Abstract
Reduction of lean mass is a primary body composition change associated with aging. Because many factors contribute to lean mass reduction, the problem has been given various names depending on the proposed cause, such as “age-related sarcopenia,” “dynapenia,” “myopenia,” “sarcopenic obesity,” or simply “sarcopenia.” There is currently no consensus on how to best diagnose the reduction of lean mass and its consequences on health. We propose that simple body composition methods can be used to indirectly evaluate sarcopenia, provided that those techniques are validated against the “quality of lean” criterion that associates muscle mass and metabolic function with the components of fat-free mass. Promising field methods include the use of stable isotopes for the evaluation of water compartments and new approaches to bioelectrical impedance analysis, which is also associated with the monitoring of water homeostasis.
Introduction
Aging results in changes to a number of metabolic and physiologic functions, with 1 consequence being a reduction in lean mass—also known as fat-free mass (FFM)5—which includes body water, skeletal and smooth muscles, and bones. (We will use the term fat-free mass as a synonym for lean body mass, although there is a slight difference between them.) Aside from its structural role, FFM is fundamental to the mobilization of metabolic substrates, essential molecular synthesis, and reaction to external stressors, among others (1–4). In addition, skeletal muscle can be considered the main component of the protein of the body and is capable of driving antibody production, wound healing, and white blood cell production during acute or chronic diseases. If muscle is depleted, there is less protein to fuel the functionality of the body, thereby enhancing the risk of disabilities and functional impairment while reducing muscle power and/or physical function (3, 5–10).
Loss of muscle is a complex process, and this complexity has led to different naming conventions (Table 1). Some authors simply suggest the term “age-related sarcopenia” (9, 11–15). Recently, the term “myopenia” was suggested to indicate the presence of clinically relevant muscle wasting (16). Taking into account the association between sarcopenia and disabilities, the term “dynapenia” has been proposed (17). Additionally, because reduction of muscle mass is accompanied by the infiltration of fat and connective tissue into the muscle, some have named the process “myosteatosis,” with the justification that this term can give a dimension of muscle quality (18). The term “sarcopenic obesity” is also used (19, 20), corroborated in part by results from the longitudinal Health, Aging, and Body Composition Study, which reported that, as the population ages, there are more obese sarcopenic individuals than lean ones (21, 22).
TABLE 1.
Definitions
| Term | Definition | Reference(s) |
| Sarcopenia | The age-related loss in skeletal muscle mass and function | Rosenberg, 1997 (11) and Evans, 1995 (79) |
| Sarcopenic obesity | A reduction in skeletal mass together with increased body fat | Baumgartner et al., 2004 (19) |
| Dynapenia | The age-related loss of strength in skeletal muscle | Clark and Manini, 2008 (17) |
| Myosteatosis | Age-associated changes in muscle quality evidenced by increased fat infiltration | Taaffe et al., 2009 (18) |
| Myopenia | A clinically relevant muscle and function loss and/or increased risk of morbidity or mortality at any age | Fearon et al., 2011 (16) |
| Sarco-ostopenia and sarco-osteoporosis | Reduction in lean mass combined with reduction of bone density | Binkley and Buehring, 2009 (80) |
Considering the number of factors associated with muscle losses that occur with aging, it seems certain that other terms will be proposed. To determine consensus, a group of experts has periodically discussed this issue. A recent document published by this group reinforced their previous definition of the term, simply calling it “sarcopenia” (23): “Sarcopenia is the age-associated loss of skeletal muscle mass and functions. Sarcopenia is a complex syndrome that is associated with muscle mass loss alone or in conjunction with increased fat mass. The causes of sarcopenia are multi-factorial and can include disuse, changing endocrine function, chronic diseases, inflammation, insulin resistance and nutritional deficiencies. While cachexia may be a component of sarcopenia, the two conditions are not the same.” (13) For didactic purposes, this is the term we have chosen to adopt throughout this paper.
