There is a common need to identify risk assessment models that may be predictive of cancer-associated venous thromboembolism in at-risk patients who might benefit from appropriate prevention measures. Assessment of pretreatment estimated glomerular filtration rate could represent a simple and cost-effective predictor of venous thromboembolic events, at no additional cost to health care systems.
Keywords: Venous thromboembolism, Platinum-based chemotherapy, Renal impairment, Toxicity, Risk prediction, Risk stratification
Abstract
Background.
Reduced estimated glomerular filtration rate (eGFR) has been associated with increased venous thromboembolism (VTE) risk in the general population. VTE incidence significantly increases in cancer patients, especially those undergoing chemotherapy. Despite the evidence that a substantial number of cancer patients have unrecognized renal impairment, as indicated by reduced eGFR in the presence of serum creatinine levels within the reference value, chemotherapy dosage is routinely adjusted for serum creatinine values. Among chemotherapies, platinum-based regimens are associated with the highest rates of VTE. A cohort study was designed to assess the value of pretreatment eGFR in the risk prediction of a first VTE episode in cancer outpatients without previous history of VTE who were scheduled for platinum-based chemotherapy.
Methods.
Serum creatinine and eGFR were evaluated before the start of standard platinum-based chemotherapy in a cohort of 322 consecutive patients with primary or relapsing/recurrent solid cancers, representative of a general practice population.
Results.
Patients who experienced a first VTE episode in the course of chemotherapy had lower mean eGFR values compared with patients who remained VTE free. Multivariate Cox analysis demonstrated that eGFR had an independent value for risk prediction of a first VTE episode during treatment, with a 3.15 hazard ratio. Indeed, 14% of patients with reduced eGFR had VTE over 1-year follow-up compared with 6% of patients with normal eGFR values.
Conclusion.
The results suggest that reductions in eGFR, even in the presence of normal serum creatinine, are associated with an increased VTE risk in cancer outpatients undergoing platinum-based chemotherapy regimens. Determining eGFR before chemotherapy could represent a simple predictor of VTE, at no additional cost to health care systems.
Implications for Practice:
All major society guidelines currently recommend no thromboprophylaxis for chemotherapy-treated cancer outpatients. Nonetheless, there is a common need to identify risk assessment models that may be predictive of cancer-associated venous thromboembolism in at-risk patients who might benefit from appropriate prevention measures. In this respect, the Khorana score correctly assigns patients to the high-risk category; however, clinical decision making remains challenging in approximately 50% of patients, who fall in the intermediate risk class. Assessment of pretreatment estimated glomerular filtration rate could represent a simple and cost-effective predictor of venous thromboembolic events, at no additional cost to health care systems.
Introduction
Venous thromboembolism (VTE) has an overall incidence rate of approximately 1.5 per 1,000 person-years in developed countries [1]. This percentage, however, dramatically increases in older age and in selected groups of patients, especially those with cancer, for whom the absolute rate of VTE has been reported to be as high as 13.9 per 1,000 person-years [2]. Rates vary greatly by cancer site, age, time from diagnosis, use of specific antineoplastic agents (cisplatin, bevacizumab) or supportive care agents (erythropoietin), and comorbidities as assessed by the Charlson Comorbidity Index score [2, 3].
Recent studies suggested that chronic kidney diseases (CKD) may be associated with increased VTE risk in the general population, but conflicting results were reported [4–8]. In particular, an association between reduced estimated glomerular filtration rate (eGFR) and an increased VTE risk was found [5, 8], even in the non-CKD range of eGFR [8], although other studies confirmed this association for only severe eGFR reductions [7].
A substantial number of cancer patients have unrecognized renal impairment, as indicated by reduced eGFR in the presence of serum creatinine (SCr) levels within the reference value [9]. Despite this finding, most oncologists still rely on SCr when assessing whether chemotherapy dosage adjustment for renal impairment is required. This is the case for cisplatin, for which indications for safe administration include normal renal function (SCr levels <1.5 mg/dL). Nonetheless, cisplatin administration is associated with a significant vascular toxicity [10] and increased VTE incidence [3, 11, 12].
Based on these considerations, we hypothesized that reduced eGFR might represent a novel risk factor for VTE onset in cancer outpatients receiving platinum-based regimens. Accordingly, a cohort study was designed to assess the value of pretreatment eGFR in the risk prediction of a first VTE episode in cancer outpatients without previous history of VTE who were scheduled for platinum-based chemotherapy.
