Abstract
Testicular germ cell cancer develops from pre-malignant intratubular germ cell neoplasia, unclassified cells that are believed to arise from failure of normal maturation of fetal germ cells from gonocytes (OCT4+/ MAGEA4−) into pre-spermatogonia (OCT4−/MAGEA4+). Intratubular germ cell neoplasia cell subpopulations based on stage of germ cell differentiation have been described, however the importance of these subpopulations in terms of invasive potential has not been reported. We hypothesised that cells expressing an immature (OCT4+/MAGEA4−) germ cell profile would exhibit an increased proliferation rate compared to those with a mature profile (OCT4+/ MAGEA4+). Therefore, we performed triple immunofluorescence and stereology to quantify the different intratubular germ cell neoplasia cell subpopulations, based on expression of germ cell (OCT4, PLAP, AP2γ, MAGEA4, VASA) and proliferation (Ki67) markers, in testis sections from patients with pre-invasive disease, seminoma and non-seminoma. We compared these subpopulations with normal human fetal testis and with seminoma cells. Heterogeneity of protein expression was demonstrated in intratubular germ cell neoplasia cells with respect to gonocyte and spermatogonial markers. It included an embryonic/fetal germ cell subpopulation lacking expression of the definitive intratubular germ cell neoplasia marker OCT4, that did not correspond to a physiological (fetal) germ cell subpopulation. OCT4+/MAGEA4- cells showed a significantly increased rate of proliferation compared with the OCT4+/MAGEA4+ population (12.8 v 3.4%, p<0.0001) irrespective of histological tumour type, reflected in the predominance of OCT4+/MAGEA4− cells in the invasive tumour component. Surprisingly, OCT4+/MAGEA4− cells in patients with pre-invasive disease showed significantly higher proliferation compared to those with seminoma or non-seminoma (18.1 v 10.2 v 7.2%, p<0.05 respectively). In conclusion, this study has demonstrated that OCT4+/MAGEA4− cells are the most frequent and most proliferative cell population in tubules containing intratubular germ cell neoplasia, which appears to be an important factor in determining invasive potential of intratubular germ cell neoplasia to seminomas.
Keywords: Testicular germ cell tumours, Cell differentiation, Cell proliferation, Germ cells, Carcinoma in situ
Introduction
Testicular germ cell cancer is the most common malignancy in young men and the incidence of these tumours is increasing worldwide [1,2]. The tumours are classified as seminoma or non-seminoma with a distinct cell of origin and pathogenesis compared with spermatocytic seminoma of late adulthood [1]. These tumours result from transformation, usually in young adulthood, of pre-invasive intratubular germ cell neoplasia (also known as carcinoma in situ) cells that arise during fetal life [3,4]. Intratubular germ cell neoplasia cells are believed to be germ cells that have failed to undergo normal maturation during fetal or early postnatal life.
In humans, during fetal life, primordial germ cells migrate into the developing gonad at around 5 weeks of gestation and become gonocytes [5]. These cells express proteins associated with pluripotency (e.g. OCT4 and NANOG) [6,7] and a number of other embryonic markers (e.g. AP2γ and PLAP) [8,9]. During the remainder of fetal life and into the early postnatal period these cells begin to express germ cell specific proteins (e.g. VASA and MAGEA4) during their transition from gonocytes into spermatogonia and this is associated with a loss of the gonocyte protein markers [7]. This transition occurs in an asynchronous manner such that cells at different stages of development may be present in an individual seminiferous cord during this period and some of these cells may co-express both gonocyte and a spermatogonial markers [10].
Intratubular germ cell neoplasia cells express many of the same proteins as gonocytes (e.g. OCT4, PLAP, AP2γ) and these are often used in conjunction with histological evaluation to diagnose the condition in testicular biopsies [11]. It is also recognised that intratubular germ cell neoplasia cells may express proteins indicative of spermatogonia (e.g. MAGEA4, VASA, TSPY) [3,12,13]. The clinical significance of the differing protein expression profiles amongst intratubular germ cell neoplasia cells is not known.
Proliferation of pre-invasive cells is important for the development of an invasive tumour and proliferation has been shown to occur in intratubular germ cell neoplasia cells prior to the development of an invasive tumour [12]. However, proliferation in the different sub-populations of intratubular germ cell neoplasia cells, based on germ cell differentiation profile, has not previously been investigated.
The aim of this study was to characterise the heterogeneous protein expression profiles of intratubular germ cell neoplasia cells using co-localisation of multiple proteins simultaneously and to compare this to the expression profiles of normal germ cells in the human fetal testis. In addition we aimed to quantify the different intratubular germ cell neoplasia sub-populations associated with different testicular germ cell cancer histological types and to investigate whether the protein expression profile of intratubular germ cell neoplasia cells is related to proliferation of these cells and hence to their invasive potential.
Materials and Methods
Tissue collection
Human intratubular germ cell neoplasia/testicular germ cell cancer tissue
Ethical approval was obtained for the use of archived human testicular tissue from the Pathology Departments at the Western General Hospital in Edinburgh (REC Reference number - 10/S1402/33) and Erasmus MC-University Medical Center, Rotterdam (Institutional review board - MEC 02.981 and CCR2041). Samples were randomly selected from the testicular germ cell tumour database and analysed by light microscopy for the presence of intratubular germ cell neoplasia cells. The diagnosis included pre-invasive disease (childhood, n=4; adulthood, n=7), seminoma (n=9) and non-seminoma (n=8). Patient details are described in Table 1. The specimens had been fixed in formalin for 24 hours.
Table 1.
Clinical characteristics of patients from whom tissue was obtained
| Sample | Age (y) | Diagnosis |
|---|---|---|
| 1 | 0.8 | Maturation delay, intra-abdominal testis |
| 2 | 1 | Maturation delay, intra-abdominal testis |
| 3 | 7 | Intratubular germ cell neoplasia, intra-abdominal testis |
| 4 | 12 | Intratubular germ cell neoplasia |
| 5 | 21 | Intratubular germ cell neoplasia only |
| 6 | 31 | Intratubular germ cell neoplasia, atrophy |
| 7 | 34 | Intratubular germ cell neoplasia only |
| 8 | 36 | Intratubular germ cell neoplasia only |
| 9 | 36 | Intratubular germ cell neoplasia only |
| 10 | 32 | Intratubular germ cell neoplasia, intra-tubular seminoma |
| 11 | Adult | Seminoma |
| 12 | Adult | Seminoma |
| 13 | Adult | Seminoma |
| 14 | Adult | Seminoma |
| 15 | Adult | Seminoma |
| 16 | Adult | Seminoma |
| 17 | Adult | Seminoma |
| 18 | Adult | Seminoma |
| 19 | Adult | Seminoma |
| 20 | Adult | Embryonal carcinoma with teratoma |
| 21 | Adult | Mixed-embryonal carcinoma, seminoma and teratoma |
| 22 | Adult | Embryonal carcinoma with teratoma |
| 23 | Adult | Embryonal carcinoma with teratoma |
| 24 | Adult | Mixed-embryonal carcinoma, yolk sac tumour, seminoma and teratoma |
| 25 | Adult | Embryonal carcinoma with teratoma |
| 26 | Adult | Teratoma |
| 27 | Adult | Teratoma |
Human Fetal Testes
Human fetal testes were obtained following termination of pregnancy during 2nd trimester (14-19 weeks, n=5). Women gave consent in accordance with national guidelines, and ethical approval was obtained from the Local Research Ethics Committee (Reference number – LREC08/S1101/1). No terminations were due to fetal abnormalities. Gestational age was determined initially by ultrasound, followed by measurement of foot length. Testes were fixed for 2h in Bouins, transferred into 70% ethanol and then embedded in paraffin. Sections of 5μm thickness were prepared.