Sarcopenia can culminate in extreme conditions, including frailty syndrome. Frailty is defined as a state of increased vulnerability caused by cumulative stressors, such as declining physiologic reserves and multisystem dysregulation (1, 5, 24). Frailty is currently identified through a screening process based on 5 criteria: 1) weight; 2) grip strength; 3) subjective fatigue; 4) physical activity; and 5) walking speed measurements (24). The result of this screening is a score that sometimes has limitations, such as its ability to detect subtle changes related to medications, diet, and physical activity. We believe that early diagnosis of sarcopenic processes is an important way to prevent or manage frailty.
Despite the clinical variations and consequences of muscle loss and the lack of agreement on an exact definition of sarcopenia, with this review, we will suggest that field methods of body composition can play a very important role in the identification and management of sarcopenia, no matter the cause. Although we will not introduce a new definition, we believe that the association of this condition with a specific measurable change in body composition will increase its utility and its contribution to preventive medicine.
Current Status of Knowledge
Metabolic and physiologic processes associated with sarcopenia: a basis to identify the best screening method
Because of the profound effects that sarcopenia exerts on health, many authors have investigated the best methods to diagnose sarcopenia in its initial stages (13). To discover better detection techniques, we believe that it is helpful to understand the effects of the physiologic and metabolic processes associated with sarcopenia. Some of these processes are described below.
Protein synthesis and degradation.
The composition of FFM results from the balance between protein synthesis and degradation. Some authors tried to explain sarcopenia as the inability of the body to synthesize proteins or control protein breakdown. However, there is not a consensus regarding these issues. In a cross-sectional study using stable isotope techniques and comparing protein synthesis between young and old men, reduced muscle was observed in the elderly, but no differences were observed in the rate of protein synthesis (25). Recent studies investigated whether protein metabolism in older individuals responds differently to feeding when compared with younger individuals, possibly as the result of a reduced sensitivity to the inhibitory effect of insulin on protein breakdown (26, 27). Wilkes et al. (26) compared protein metabolism between young and old men and women, using [D5]phenylalanine and [1,2–13C2]leucine techniques. The authors found that, at moderate availability, the effect of insulin on leg protein breakdown was reduced in older individuals, and this effect may have been mediated by blunted Akt–protein kinase B activation. However, Chevalier et al. (28) also compared young and old women from a simulated fed-state clamp and using whole-body [3H]glucose and protein [13C]leucine kinetics and muscle protein fractional synthesis rate [[2H5]phenylalanine]. They observed that suppression of protein breakdown was lower in elderly women; however, this difference disappears when adjusted for serum insulin. In general, protein degradation is expected to exceed synthesis in the elderly, possibly because of a higher protein breakdown. However, more studies are needed to investigate the details of protein metabolism in aging.
Genetics.
Sarcopenic progression is a result of the interaction between genetic and environmental factors. Genetic aspects possibly related to growth hormone (GH), its receptors, and transporters or myostatin and cytokine expression, among others, may account for individual differences in protein turnover. In other words, genetic factors might explain why some individuals or groups are more susceptible to developing sarcopenia than others (29).
Natural reduction of secretion and action of GH, testosterone, and insulin-like growth factor-1.
Some of the theories that try to explain aging adopt the expression “gradual imbalance,” which assumes an association between senescence and homeostatic imbalances, justified by changes in the endocrine, immune, and central nervous systems. In this context, aging is associated with reduced activity of the hormonal axis, including the somatotropic axis (GH/insulin-like growth factor-1), which is closely associated with protein metabolism and rates of muscle excitation and contraction (30, 31).
Increase in oxygen-free radicals.
Mitochondrial dysfunction is associated with aging and can contribute to oxidative stress (OS) and the apoptosis of cells (32, 33). These dysfunctions can include the following: 1) reductions in redox active iron; 2) increases in membrane lipid peroxidation, cellular hydrogen peroxide, and lipofuscin accumulation; and 3) alterations in membrane lipids. Additionally, there is a decline in endogenous antioxidant protection, such as changes in the ratio of oxidized-to-total glutathione and reduced glutamine synthetase. Some specific tissues/systems are particularly vulnerable to the increases in OS, including motoneurons (33).
Increase of cytokine concentrations.