Patients and Methods
A cohort of 322 consecutive patients with primary or relapsing/recurrent solid cancers who were receiving different standard combinations of platinum-based regimens in the outpatient department setting of the Medical Oncology Unit of Tor Vergata Clinical Center in Rome, Italy, was prospectively followed between January 2007 and December 2012. The study outcome was defined as the occurrence of a first symptomatic or asymptomatic VTE episode, either deep venous thrombosis (DVT) or pulmonary embolism (PE), during active treatment.
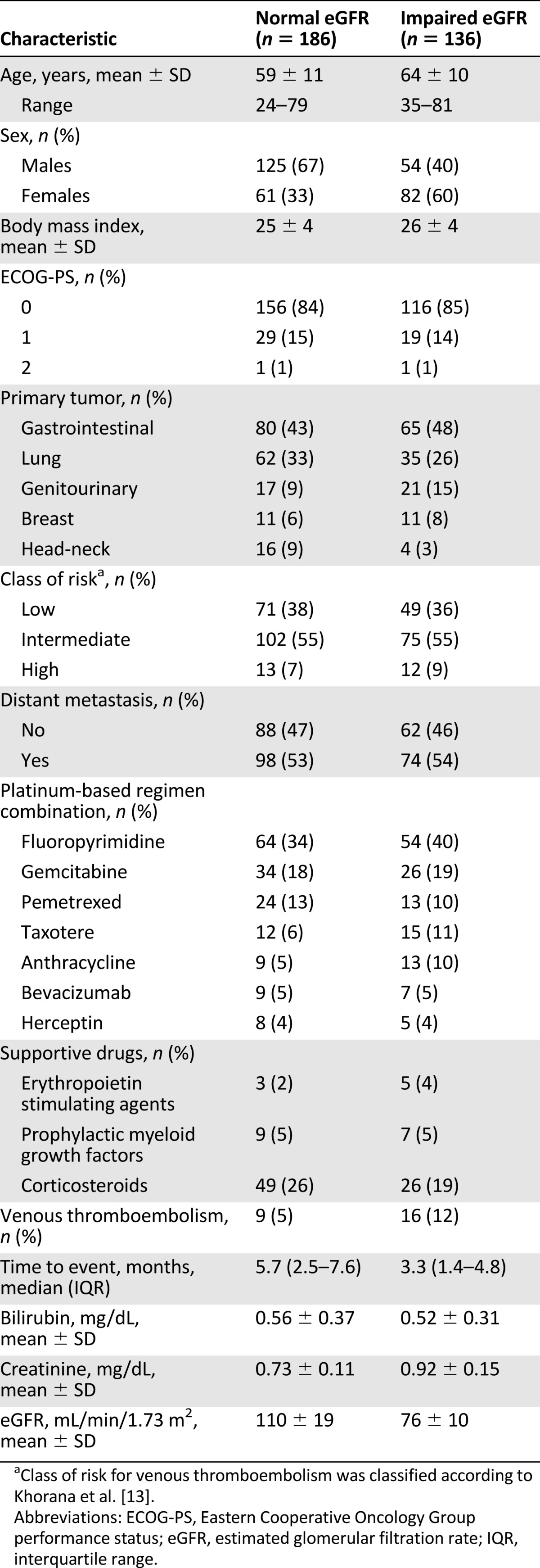
All patients were required to be at the start of a new chemotherapy regimen (6% neoadjuvant, 38% adjuvant, and 56% metastatic treatments), and 14 patients (4%) received concurrent radiotherapy. Eligibility criteria included histologically confirmed diagnosis of malignancy; age >18 years; no previous VTE; Eastern Cooperative Oncology Group performance status (ECOG-PS) 0–2, absolute neutrophil count ≥2,000 mm−3, platelet count ≥100,000 mm−3, hemoglobin level ≥9.5 g/dL; bilirubin level ≤1.5 times the upper limit of normal (ULN), alanine aminotransferase and aspartate aminotransferase ≤2.5 times the ULN in the absence of liver metastasis, or ≤5 times the ULN in the presence of liver metastasis; and SCr ≤1.2 mg/dL. Exclusion criteria were therapeutic doses of any heparin before enrollment or concomitant treatment with anticoagulant or antiplatelet drugs. No patient received prophylactic treatment with any anticoagulant after chemotherapy start. Patients’ characteristics are summarized in Table 1.
Table 1.
Patient characteristics
VTE risk was scored according to the model developed by Khorana et al., taking into account the site of cancer (2 points for very high-risk stomach, pancreas, or brain cancer; 1 point for high-risk lung or kidney cancer; and 0 points for all other solid cancer sites), platelet count of 350 × 109/L or more, leukocyte count above 11 × 109/L, hemoglobin lower than 10 g/dL and/or use of erythropoiesis-stimulating agents, and body mass index of 35 kg/m2 or more (1 point each) [13].