Immunohistochemistry
Details of antibodies, dilutions and requirement for antigen retrieval are shown in Table 2. Sections were dewaxed in xylene, rehydrated in graded alcohols and washed in tap water. Antigen retrieval involved pressure cooking in 0.01M citrate (pH 6.0) buffer as described previously [14]. Sections were treated with 3% (v/v) H2O2 in methanol for 30 min and washed in water, followed by Tris-buffered saline (TBS, 0.05M Tris and 0.85% NaCl, pH 7.6) for a further 5 min. Endogenous biotin was blocked using an avidin/biotin blocking kit (Vector Laboratories, Peterborough, UK), according to the manufacturers instructions. Sections were incubated in appropriate normal serum (diluted 1:5 with TBS containing 5% (w/v) bovine serum albumin (BSA) (Sigma, Poole, Dorset, UK) for 30 min. Sections were incubated overnight with primary antibody diluted in serum at 4oC in a humidified chamber. Sections were washed in TBS (2×5min) and incubated for 30 min with the appropriate biotinylated secondary antibody (swine anti-rabbit, rabbit anti-mouse; both Dako, Ely, UK or rabbit anti-goat; Vector Laboratories), diluted in normal serum. This was followed by two further 5 min washes in TBS and incubation for 30 min with Streptavidin-HRP at 1:1000 (Dako), diluted in TBS.
Table 2.
Antibodies and conditions for immunohistochemistry. All antibodies were raised against human peptide sequences
| Antigen | Retrieval | Source | Species | Dilution | |
|---|---|---|---|---|---|
| DAB | Fluorescence | ||||
| AP-2γ | Y | Santa Cruza | Mouse | 1:20 | 1:60 |
| OCT 4 | Y | Santa Cruza | Goat | 1:50 | 1:150 |
| PLAP | Y | Abcamb | Rabbit | 1:100 | 1:200 |
| VASA | Y | Abcamb | Rabbit | 1:200 | 1:500 |
| MAGEA4 | N | Giftc | Mouse | 1:20 | 1:100 |
| Ki67 | Y | Dakod | Rabbit | 1:40 | 1:100 |
Santa Cruz Biotechnology, CA, USA
Abcam, Cambridge, UK.
Dr. Guilio Spagoli, University Hospital, Basel, Switzerland
Dako, Ely, UK
Visualisation was performed using 3,3-diaminobenzidine tetrahydrochloride (DAB) (Dako) and sections were counterstained with haematoxylin, dehydrated in graded alcohols, immersed in xylene and mounted in Pertex medium (CellPath, Hemel Hempstead, UK). For each experiment a negative control (primary antibody replaced with the appropriate normal serum) was included. Images were captured using an Olympus Provis microscope (Olympus, London, UK) and Canon DS126131 camera with Canon EOS image capture software (Canon, Woodhatch, Surrey, UK).
Immunofluorescence
Sections were initially treated as described for single staining as far as the primary antibody stage, with Phosphate Buffered Saline (PBS; Sigma) washes between each step. Antigen retrieval was required for all experiments. Details of antibodies, serum and visualisation method are listed in Table 2.
Following overnight incubation with primary antibody in serum, sections were incubated with secondary antibody for 30 min, followed by fluorescently labelled Tyramide (1:50; Perkin Elmer, Cambridge, UK) in dilution buffer. For this and subsequent steps, the sections were kept in darkness. Sections were then microwaved in 0.01M citrate (pH 6.0) for 2.5 min and left to cool for 30 min, before being washed in water and PBS for 5 min. Sections were incubated for 30 min in serum. They were incubated with secondary antibody for 30 min, followed by the labelled Tyramide (1:50) using a different fluorescent label. After the second visualisation sections were microwaved again as described above and incubated with the third secondary antibody for 30 min, followed by the third labelled Tyramide (1:50). DAPI (Sigma) was applied to the sections at 1:1000 in PBS for 10 min and the slides were mounted using Permafluor (Immunotech, Marseille, France). Images were captured using an LSM 510 Confocal microscope (Carl Zeiss, Hertfordshire, UK).
Quantification of germ cell differentiation and proliferation
Quantification of germ cell subpopulations and proliferation indices were performed for the triple-stained sections as previously described [10]. For each sample, a minimum of 10 randomly selected fields with tubules containing intratubular germ cell neoplasia were counted and included an average of 1000 cells per section. Images were obtained using an Axiovert 200M microscope with attached Axiocam HRc camera and Axiovision 4.6 software (all Carl Zeiss). All germ cells within each section were manually counted and quantified according to their protein expression profile and proliferation status by marking cells in layered images using Adobe Photoshop 7.0 (Adobe, San Jose, CA, USA).
Statistics
Statistical analysis was performed using Graphpad Prism 5 software (La Jolla, CA, USA). Groups were compared using Students t-test. Multiple groups were analysed using one-way analysis of variance (ANOVA). Statistical significance was set at P<0.05.
Results
In order to characterise the heterogeneity of expression of germ cell proteins in putative intratubular germ cell neoplasia cells we first compared the expression of a range of germ cell-specific proteins in testicular tissue from patients with testicular germ cell cancer (including tubules containing intratubular germ cell neoplasia cells and those with apparently normal spermatogenesis) with that of the normal human fetal testis.
Expression of gonocyte markers in human fetal testis, intratubular germ cell neoplasia and spermatogonia
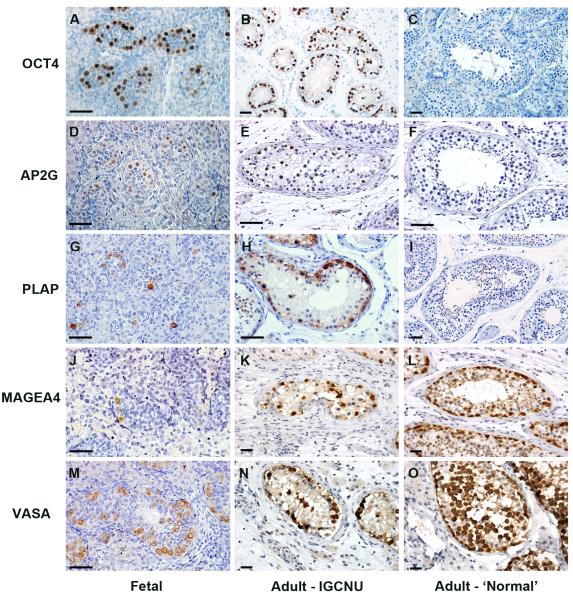
OCT4, AP2γ and PLAP were expressed in germ cells (gonocytes) in the human fetal testis. In sections from patients with testicular germ cell cancer these proteins were also expressed in intratubular germ cell neoplasia cells; however none of the proteins were expressed in spermatogonia in tubules that contained active spermatogenesis (Fig. 1A-I).
