 1985;35(3):223–235. doi: 10.1016/0378-1119(85)90001-0. [DOI] [PubMed] [Google Scholar]
- Lynen F. On the structure of fatty acid synthetase of yeast. Eur J Biochem. 1980 Dec;112(3):431–442. doi: 10.1111/j.1432-1033.1980.tb06105.x. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Hallam S. E., Kieser H. M., Motamedi H., Hutchinson C. R., Butler M. J., Sugden D. A., Warren M., McKillop C., Bailey C. R. Homology between Streptomyces genes coding for synthesis of different polyketides used to clone antibiotic biosynthetic genes. 1987 Feb 26-Mar 4Nature. 325(6107):818–821. doi: 10.1038/325818a0. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Hopwood D. A. Molecular cloning of the whole biosynthetic pathway of a Streptomyces antibiotic and its expression in a heterologous host. 1984 May 31-Jun 6Nature. 309(5967):462–464. doi: 10.1038/309462a0. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Hopwood D. A. Physical and genetic characterisation of the gene cluster for the antibiotic actinorhodin in Streptomyces coelicolor A3(2). Mol Gen Genet. 1986 Oct;205(1):66–73. doi: 10.1007/BF02428033. [DOI] [PubMed] [Google Scholar]
- Mohamed A. H., Chirala S. S., Mody N. H., Huang W. Y., Wakil S. J. Primary structure of the multifunctional alpha subunit protein of yeast fatty acid synthase derived from FAS2 gene sequence. J Biol Chem. 1988 Sep 5;263(25):12315–12325. [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Rodicio M. R., Bruton C. J., Chater K. F. New derivatives of the Streptomyces temperate phage phi C31 useful for the cloning and functional analysis of Streptomyces DNA. Gene. 1985;34(2-3):283–292. doi: 10.1016/0378-1119(85)90137-4. [DOI] [PubMed] [Google Scholar]
- Rudd B. A., Hopwood D. A. Genetics of actinorhodin biosynthesis by Streptomyces coelicolor A3(2). J Gen Microbiol. 1979 Sep;114(1):35–43. doi: 10.1099/00221287-114-1-35. [DOI] [PubMed] [Google Scholar]
- Rüther U., Koenen M., Otto K., Müller-Hill B. pUR222, a vector for cloning and rapid chemical sequencing of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4087–4098. doi: 10.1093/nar/9.16.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer M., Roberts L. M., Höltke H. J., Takabayashi K., Höllerer E., Hoffmann B., Müller G., Köttig H., Schweizer E. The pentafunctional FAS1 gene of yeast: its nucleotide sequence and order of the catalytic domains. Mol Gen Genet. 1986 Jun;203(3):479–486. doi: 10.1007/BF00422073. [DOI] [PubMed] [Google Scholar]
- Schüz R., Heller W., Hahlbrock K. Substrate specificity of chalcone synthase from Petroselinum hortense. Formation of phloroglucinol derivatives from aliphatic substrates. J Biol Chem. 1983 Jun 10;258(11):6730–6734. [PubMed] [Google Scholar]
- Siggaard-Andersen M. Role of Escherichia coli beta-ketoacyl-ACP synthase I in unsaturated fatty acid synthesis. Carlsberg Res Commun. 1988;53(6):371–379. doi: 10.1007/BF02983312. [DOI] [PubMed] [Google Scholar]
- Wakil S. J. The relationship between structure and function for and the regulation of the enzymes of fatty acid synthesis. Ann N Y Acad Sci. 1986;478:203–219. doi: 10.1111/j.1749-6632.1986.tb15532.x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zalkin H., Ebbole D. J. Organization and regulation of genes encoding biosynthetic enzymes in Bacillus subtilis. J Biol Chem. 1988 Feb 5;263(4):1595–1598. [PubMed] [Google Scholar]