All patients were regularly seen at their scheduled chemotherapy visits or at the occurrence of clinically suspected VTE. Clinical data were collected in a computerized database by two independent oncologists, and patient management was discussed by an interdisciplinary team including different residency specialists (oncologists, surgeons, pathologists, radiologists, and radiotherapists). No patient underwent surgery during follow-up, and none were admitted to the clinic for acute medical illness requiring thromboprophylaxis. All patients were followed up for a median period of 9.3 months, during which outcomes were prospectively recorded. DVT was confirmed by venography or color-coded duplex sonography (in proximal DVT only). Diagnosis of PE was established by spiral computed tomography displaying one low-attenuation area or several that partly or completely filled the lumen of an opacified vessel.
Toxicity was scored according to the World Health Organization toxicity scale (grade 0–4) [14]. Nephrotoxicity was evaluated by both SCr and creatinine clearance. In the case of a creatinine clearance rate lower than 60 mL/minute, the dosage was reduced by 50% of the planned dose. Chemotherapy was not infused when creatinine clearance dropped below 40 mL/minute. Incidence and severity of adverse effects were evaluated before each chemotherapy cycle. All patients were included in the final analysis, regardless of whether their planned treatment was completed or uncompleted.
The study was performed in accordance with the principles embodied in the Declaration of Helsinki. All patients provided written informed consent, which was previously approved by our institutional ethics committees.
Blood Samples and Laboratory Tests
Blood samples were obtained prior to chemotherapy for all recruited subjects and stored at −80°C in the facilities of the interinstitutional multidisciplinary biobank of the Istituto di Ricovero e Cura a Carattere Scientifico San Raffaele Pisana in Rome. Serum samples were immediately analyzed for routine blood chemistry (Accelerator Total Laboratory Automation; Abbott Laboratories, Abbott Park, IL, http://www.abbott.com). SCr was measured using a modified kinetic Jaffe reaction on an ARCHITECT c8000 System (Abbott Laboratories). eGFR estimation was conducted as a retrospective procedure using the simplified Modification Diet of Renal Disease study equation: eGFR (mL/minute per 1.73 m2) = 186 × (SCr)−1.154 × (age)−0.203 × (0.742 if female) [15]. Renal function was classified according to the National Kidney Foundation using these ranges: eGFR >90 mL/minute per 1.73 m2 (normal renal function), eGFR of 60–89 mL/minute per 1.73 m2 (mild renal dysfunction), eGFR of 30–59 mL/minute per 1.73 m2 (moderate renal dysfunction), and eGFR <30 mL/minute per 1.73 m2 (severe renal dysfunction) [16]. Accordingly, the cutoff value of 90 mL/minute per 1.73 m2 was used for categorization analyses.
Statistical Analysis
A total of 322 patients entered the study based on the hypothesis that this approach would detect a difference with a probability of >95%, at a two-sided 5% significance level, if the true hazard ratio is 2. This was based on the assumption of an accrual period of at least 2 years, an elapsed time between cycles within 30 days, and a median time to event of 2.5 months. Data are presented as percentages, mean ± SD, or median and interquartile range. Differences between percentages were assessed by the χ2 test. Student’s t test, analysis of variance test, and Pearson’s correlation analysis were used for normally distributed variables. Appropriate nonparametric tests (Mann-Whitney test, Kruskal-Wallis test, and Spearman’s correlation test) were used for all other variables. Logistic regression analysis was performed to quantify the relationship between clinical and biochemical variables. Survival curves were calculated by the Kaplan-Meier and log-rank methods. Cox proportional hazards analysis was used to evaluate the association between clinical variables and time to event (TTE). TTE was calculated from the date of enrollment until the event date (any VTE, either DVT or PE) or the study end. For patients receiving neoadjuvant chemotherapy, follow-up was stopped at completion of an entire antiblastic treatment and before surgery. All tests were two-tailed, and only p values <.05 were regarded as statistically significant. Calculations were performed using a computer software package (Statistica 8.0; StatSoft, Tulsa, OK, http://www.statsoft.com) or free Web-based applications Interactive Statistical Pages http://statpages.org/).
Results
In January 2007, the Medical Oncology Unit of the Tor Vergata Clinical Center started recruiting ambulatory patients with primary or relapsing/recurrent solid cancers who were prospectively followed under the appropriate institutional ethics approvals to investigate possible predictors of cancer-associated VTE. As of December 31, 2012, a total of 655 consecutive eligible patients had been enrolled. Of these, 344 patients were at the start of a new platinum-based chemotherapy regimen. Sixteen patients (4.7%) had a baseline SCr >1.2 mg/dL, and 6 patients (1.7%) withdrew informed consent, leaving a total of 322 participants for the final analyses.