Figure 1.
Expression of gonocyte markers (OCT4, AP2γ and PLAP; A-I) and spermatogonial markers (MAGEA4 and VASA; J-O) in human fetal testis, intratubular germ cell neoplasia containing tubules (Adult – intratubular germ cell neoplasia) and tubules from adult testis with active spermatogenesis (Adult – ‘Normal’). Gonocyte proteins are detected in human fetal germ cells and intratubular germ cell neoplasia cells, but are absent from the germ cells in tubules with apparently normal spermatogenesis; whilst spermatogonial proteins are expressed in germ cells in all tissue types. Human fetal samples are 14 (A,D), 16 (J) and 18 weeks (G,M) gestation. Scale bar = 50μm.
Expression of spermatogonial markers in human fetal testis, intratubular germ cell neoplasia and spermatogonia
MAGEA4 and VASA were expressed in germ cells (pre-spermatogonia) in the human fetal testis. These proteins were also expressed in intratubular germ cell neoplasia cells. There was also expression of these proteins in germ cells (MAGEA4 in spermatogonia and early spermatocytes, VASA in all germ cells) of tubules that contained active spermatogenesis in patients with testicular germ cell cancer (Fig 1J-O).
Co-expression of gonocyte and spermatogonial markers in intratubular germ cell neoplasia cells and normal testis
For the identification of intratubular germ cell neoplasia cells and to attempt to distinguish these cells from normal germ cells, AP2γ and VASA co-expression was investigated (Fig 2). In tubules with normal-appearing spermatogenesis there was expression of VASA in the cytoplasm of germ cells, but no expression of AP2γ (Fig 2A). In tubules containing a mixture of germ cells characteristic of either intratubular germ cell neoplasia or normal spermatogonia the putative intratubular germ cell neoplasia cells (located on the basement membrane) were identified as AP2γ+/VASA−, whilst a small proportion of these cells were AP2γ+/VASA+ (Fig 2B). AP2γ−/VASA+cells, located nearer the lumen were also identified in intratubular germ cell neoplasia tubules. These putative spermatocytes were also identified in tubules in which the majority of the cells were intratubular germ cell neoplasia cells (AP2γ+/VASA−; Fig 2C). Similar populations of germ cells were also identified within the human fetal testis, based on co-staining for OCT4 and VASA (Supp. Fig. 1; [7]).
Figure 2.
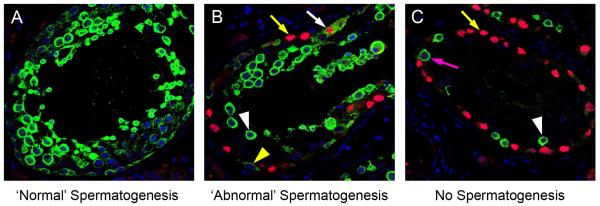
Representative image for expression of VASA (green) and AP2γ (red) in tubules from adult patients with testicular germ cell cancer. A) Tubule with apparently normal spermatogenesis: VASA is expressed in the germ cells with no expression of the intratubular germ cell neoplasia/gonocyte protein AP2γ. B) Tubule with abnormal spermatogenesis: VASA expression is seen in the presumptive spermatocytes towards the lumen (white arrowhead), however germ cells along the basement membrane express VASA (yellow arrowhead) or AP2γ (yellow arrow). A small proportion of cells co-express VASA and AP2γ (white arrow). C) Intratubular germ cell neoplasia tubule: The majority of cells express the intratubular germ cell neoplasia protein AP2γ (yellow arrow) with a small number of cells adjacent to the basement membrane expressing VASA (presumptive spermatogonia; pink arrow). A small number of germ cells expressing VASA are located towards the lumen (presumptive spermatocytes; white arrowhead).
Heterogeneity of expression of ‘classical’ intratubular germ cell neoplasia markers in patients with testicular germ cell cancer
In order to demonstrate the heterogeneity of expression of gonocyte proteins in intratubular germ cell neoplasia, co-localisation of OCT4, AP2γ and PLAP was undertaken. OCT4 and AP2γ were always co-expressed and were localised to the nuclei of intratubular germ cell neoplasia cells (Supp. Fig. 2). A similar pattern of co-expression was demonstrated for OCT4 and PLAP, with co-expression of OCT4 (nuclear) and PLAP (cytoplasm +/− nuclear) in the majority of cells (OCT4+/PLAP+) within these tubules, however there were also germ cells that were OCT4+/PLAP− (Fig. 3A). These two populations were also identified in the human fetal testis (Fig. 3B). However within the intratubular germ cell neoplasia containing tubules we also identified a rare population of cells that were OCT4−/PLAP+ (Fig. 3C), whilst this population of germ cells was not identified in any of the human fetal testis sections (Fig. 3B).
Figure 3.
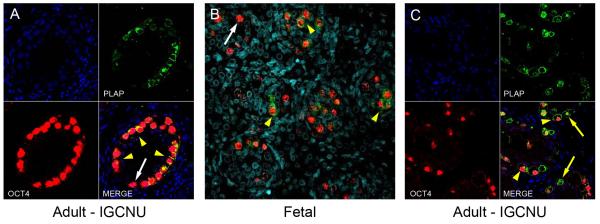
Expression of OCT4 (red) and PLAP (green) in intratubular germ cell neoplasia containing tubules from men with testicular germ cell cancer (A,C) and in normal human fetal testis tissue (B). The majority of intratubular germ cell neoplasia cells co-express OCT4 and PLAP (A,C; yellow arrowheads). Occasional OCT4 positive intratubular germ cell neoplasia cells are negative for PLAP expression (A; white arrow). Similar sub-populations of OCT4+/PLAP− (B, white arrow) and OCT4+/PLAP+ (B, yellow arrowheads) cells are also identified in the human fetal testis. In tubules with intratubular germ cell neoplasia, occasional OCT4−/PLAP+ cells are identified (C; yellow arrows), however no similar population is seen in the human fetal testis. Counterstain (DAPI; blue).