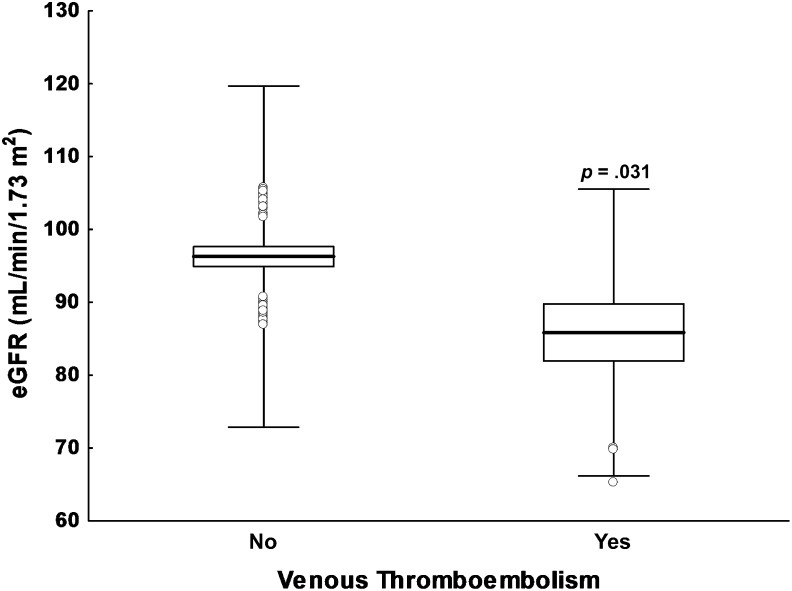
Mean pretreatment SCr and eGFR of the recruited patients are reported in Table 1. Of interest is that patients who experienced a first VTE episode in the course of chemotherapy had lower mean eGFR values (86 ± 20 mL/minute per 1.73 m2) compared with patients who remained VTE free (96 ± 23 mL/minute per 1.73 m2; p = .031) (Fig. 1). Renal function was classified according to the National Kidney Foundation. Fifty-eight percent of patients had normal renal function, as demonstrated by an eGFR >90 mL/minute per 1.73 m2, whereas 37% of patients had mild renal dysfunction (eGFR of 60–89 mL/minute per 1.73 m2). Only 5% of cases had moderate impairment of kidney functionality and an eGFR <60 mL/minute per 1.73 m2, suggesting the presence of stage 3 CKD. No patient had stage 4 CKD (eGFR <30 mL/minute per 1.73 m2).
Figure 1.
Box-plot analysis of pretreatment eGFR distribution. Comparison of cancer patients who experienced a first episode of venous thromboembolism during platinum-based chemotherapy and those who did not. Data are presented as mean values (solid lines), standard errors (columns), and standard deviations (whiskers). Open circles indicate outliers.
Abbreviation: eGFR, estimated glomerular filtration rate.
Kidney toxicity occurred in seven patients (2%), three of whom (1.6%) had normal kidney function (by both baseline SCr and eGFR), three of whom (2.5%) had mild renal dysfunction (one male with SCr of 1.2 mg/dL and eGFR of 64.3 mL/minute per 1.73 m2, one female with SCr of 0.75 mg/dL and eGFR of 80.3 mL/minute per 1.73 m2, and one male with SCr of 1.1 mg/dL and eGFR of 71.3 mL/minute per 1.73 m2), and one female patient (6.7%) who had moderate renal dysfunction (SCr of 1.0 mg/dL and eGFR of 55.4 mL/minute/1.73 m2). However, the small number of toxic events recorded did not allow any association analysis between renal function measured by eGFR and incidence of toxicity. No patient required platinum-dose modification at baseline secondary to renal insufficiency, whereas four patients had a dose reduction of 50% and one patient had a dose reduction of 25% of full dosage because of the development of kidney toxicity during treatment.
VTE occurred during chemotherapy in 8% of patients (10 with PE and 17 with DVT; median TTE: 3.5 months). Nine of 23 patients were diagnosed with incidental VTE (seven with PE) at time of restaging during treatment. Sixteen of 136 patients (11.8%, 15 with mild and 1 with moderate renal dysfunction) with impaired eGFR (<90 mL/minute per 1.73 m2) had VTE during the course of chemotherapy compared with 4.8% (9 of 186) with normal kidney function (two-tailed Fisher’s exact test, p = .033). No VTE events were recorded in the seven patients who developed kidney toxicity during treatment.
To further assess the possible determinants of VTE among clinical-pathological variables and eGFR, a logistic regression analysis was performed including, as predictor variables, age, gender, site of primary tumor, presence of metastasis, ECOG-PS, class of risk according to Khorana et al. [13], anticancer and supportive drugs used, and eGFR (Table 2). The latter was categorized as normal or impaired according to the cutoff of 90 mL/minute per 1.73 m2. The results obtained showed that a reduction of eGFR (odds ratio: 3.44; p = .015) was the only independent predictor of VTE during platinum-based chemotherapy. When the same variables were included in a multivariate Cox proportional-hazards regression model, only eGFR retained an independent value for risk prediction of a first VTE episode during treatment (hazard ratio: 3.15 [95% confidence interval: 1.22–8.14]; p = .018].
Table 2.
Multivariate logistic regression analysis of the predictive value of clinical-pathological variables and estimated glomerular filtration rate on venous thromboembolism occurrence
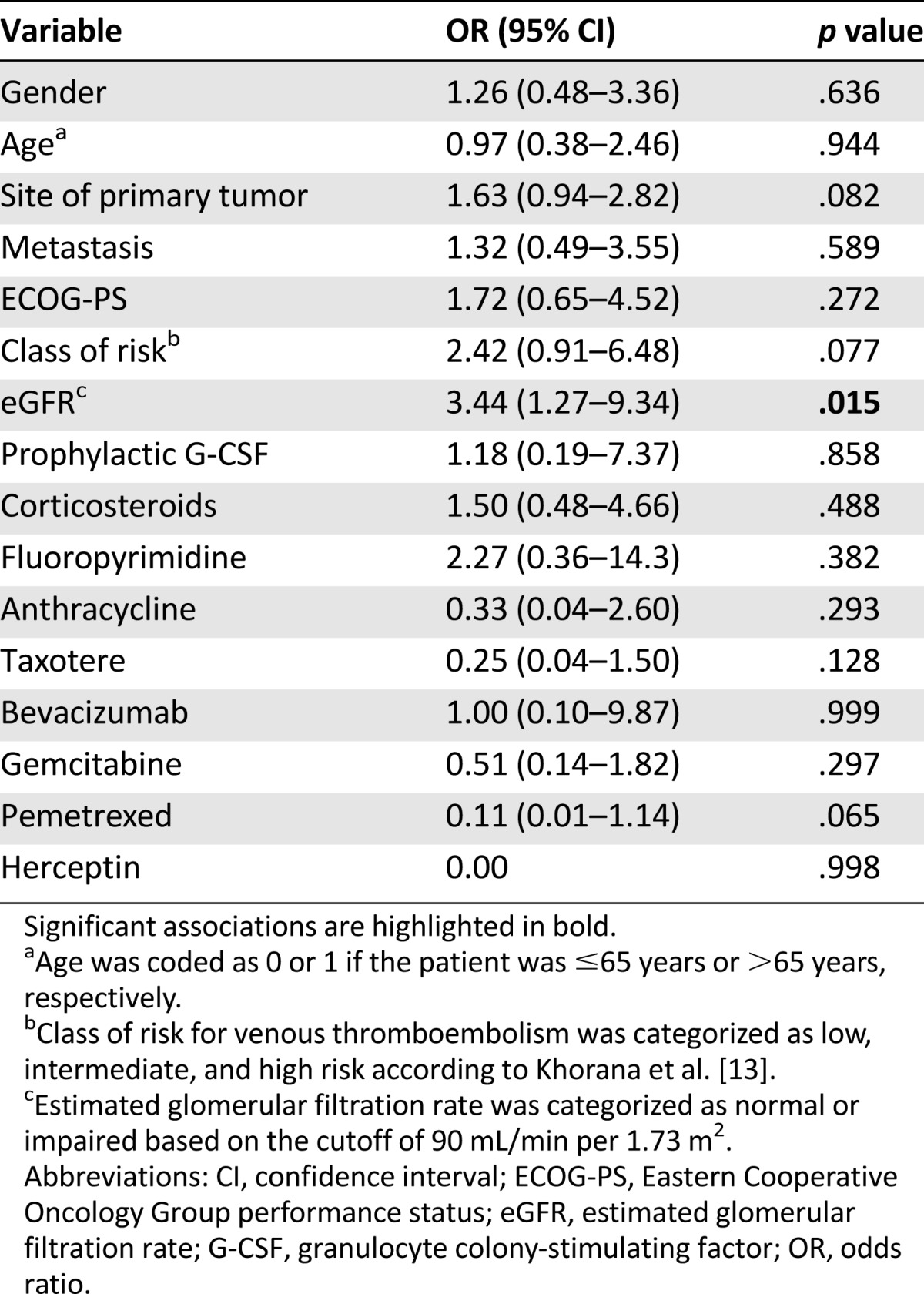
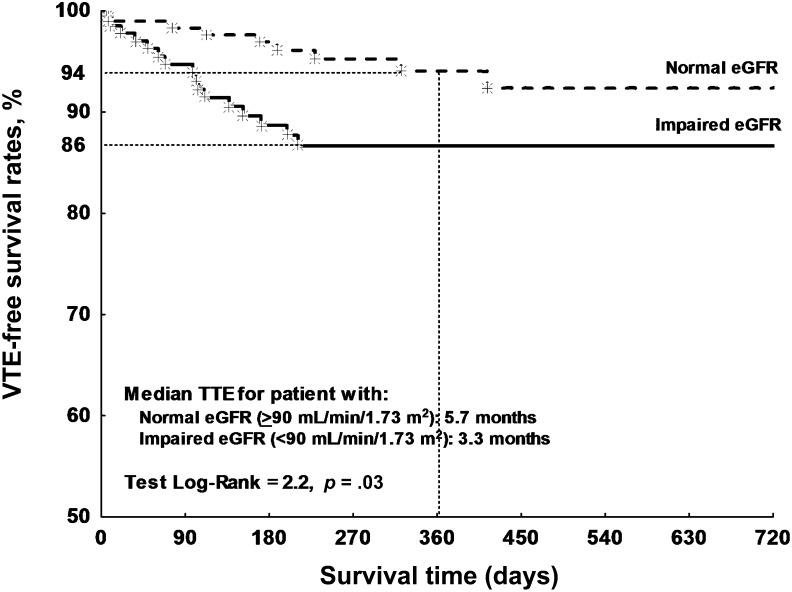
Figure 2 reports the Kaplan-Meier curves for patients stratified on the basis of pretreatment eGFR values. As shown, patients with reduced eGFR had an 86% VTE-free survival rate during 1-year follow-up (median TTE: 3.3 months), compared with 94% of patients with normal renal function (median TTE: 5.7 months).
Figure 2.
Kaplan-Meier analysis of VTE-free survival rates. Comparison is of VTE-free survival time of cancer patients categorized on the basis of eGFR prior to chemotherapy start.
Abbreviations: eGFR, estimated glomerular filtration rate; TTE, time to event; VTE, venous thromboembolism.
Discussion
The possibility that reduced eGFR may have a predictive value for VTE in the general population has been raised recently [5, 8]. In this paper, for the first time to our knowledge, we demonstrate that reduced eGFR values are associated with an increased VTE risk in patients undergoing platinum-based chemotherapy regimens, suggesting that eGFR assessment prior to treatment may help in VTE risk stratification. The use of a general screening strategy is appealing because it could provide the opportunity for prophylactic treatment, reducing the event rates in at-risk cancer patients [17, 18]. In this context, the availability of a predictive variable such as eGFR is captivating. In