The expression of spermatogonial markers in putative intratubular germ cell neoplasia cells
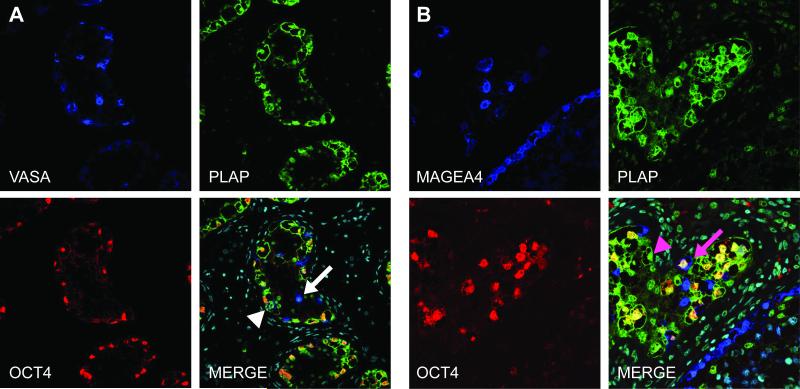
To further characterise the germ cells within intratubular germ cell neoplasia containing tubules that either did or did not express OCT4, triple immunofluorescence staining was undertaken for MAGEA4/VASA, OCT4 and PLAP (Fig. 4). In tubules containing intratubular germ cell neoplasia cells, the majority of germ cells expressed nuclear OCT4 and most of these were co-stained with PLAP (cytoplasmic +/− nuclear). A small proportion of presumptive spermatocytes expressed VASA only (Fig. 4A). However there were infrequent germ cells which expressed VASA and PLAP, but which did not express OCT4 (Fig. 4A), suggesting that a proportion of the VASA expressing cells (VASA+/OCT4−) are not ‘normal’ spermatogonia/spermatocytes and may represent gonocytes that have downregulated OCT4 and begun to express VASA but retain PLAP expression (VASA+/OCT4−/PLAP+). Similar sub-populations were found when the co-expression of MAGEA4 with OCT4 and PLAP was undertaken (Figure 4B).
Figure 4.
Representative image for expression of OCT4 (red), VASA (A; blue), MAGEA4 (B; blue) and PLAP (green) in intratubular germ cell neoplasia containing tubules from patients with testicular germ cell cancer. A) VASA expression is demonstrated in putative ‘spermatogenic’ germ cells that are negative for intratubular germ cell neoplasia cell proteins PLAP and OCT4 (white arrow). A small proportion of the VASA+ cells that are negative for OCT4 express PLAP (white arrowhead). B) The majority of cells co-express OCT4 and PLAP without MAGEA4, other sub-populations are identified including PLAP+/OCT4+/MAGEA4+ (pink arrow) and PLAP+/OCT4−/MAGEA4− (pink arrowhead). Counterstain (DAPI; pale blue) in merged panels.
Quantification of intratubular germ cell neoplasia phenotypes depending on histological testicular germ cell cancer type
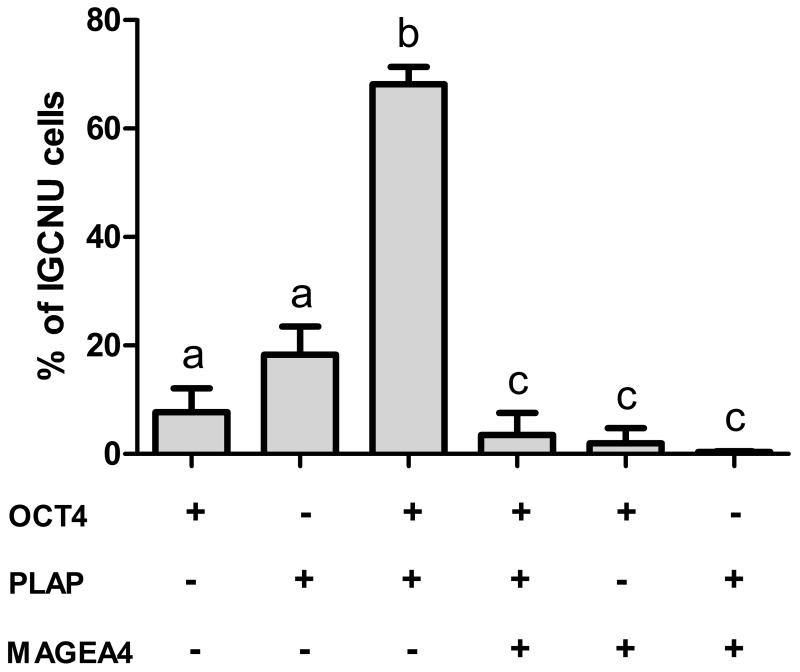
The proportion of intratubular germ cell neoplasia cells (identified by expression of OCT4 and/or PLAP) with different expression profiles based on OCT4/PLAP/MAGEA4 co-staining was determined (Fig. 5). By far the most common phenotype was OCT4+/PLAP+/MAGEA4−, which was found in a significantly higher proportion (68%) of cells compared with the other phenotypes (Fig. 5; b versus a,c). A smaller proportion (7.7%) of intratubular germ cell neoplasia cells were OCT4+/PLAP−/MAGEA4−. Overall 82% of intratubular germ cell neoplasia cells expressed OCT4 with the remaining 18% of putative intratubular germ cell neoplasia cells expressing PLAP (but no detectable OCT4). In terms of spermatogonial markers, MAGEA4 expression was found in 6% of putative intratubular germ cell neoplasia cells (defined by expression of OCT4 and/or PLAP; Fig. 5) and this represented a significantly lower proportion of cells compared to those not expressing MAGEA4 (Fig 5; c versus a,b). There was a shift towards an increasing proportion of these MAGEA4+ cells from pre-invasive (child) to pre-invasive (adult) and seminoma, whilst very few putative intratubular germ cell neoplasia cells in non-seminoma expressed this protein profile, however the differences in expression were not significant (Supp. Fig. 3).
Figure 5.
Quantification of putative intratubular germ cell neoplasia phenotypes. Expression (+) of OCT4, PLAP and MAGEA4 for intratubular germ cell neoplasia containing tubules (n=9; pre-invasive, seminoma and non-seminoma; n=3 each). Bars with different letters are significantly different from each other (p<0.05). Mean +/− SEM.
Proliferation of intratubular germ cell neoplasia cells according to germ cell expression profile and histological testicular germ cell cancer type
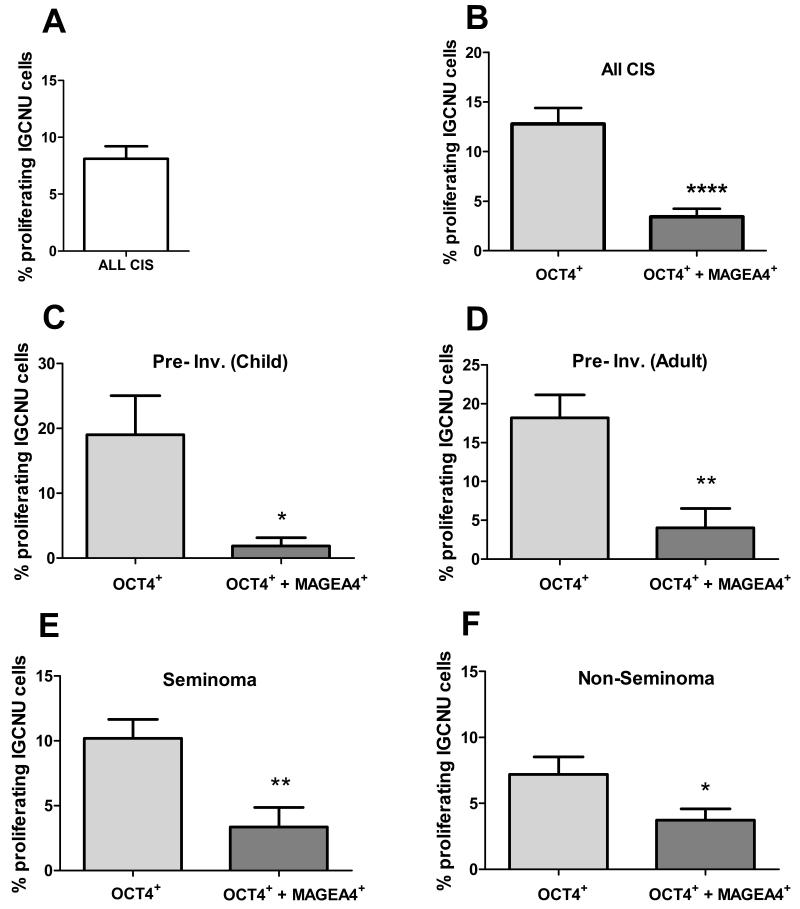
In order to investigate whether the germ cell expression profile of the putative intratubular germ cell neoplasia cells might determine their proliferation rate, which might in turn affect their invasive potential, triple immunofluorescence for OCT4/MAGEA4/Ki67 was performed (Supp. Fig. 4). Overall, the proportion of proliferating (Ki67+/OCT4+) intratubular germ cell neoplasia cells was 8.1% (Fig. 6A). There was a shift towards increased proliferation in intratubular germ cell neoplasia cells from patients with pre-invasive disease compared with those with a seminoma or non-seminoma, but this was not statistically significant (Supp. Fig. 5). However, when intratubular germ cell neoplasia (identified by expression of OCT4+) cell proliferation was analysed according to whether or not the cells also expressed MAGEA4, a significantly higher proliferation rate was found for the OCT4+/MAGEA4− population compared to OCT4+/MAGEA4+ intratubular germ cell neoplasia cells (12.8 v 3.4%, p<0.0001; Fig. 6B). Moreover, the significant difference in proliferation rate between the two intratubular germ cell neoplasia phenotypes was consistent when the same analysis was performed according to whether the intratubular germ cell neoplasia cells were from patients with pre-invasive disease (child or adult), seminoma or non-seminoma (Fig. 6C-F). Furthermore, when the proliferation rates in the two sub-populations of intratubular germ cell neoplasia cells (OCT4+/MAGEA4− or OCT4+/MAGEA4+) were compared according to the histology of the adjacent testicular germ cell cancer, there was a significantly higher proliferation rate in the OCT4+/MAGEA4− cells in pre-invasive disease compared with these cells in seminoma and non-seminoma (Fig. 7A); in contrast, there was no difference in the proportion of OCT4+/MAGEA4+ cells that were proliferating for the different tumour types (Fig. 7B).
Figure 6.
Proliferation in putative intratubular germ cell neoplasia cells. A) Overall proliferation in all intratubular germ cell neoplasia cells. B) Proliferation (Ki67+) of intratubular germ cell neoplasia (OCT4+) cells based on the co-expression with MAGEA4 in tubules from all patients (B), children with pre-invasive disease (C; Pre-Inv. Child; n=4), adults with pre-invasive disease (D; Pre-Inv. Adult; n=6), seminoma (n=7) and non-seminoma (n=8). Mean +/− SEM. * p<0.05, ** p<0.01, **** p<0.0001.
Figure 7.
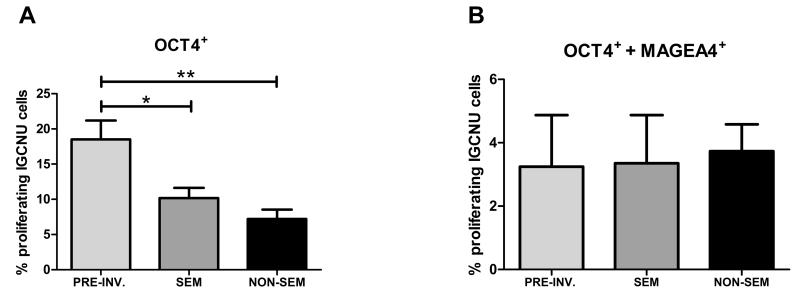
Proliferation of intratubular germ cell neoplasia cells based on diagnosis of pre-invasive disease (PRE INV; n=7), seminoma (SEM; n=7) or non-seminoma (NON-SEM; n=8). A) Proliferation of OCT4+ intratubular germ cell neoplasia cells. B) Proliferation of OCT4+/MAGEA4+ intratubular germ cell neoplasia cells. Mean +/− SEM. * p<0.05, ** p<0.01, in comparison with pre-invasive intratubular germ cell neoplasia.
Proliferation of seminoma cells according to germ cell expression profile
Given that the OCT4+/MAGEA4− population of intratubular germ cell neoplasia cells were more proliferative than the OCT4+/MAGEA4+ population, we investigated MAGEA4, OCT4 and PLAP expression in seminoma cells in order to determine whether this results in a predominance of OCT4+/MAGEA4− cells in the resulting tumours. Indeed we found that in the majority of intra-tubular seminoma cells MAGEA4 was not expressed and that the majority of cells were OCT4+/PLAP+/MAGEA4− (Fig. 8A). MAGEA4 positive cells were seen in tubules with normal appearance adjacent to areas of invasive seminoma (Fig. 8B), however MAGEA4 expression was not seen in the invasive seminoma cells (Fig. 8C). The OCT4+/MAGEA4− seminoma cells were highly proliferative, whilst a smaller proportion of OCT4+/MAGEA4− intratubular germ cell neoplasia cells were proliferative. In contrast MAGEA4 expressing cells were rarely proliferative (Supp. Fig. 6).
Figure 8.
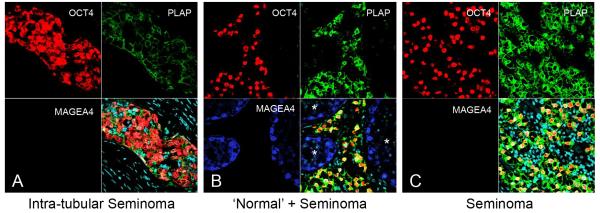
Representative images for expression of OCT4 (red), PLAP (green) and MAGEA4 (blue) in testis sections from patients with A) Intra-tubular seminoma, B) Seminoma with surrounding ‘normal’ (*) tubules and C) Seminoma. Note the lack of expression of MAGEA4 in both intra-tubular seminoma and invasive seminoma. Counterstain (DAPI; pale blue) in merged panels (bottom right).