contrast to the majority of the prothrombotic markers that have been proposed in recent years [19–24], eGFR determination does not require second-level test-performing laboratories and/or skilled personnel; it is reliably evaluated by routine tests. It necessitates only SCr determination, which is mandatory before chemotherapy start, and does not represent an adjunctive cost for the health care systems.
Chemotherapy-related kidney toxicity was more frequent in patients with reduced eGFR values, even in the presence of SCr levels within the normal range. Nephrotoxicity rates increased from 2% in patients with normal kidney function to approximately 7% in patients with moderate renal dysfunction. Although the difference was not significant, possibly because of the low number of toxic events, this observation is in agreement with recent findings suggesting that the presence of unrecognized renal impairment could be responsible for chemotherapy-associated adverse effects [9] and highlights the significance of eGFR assessment before the initiation of anticancer therapy.
Among anticancer drugs, platinum-based compounds are characterized not only by a dose-related and cumulative nephrotoxicity but also by significant vascular toxicity [10] and increased VTE incidence [3, 11, 12], possibly related to cisplatin-induced generation of reactive oxygen species and failure of the antioxidant defense mechanism against oxidative damage. It is conceivable to hypothesize that the administration of platinum compounds further contributes to a vicious cycle of oxidative stress and vascular dysfunction that is already instituted in patients with impaired renal function and that ultimately leads to an increased risk of VTE; however, this hypothesis is merely speculative. Studies specifically designed to address this issue are required.
Some limitations must be acknowledged. First, eGFR estimation was conducted as a retrospective procedure; however, all eligible consecutive patients within the designated time frame were included, and all measurements were performed while blinded to the patient outcome. Moreover, recruitment was performed at a single institution, which might have posed further limitations because the primary and most obvious shortcoming of single-center studies is their potentially limited external validity. A final issue is the relatively small sample size, ultimately leading to a small number of recorded events.
Presently, all major society guidelines recommend no thromboprophylaxis for cancer outpatients treated with chemotherapy. Nonetheless, there is increasing awareness that the use of appropriate prevention measures in high-risk cancer patients with VTE risk factors could provide an important opportunity to substantially improve time-delivery chemotherapy and quality of life, decreasing morbidity, mortality, and use of health care resources. We are witnessing a sustained search for the identification of risk assessment models that may be predictive of cancer-associated VTE, at least in selected patient populations. In this respect, the Khorana score [13] correctly assigns patients to the high-risk category, in whom, according to the latest guidelines issued by the American Society for Clinical Oncology, “clinicians may consider [low-molecular-weight heparin] prophylaxis on a case-by-case basis” after discussing with the patient “benefits and harms as well as dose and duration of prophylaxis in this setting” [25]. However, the Khorana score [13] fails to classify approximately 50% of patients (intermediate risk), for whom clinical decision making remains challenging. Expanded risk-scoring models have been proposed recently, in which points were added based either on laboratory tests [20] or administered drugs [26]. Although an expansion of the score did not fall within the aims of our study, the results reported demonstrate that reduced eGFR is associated with an increased VTE risk in ambulatory cancer patients undergoing platinum-based chemotherapy regimens and might be used as an adjunctive criterion for the definition of the risk class of an individual patient.
Conclusion
Pretreatment eGFR could represent a simple and cost-effective predictor of venous thromboembolic events, at no additional cost to health care systems. Future multicenter prospective studies specifically designed to address the issue of whether reduced kidney function could represent a VTE predictor in the course of platinum-based chemotherapy regimens will help to improve our knowledge on treatment-associated risks and their impact on patients' quality of life.
Acknowledgments
This work has been performed within the Programs XXVI and XXVII Ciclo and was partially supported by the Italian Ministry of Health Grants MERIT RBNE08NKH7 and PO FESR 2007/2013 Linea di Intervento 4.1.1.1 - SIASOP. P.F. and F.G. contributed equally as first authors.
Footnotes
For Further Reading: Gary H. Lyman, Laurent Eckert, Yanxin Wang et al. Venous Thromboembolism Risk in Patients With Cancer Receiving Chemotherapy: A Real-World Analysis. The Oncologist 2013;18:1321–1329.
Implications for Practice: This large observational study of unselected patients receiving cancer chemotherapy demonstrates considerably greater rates of venous thromboembolism (VTE) than commonly reported in patients accrued to clinical trials. The risk of VTE appears to increase progressively over the year following initiation of treatment. Cancer patients developing VTE also experience a greater risk of major bleeding and greater health care costs than patients without VTE. Patients considered at high risk for VTE should be considered for thromboprophylaxis after assessing the balance of potential benefits and harms.
Author Contributions
Conception/Design: Patrizia Ferroni, Fiorella Guadagni, Antonio Russo, Giovanni Davì, Mario Roselli
Collection and/or assembly of data: Anastasia Laudisi, Matteo Vergati, Silvia Riondino
Data analysis and interpretation: Patrizia Ferroni, Fiorella Guadagni, Anastasia Laudisi, Matteo Vergati, Silvia Riondino, Antonio Russo, Mario Roselli
Manuscript writing: Patrizia Ferroni, Fiorella Guadagni, Giovanni Davì, Mario Roselli
Final approval of manuscript: Patrizia Ferroni, Fiorella Guadagni, Anastasia Laudisi, Matteo Vergati, Silvia Riondino, Antonio Russo, Giovanni Davì, Mario Roselli
Disclosures
The authors indicated no financial relationships.