Discussion
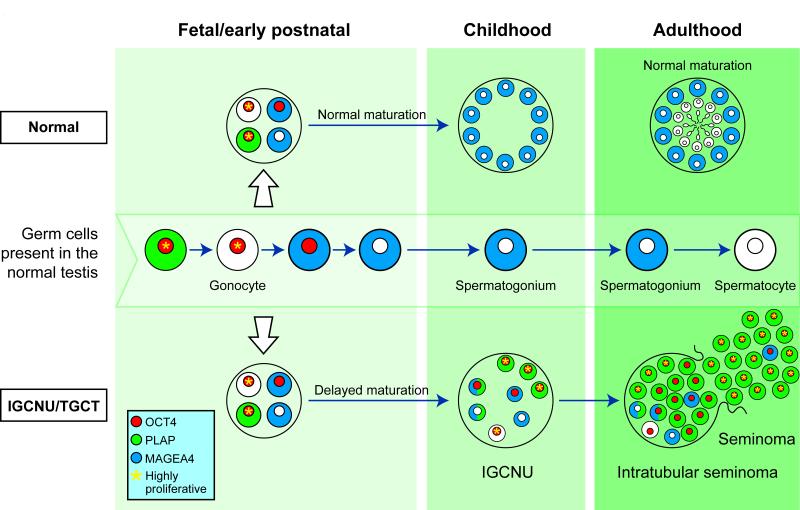
The present study has characterised the heterogeneity of germ cell protein expression in the human testis based on co-expression of germ cell proteins involved in differentiation from gonocyte to pre-spermatogonia. We have demonstrated an infrequent population of cells within intratubular germ cell neoplasia containing tubules with an expression profile distinct from germ cells in the normal human fetal testis. We have also demonstrated that the most common sub-population in intratubular germ cell neoplasia containing tubules, which displays a ‘gonocyte’ expression profile (OCT4+/MAGEA4−), is associated with an increased proliferation rate compared to the subpopulation expressing a ‘pre-spermatogonial’ profile (OCT4+/MAGEA4+), and that this (OCT4+/MAGEA4−) population represents the true intratubular germ cell neoplasia cell and precursor for invasive seminoma and non-seminoma. The findings of the present study are summarised in Fig 9.
Figure 9.
Schematic for germ cell maturation and proliferation in germ cells during transition from gonocyte to intratubular germ cell neoplasia and testicular germ cell cancer (bottom). Germ cell maturation from gonocyte to initiation of spermatogenesis is represented in the testis during the different stages of life (middle). For comparison, germ cell differentiation in the normal testis is also shown (top). Germ cells in the fetal testis may exhibit delayed maturation with persistence of gonocyte markers through childhood. A variety of germ cell protein profiles are present in the intratubular germ cell neoplasia tubule, however it is the cells expressing exclusively gonocyte proteins (with no spermatogonial proteins) that are more proliferative and contribute to the majority of the cells in intratubular seminoma and subsequently invasive seminoma. Cells expressing spermatogonial proteins are occasionally seen in the tubule or resultant tumour but exhibit low proliferation rates. Expression of OCT4 (red), PLAP (green) and MAGEA4 (blue) is shown for individual cells and cells with high rates of proliferation are indicated (yellow asterisk)
Intratubular germ cell neoplasia cells are thought to originate in fetal life from abnormally differentiated gonocytes [3,15]. This is supported by similarities in morphological, immunohistochemical and genetic profiles [3,16,17]. Intratubular germ cell neoplasia cells share expression of a variety of proteins that are involved in pluripotency and early germ cell fate, such as OCT4 [18-20], PLAP [9,21] and AP2γ [8]. As gonocytes differentiate into spermatogonia during fetal life these markers have been shown to be downregulated [3,6,7,20,22,23]. OCT4, AP2γ and PLAP are described as ‘classical’ markers of intratubular germ cell neoplasia cells in adulthood and persistence of expression of these proteins is routinely used for diagnostic purposes for patients at risk of, or with suspected, testicular germ cell cancer [11]. These markers are considered highly sensitive and specific for intratubular germ cell neoplasia cells. Whilst we found co-expression of AP2γ and OCT4 in intratubular germ cell neoplasia cells with no cells expressing a single marker alone, there was heterogeneity in the co-expression of PLAP and OCT4 in intratubular germ cell neoplasia cells. Expression of OCT4 has been reported to be present in all intratubular germ cell neoplasia cells [19], whilst PLAP has been reported to be expressed in 83-99% of intratubular germ cell neoplasia cells [9]. The present co-localisation studies demonstrate that the majority of the cells expressing OCT4 also express PLAP. Overall co-localisation was seen in 68% of cells in tubules with intratubular germ cell neoplasia. Both of these sub-populations are also present in the normal human fetal testis during the transition from gonocyte to spermatogonia. However, our co-localisation studies have demonstrated the presence of a sub-population of intratubular germ cell neoplasia cells with a protein expression profile distinct from the germ cells in the normal human fetal testis. This OCT4−/PLAP+ sub-population represented 18% of the total cells in tubules with intratubular germ cell neoplasia. PLAP is expressed in most of the germ cells in a first trimester fetal testis, but is rare by the start of the second trimester [23]. OCT4 is also present in most of the germ cells of the first trimester, but is downregulated later in gestation in comparison to PLAP [24]. This study has shown that an OCT4−/PLAP+ population can be identified in tubules with intratubular germ cell neoplasia, whilst our results confirm that PLAP is not expressed without co-expression of OCT4 in the normal human fetal testis [23]. As these cells do not occur as part of normal germ cell development they may represent impaired maturation of gonocytes with loss of OCT4 and retention of PLAP expression as a result of an altered germ cell niche.
In addition to the proteins that are found in undifferentiated germ cells, intratubular germ cell neoplasia cells have also been reported to express proteins characteristic of differentiated germ cells such as VASA [25] and MAGEA4 [26]. These markers begin to be expressed in germ cells during fetal life in increasing proportions as the cells differentiate [24,26-28]. We have shown that the majority of intratubular germ cell neoplasia cells (based on the expression of OCT4 and/or PLAP) do not express the spermatogonial proteins MAGEA4 and VASA. We have quantified the expression of these differentiated germ cell markers in putative intratubular germ cell neoplasia for the first time and shown that MAGEA4 is only expressed in 6% of OCT4 and/or PLAP-expressing cells and therefore is not a common phenotype for putative intratubular germ cell neoplasia cells. Heterogeneous expression of MAGEA4 in intratubular germ cell neoplasia has been described previously, however VASA expression was reported to be expressed in all intratubular germ cell neoplasia cells [3]. We found that VASA was expressed heterogeneously in a similar proportion of putative intratubular germ cell neoplasia cells as those expressing MAGEA4.
Previous studies have indicated that differentiated germ cells (e.g. spermatogonia) may be present within intratubular germ cell neoplasia containing tubules [12,22]. Co-staining for OCT4 and VASA/MAGEA4 identified cells that had an OCT4−/VASA+ phenotype [22]. These cells would be considered differentiated germ cells rather than intratubular germ cell neoplasia cells. We have described similar populations in our samples, however triple co-localisation has demonstrated that some VASA or MAGEA4 expressing cells that do not express OCT4, express PLAP and therefore may not represent ‘normal’ spermatogonia. As a result it is likely that only cells expressing neither OCT4 nor PLAP may represent normally matured germ cells that have not undergone pre-invasive change. The OCT4−/VASA+/PLAP+ or OCT4−/MAGEA4+/PLAP+ populations may represent pre-invasive germ cells that have undergone a degree of maturation towards pre-spermatogonia (due to downregulation of OCT4 and expression of VASA/MAGEA4), alternatively they may represent presprematogonia that have aberrantly retained PLAP expression following the downregulation of OCT4. In order to determine whether these populations could represent intratubular germ cell neoplasia cells we investigated expression during the development of invasive disease. OCT4 (without MAGEA4) was expressed in all intratubular and invasive seminomas, indicating that intratubular germ cell neoplasia cells with invasive potential express OCT4 and do not express MAGEA4. Therefore we conclude that the OCT4−/MAGEA4+/PLAP+ cells do not represent intratubular germ cell neoplasia cells with malignant potential and are more likely to be a separate population of abnormally differentiated germ cells that are present in intratubular germ cell neoplasia containing tubules.