References
- 1.Naess IA, Christiansen SC, Romundstad P, et al. Incidence and mortality of venous thrombosis: A population-based study. J Thromb Haemost. 2007;5:692–699. doi: 10.1111/j.1538-7836.2007.02450.x. [DOI] [PubMed] [Google Scholar]
- 2.Walker AJ, Card TR, West J, et al. Incidence of venous thromboembolism in patients with cancer - a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49:1404–1413. doi: 10.1016/j.ejca.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Khorana AA, Dalal M, Lin J, et al. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer. 2013;119:648–655. doi: 10.1002/cncr.27772. [DOI] [PubMed] [Google Scholar]
- 4.Mahmoodi BK, Gansevoort RT, Veeger NJ, et al. Microalbuminuria and risk of venous thromboembolism. JAMA. 2009;301:1790–1797. doi: 10.1001/jama.2009.565. [DOI] [PubMed] [Google Scholar]
- 5.Wattanakit K, Cushman M, Stehman-Breen C, et al. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol. 2008;19:135–140. doi: 10.1681/ASN.2007030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folsom AR, Lutsey PL, Astor BC, et al. Chronic kidney disease and venous thromboembolism: A prospective study. Nephrol Dial Transplant. 2010;25:3296–3301. doi: 10.1093/ndt/gfq179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parikh AM, Spencer FA, Lessard D, et al. Venous thromboembolism in patients with reduced estimated GFR: A population-based perspective. Am J Kidney Dis. 2011;58:746–755. doi: 10.1053/j.ajkd.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahmoodi BK, Gansevoort RT, Næss IA, et al. Association of mild to moderate chronic kidney disease with venous thromboembolism: Pooled analysis of five prospective general population cohorts. Circulation. 2012;126:1964–1971. doi: 10.1161/CIRCULATIONAHA.112.113944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lotan E, Leader A, Lishner M, et al. Unrecognized renal insufficiency and chemotherapy-associated adverse effects among breast cancer patients. Anticancer Drugs. 2012;23:991–995. doi: 10.1097/CAD.0b013e328355dd8a. [DOI] [PubMed] [Google Scholar]
- 10.Ferroni P, Della-Morte D, Palmirotta R, et al. Platinum-based compounds and risk for cardiovascular toxicity in the elderly: Role of the antioxidants in chemoprevention. Rejuvenation Res. 2011;14:293–308. doi: 10.1089/rej.2010.1141. [DOI] [PubMed] [Google Scholar]
- 11.Barni S, Labianca R, Agnelli G, et al. Chemotherapy-associated thromboembolic risk in cancer outpatients and effect of nadroparin thromboprophylaxis: Results of a retrospective analysis of the PROTECHT study. J Transl Med. 2011;9:179–185. doi: 10.1186/1479-5876-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roselli M, Ferroni P, Riondino S, et al. Impact of chemotherapy on activated protein C-dependent thrombin generation—association with VTE occurrence. Int J Cancer. 2013;133:1253–1258. doi: 10.1002/ijc.28104. [DOI] [PubMed] [Google Scholar]
- 13.Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Alliance for Patient Safety. WHO draft guidelines for adverse event reporting and learning systems: From information to action. Available at http://www.who.int/patientsafety/events/05/Reporting_Guidelines.pdf. Last accessed March 20th, 2014.
- 15.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(suppl 1):S1–S266. [PubMed] [Google Scholar]
- 17.Agnelli G, Gussoni G, Bianchini C, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: A randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009;10:943–949. doi: 10.1016/S1470-2045(09)70232-3. [DOI] [PubMed] [Google Scholar]
- 18.Lyman GH. Thromboprophylaxis with low-molecular-weight heparin in medical patients with cancer. Cancer. 2009;115:5637–5650. doi: 10.1002/cncr.24665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ay C, Vormittag R, Dunkler D, et al. D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: Results from the Vienna Cancer and Thrombosis Study. J Clin Oncol. 2009;27:4124–4129. doi: 10.1200/JCO.2008.21.7752. [DOI] [PubMed] [Google Scholar]
- 20.Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377–5382. doi: 10.1182/blood-2010-02-270116. [DOI] [PubMed] [Google Scholar]
- 21.Ferroni P, Martini F, Portarena I, et al. An activated protein C-dependent thrombin generation assay predicts chemotherapy-associated venous thromboembolism in cancer patients. Thromb Haemost. 2011;105:931–932. doi: 10.1160/TH10-11-0757. [DOI] [PubMed] [Google Scholar]
- 22.Ay C, Dunkler D, Simanek R, et al. Prediction of venous thromboembolism in patients with cancer by measuring thrombin generation: Results from the Vienna Cancer and Thrombosis Study. J Clin Oncol. 2011;29:2099–2103. doi: 10.1200/JCO.2010.32.8294. [DOI] [PubMed] [Google Scholar]
- 23.van Doormaal F, Kleinjan A, Berckmans RJ, et al. Coagulation activation and microparticle-associated coagulant activity in cancer patients. An exploratory prospective study. Thromb Haemost. 2012;108:160–165. doi: 10.1160/TH12-02-0099. [DOI] [PubMed] [Google Scholar]
- 24.Zwicker JI, Liebman HA, Bauer KA, et al. Prediction and prevention of thromboembolic events with enoxaparin in cancer patients with elevated tissue factor-bearing microparticles: A randomized-controlled phase II trial (the Microtec study) Br J Haematol. 2013;160:530–537. doi: 10.1111/bjh.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2189–2204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]
- 26.Verso M, Agnelli G, Barni S, et al. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: The Protecht score. Intern Emerg Med. 2012;7:291–292. doi: 10.1007/s11739-012-0784-y. [DOI] [PubMed] [Google Scholar]