Uncontrolled proliferation of cells is a hallmark of invasive tumours [29]. Previous studies have demonstrated proliferation in intratubular germ cell neoplasia and overt testicular germ cell cancer [12,30-32], and a previous study has shown that Ki67 expression is found in intratubular germ cell neoplasia cells in 14/16 non-seminomas and 14/17 seminomas, although the proportion of intratubular germ cell neoplasia cells that were proliferating was not quantified [31]. A detailed analysis of proliferation in the various intratubular germ cell neoplasia sub-populations in relation to the underlying tumour type has not previously been performed. Intratubular germ cell neoplasia cells have previously been reported to proliferate at a relatively high rate. In a study of sections taken from patients with testicular germ cell cancer, using Ki67 as a marker of proliferation, 17.42% of intratubular germ cell neoplasia cells were found to be Ki67 positive [30]. Overall we found that 8.1% of intratubular germ cell neoplasia cells were positive for Ki67, however we have shown that the proliferation rate is dependent on which intratubular germ cell neoplasia sub-population is investigated. We have shown that PLAP is not expressed in ~20% of intratubular germ cell neoplasia (OCT4+) cells and this may partially explain the differences seen between proliferation of intratubular germ cell neoplasia cells in the present study compared to previous studies which relied on PLAP expression to identify intratubular germ cell neoplasia cells [12,30]. The OCT4+/MAGEA4+ (and also OCT4−/MAGEA4+, not shown) populations are less proliferative than those expressing OCT4+/MAGEA4− which provides further evidence supporting the view that the OCT4+/MAGEA4− cells have more invasive potential than those expressing a more mature phenotype. This hypothesis is supported by our finding of little or no expression of MAGEA4 in the OCT4+ seminoma cells of an invasive tumour. We therefore propose that the OCT4+/MAGEA4− population of intratubular germ cell neoplasia cells give rise to the invasive tumour, whilst the OCT4+/MAGEA4+ population has a lower capacity to progress to invasiveness and may represent germ cells that are arrested in the transition from gonocyte to spermatogonia and do not contribute to the invasive tumour. OCT4−/MAGEA4+ cells are occasionally seen within the seminomatous component but are likely to represent spermatogonia that have become enclosed in the invasive tumour.
Differences in the proliferation of intratubular germ cell neoplasia cells have recently been investigated with respect to one of the key regulators of the mitosis-meiosis switch, DMRT1 [12]. This study demonstrated that intratubular germ cell neoplasia cells expressing DMRT1 were significantly less proliferative than those intratubular germ cell neoplasia cells that did not express DMRT1 and that progression from ‘early-stage’ (Ki67−) intratubular germ cell neoplasia cell to invasive disease (Ki67+) is associated with a down-regulation of DMRT1. In order to test the hypothesis that certain subpopulations of intratubular germ cell neoplasia cells display differences in invasive potential, future studies involving isolation of the different intratubular germ cell neoplasia sub-populations followed by germ cell transplantation or xenografting may be performed.
The present study has demonstrated the presence of proliferating intratubular germ cell neoplasia cells in testis tissue from patients with pre-invasive disease, seminoma and non-seminoma with a higher rate of proliferation in the OCT4+/MAGEA4− population in pre-invasive samples compared to those with an invasive tumour (either seminoma or non-seminoma). The finding of higher rates of proliferation of intratubular germ cell neoplasia cells in pre-invasive disease compared to tumours samples might be considered surprising given previous reports of low rates of proliferation in pre-invasive intratubular germ cell neoplasia cells [12]. In adults with pre-invasive disease this may be explained by an increase in proliferation around the time of progression to invasive disease, however this would not explain the proliferation rate for intratubular germ cell neoplasia cells in the pre-invasive childhood patients in which it might be expected that these cells are relatively quiescent. However, we have demonstrated previously that a higher proportion of OCT4+ germ cells in the second trimester human fetal testis are proliferating compared with the MAGEA4+ population and that the rates of proliferation in the present study are similar to those in the normal human fetal testis for each sub-population [10], indicating that the proliferation in the germ cell sub-populations in children with pre-invasive disease may simply reflect the proliferation rates in the normal human fetal testis.
In conclusion, we have described in detail the heterogeneity of germ cell protein expression in cells within intratubular germ cell neoplasia tubules. We have demonstrated sub-populations of OCT4− cells that do not correspond to an equivalent stage of normal human fetal germ cell differentiation, suggesting that these cells may have lost expression of proteins that may determine their malignant potential. We have also demonstrated that a more undifferentiated/pluripotent expression profile is associated with an increased proliferation rate compared with a differentiated phenotype. These results indicate that germ cells expressing an OCT4+/MAGEA4− phenotype are those that will ultimately lead to tumour formation.
Supplementary Material
Acknowledgements
We are grateful to Sheila Macpherson for her assistance during the present study and to Ronnie Grant for his assistance with illustrations. The MAGEA4 antibody was a kind gift from Dr. Giulio Spagnoli. This study was supported by the Wellcome Trust (Grant Code - 098522) and the Medical Research Council (Career Development Fellowship).
Footnotes
Disclosure/Conflict of Interest: The authors have no conflicts of interest to disclose.
Supplementary information is available at Modern Pathology’s website
References
- 1.McGlynn KA, Devesa SS, Sigurdson AJ, et al. Trends in the incidence of testicular germ cell tumors in the United States. Cancer. 2003;97:63–70. doi: 10.1002/cncr.11054. [DOI] [PubMed] [Google Scholar]
- 2.Richiardi L, Bellocco R, Adami HO, et al. Testicular cancer incidence in eight northern European countries: secular and recent trends. Cancer Epidemiol Biomarkers Prev. 2004;13:2157–2166. [PubMed] [Google Scholar]
- 3.Rajpert-De Meyts E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum Reprod Update. 2006;12:303–323. doi: 10.1093/humupd/dmk006. [DOI] [PubMed] [Google Scholar]
- 4.Skakkebaek NE. Possible carcinoma-in-situ of the testis. Lancet. 1972;2:516–517. doi: 10.1016/s0140-6736(72)91909-5. [DOI] [PubMed] [Google Scholar]
- 5.Waters BL, Trainer TD. Development of the human fetal testis. Pediatr Pathol Lab Med. 1996;16:9–23. [PubMed] [Google Scholar]
- 6.Hoei-Hansen CE, Almstrup K, et al. Stem cell pluripotency factor NANOG is expressed in human fetal gonocytes, testicular carcinoma in situ and germ cell tumours. Histopathology. 2005;47:48–56. doi: 10.1111/j.1365-2559.2005.02182.x. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell RT, Cowan G, et al. Germ cell differentiation in the marmoset (Callithrix jacchus) during fetal and neonatal life closely parallels that in the human. Hum Reprod. 2008;23:2755–2765. doi: 10.1093/humrep/den295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoei-Hansen CE, Nielsen JE, et al. Transcription factor AP-2gamma is a developmentally regulated marker of testicular carcinoma in situ and germ cell tumors. Clin Cancer Res. 2004;10:8521–8530. doi: 10.1158/1078-0432.CCR-04-1285. [DOI] [PubMed] [Google Scholar]
- 9.Jorgensen N, Rajpert-De Meyts E, Graem N, et al. Expression of immunohistochemical markers for testicular carcinoma in situ by normal human fetal germ cells. Lab Invest. 1995;72:223–231. [PubMed] [Google Scholar]
- 10.Mitchell RT, Saunders PT, Childs AJ, et al. Xenografting of human fetal testis tissue: a new approach to study fetal testis development and germ cell differentiation. Hum Reprod. 2010;25:2405–2414. doi: 10.1093/humrep/deq183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer. 2005;5:210–222. doi: 10.1038/nrc1568. [DOI] [PubMed] [Google Scholar]
- 12.Jorgensen A, Nielsen JE, Almstrup K, et al. Dysregulation of the mitosis-meiosis switch in testicular carcinoma in situ. J Pathol. 2013;229:588–598. doi: 10.1002/path.4154. [DOI] [PubMed] [Google Scholar]
- 13.Kersemaekers AM, Honecker F, Stoop H, et al. Identification of germ cells at risk for neoplastic transformation in gonadoblastoma: an immunohistochemical study for OCT3/4 and TSPY. Hum Pathol. 2005;36:512–521. doi: 10.1016/j.humpath.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Norton AJ, Jordan S, Yeomans P. Brief, high-temperature heat denaturation (pressure cooking): a simple and effective method of antigen retrieval for routinely processed tissues. J Pathol. 1994;173:371–379. doi: 10.1002/path.1711730413. [DOI] [PubMed] [Google Scholar]
- 15.Skakkebaek NE, Berthelsen JG, Giwercman A, et al. Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl. 1987;10:19–28. doi: 10.1111/j.1365-2605.1987.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 16.Almstrup K, Hoei-Hansen CE, Wirkner U, et al. Embryonic stem cell-like features of testicular carcinoma in situ revealed by genome-wide gene expression profiling. Cancer Res. 2004;64:4736–4743. doi: 10.1158/0008-5472.CAN-04-0679. [DOI] [PubMed] [Google Scholar]
- 17.Looijenga LH, Gillis AJ, Stoop HJ, et al. Chromosomes and expression in human testicular germ-cell tumors: insight into their cell of origin and pathogenesis. Ann N Y Acad Sci. 2007;1120:187–214. doi: 10.1196/annals.1411.000. [DOI] [PubMed] [Google Scholar]
- 18.Jones TD, Ulbright TM, Eble JN, et al. OCT4: A sensitive and specific biomarker for intratubular germ cell neoplasia of the testis. Clin Cancer Res. 2004;10:8544–8547. doi: 10.1158/1078-0432.CCR-04-0688. [DOI] [PubMed] [Google Scholar]
- 19.Looijenga LH, Stoop H, de Leeuw HP, et al. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 2003;63:2244–50. [PubMed] [Google Scholar]
- 20.Rajpert-De Meyts E, Hanstein R, Jorgensen N, et al. Developmental expression of POU5F1 (OCT-3/4) in normal and dysgenetic human gonads. Hum Reprod. 2004;19:1338–1344. doi: 10.1093/humrep/deh265. [DOI] [PubMed] [Google Scholar]
- 21.Hustin J, Collette J, Franchimont P. Immunohistochemical demonstration of placental alkaline phosphatase in various states of testicular development and in germ cell tumours. Int J Androl. 1987;10:29–35. doi: 10.1111/j.1365-2605.1987.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 22.Cools M, van Aerde K, Kersemaekers AM, et al. Morphological and immunohistochemical differences between gonadal maturation delay and early germ cell neoplasia in patients with undervirilization syndromes. J Clin Endocrinol Metab. 2005;90:5295–5303. doi: 10.1210/jc.2005-0139. [DOI] [PubMed] [Google Scholar]
- 23.Honecker F, Stoop H, de Krijger RR, et al. Pathobiological implications of the expression of markers of testicular carcinoma in situ by fetal germ cells. J Pathol. 2004;203:849–857. doi: 10.1002/path.1587. [DOI] [PubMed] [Google Scholar]
- 24.Gaskell TL, Esnal A, Robinson LL, et al. Immunohistochemical profiling of germ cells within the human fetal testis: identification of three subpopulations. Biol Reprod. 2004;71:2012–2021. doi: 10.1095/biolreprod.104.028381. [DOI] [PubMed] [Google Scholar]
- 25.Rajpert-De Meyts E, Bartkova J, Samson M, et al. The emerging phenotype of the testicular carcinoma in situ germ cell. APMIS. 2003;111:267–278. doi: 10.1034/j.1600-0463.2003.11101301.x. discussion 278-269. [DOI] [PubMed] [Google Scholar]
- 26.Aubry F, Satie AP, Rioux-Leclercq N, et al. MAGEA4, a germ cell specific marker, is expressed differentially in testicular tumors. Cancer. 2001;92:2778–2785. doi: 10.1002/1097-0142(20011201)92:11<2778::aid-cncr10125>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 27.Castrillon DH, Quade BJ, Wang TY, et al. The human VASA gene is specifically expressed in the germ cell lineage. Proc Natl Acad Sci U S A. 2000;97:9585–9590. doi: 10.1073/pnas.160274797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeeman AM, Stoop H, Boter M, et al. VASA is a specific marker for both normal and malignant human germ cells. Lab Invest. 2002;82:159–166. doi: 10.1038/labinvest.3780408. [DOI] [PubMed] [Google Scholar]
- 29.Albers P, Ulbright TM, Albers J, et al. Tumor proliferative activity is predictive of pathological stage in clinical stage A nonseminomatous testicular germ cell tumors. J Urol. 1996;155:579–586. [PubMed] [Google Scholar]
- 30.Almstrup K, Nielsen JE, Mlynarska O, et al. Carcinoma in situ testis displays permissive chromatin modifications similar to immature foetal germ cells. Br J Cancer. 2010;103:1269–1276. doi: 10.1038/sj.bjc.6605880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Datta MW, Renshaw AA, Dutta A, et al. Evaluation of cyclin expression in testicular germ cell tumors: cyclin E correlates with tumor type, advanced clinical stage, and pulmonary metastasis. Mod Pathol. 2000;13:667–672. doi: 10.1038/modpathol.3880117. [DOI] [PubMed] [Google Scholar]
- 32.Chen YT, Cao D, Chiu R, et al. Chromosome X-encoded Cancer/Testis antigens are less frequently expressed in non-seminomatous germ cell tumors than in seminomas. Cancer Immun. 2013;13:10. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.