OS induces the expression of proinflammatory cytokines through activation of OS-sensitive nuclear factors, which upregulate the inflammatory response. In turn, this can lead to additional increases in reactive oxygen species, OS, and inflammation, a vulnerability to additional stressors (32). Thus, the OS typical of aging is associated with inflammation, which implies a higher systemic concentration of cytokines and acute-phase proteins in elderly individuals as opposed to younger individuals (34). This phenomenon of chronic low-grade inflammation with aging has been named “inflammaging” by some authors and is shown to have a complex integration with different body tissues and systems, somato-senescence, and calcium senescence (35). Epidemiologic studies demonstrated a significant association between sarcopenia and inflammatory factors (36–39).
Loss of motoneurons.
As one ages, the body undergoes a relatively balanced, continuous process of muscular denervation and reinnervation. As aging progresses, this balance is broken and directed to denervation (40). The mechanisms behind these changes are not fully understood but are probably associated with specific proteins governing motoneuron differentiation (41). In addition, there may be a reduction in satellite cells, which act as myogenic precursors that differentiate in myoblasts (42).
Anorexia and undernutrition.
Appetite reduction, considered by some authors to be a normal consequence of aging, is called “anorexia of aging” (4). Physiologic factors associated with this phenomenon include modifications in taste and smell, decrease in rates of gastric emptying, changes in gastric muscle extension ability, and reduction of the synthesis and action of gastric hormones (4, 43). In many cases, we observe a progressive loss of muscle and increase in percentage fat (44). With aging, people become more sedentary, which accelerates muscle loss. This reduction modifies the responsiveness to insulin, which marks the beginning of insulin resistance. In turn, insulin resistance could be an open window to increased body fat and metabolic syndrome. The body fat is associated with higher serum and tissue adipokines (for instance, tumor necrosis factor-α and interleukin-6), which begin again the insulin resistance, constituting a vicious cycle (45). Malnutrition and the possible risk of macronutrient and micronutrient deficiencies are associated with gut microbiota modification, which can contribute to inflammation caused by endotoxemia (46), which in turn contributes to a reduction in both body mass and muscle activity, as well as risk for immune dysfunction. The consequences of these factors are decline in functionality, elevated risk of frailty, and increase in hospitalization and mortality rates (43).
Physical activity.
Because physical activity is modifiable, it is important to highlight its role in sarcopenia. We currently have no consensus regarding the definition of physical activity when considering sarcopenia. In 2 studies, an increase in any type of physical activity (including everyday activities, such as domestic work) significantly contributed to the prevention of sarcopenia (10, 47). In addition, the benefits of systematic physical activity programs are well known, and there are well-designed studies pointing to the positive effects of aerobic resistance exercises (48, 49). Hypertrophy exercises may be more beneficial than aerobic exercises to reduce muscle loss because of the neural adaptations to these kinds of exercises (50). The recent publication from the International Working Group on Sarcopenia pointed to the importance of developing randomized studies investigating the best physical activity protocols (13). The group stated that there has been no consensus regarding the type, frequency, and intensity of physical activity/physical exercise required to control or prevent sarcopenia. In fact, this working group singled out a paucity of appropriate methods and techniques as 1 of the reasons for this lack of consensus.
Body composition analysis for evaluating sarcopenia
A simple approach to monitoring sarcopenia is to observe the loss of muscle mass with age. However, if metabolic and physiologic processes related to protein synthesis and degradation are considered, a more detailed description of body composition that is closely related to the metabolic capability of the FFM is required. We suggest that the principle of the “quality of lean mass,” described later, be used for this purpose. Even if it is not always practical to directly measure body composition, it can serve as the reference method against which surrogate field techniques are compared for validity.
Primary observations.
In a cross-sectional study, Kehayias et al. (44) studied total body potassium (TBK) of healthy volunteers in Boston, Massachusetts, aged 20–89 y by counting the natural radioactivity of the body as a result of the isotope 40K. TBK is a measure of body cell mass, the metabolizing and oxygen-consuming portion of FFM that is closely related to skeletal muscle mass and its function (51). The findings support the hypothesis that there is a systematic and continuous decline of cell and muscle mass throughout adult life. The observed decline in TBK content was 7.20 ± 1.00 and 9.16 ± 0.96 mg of potassium per kilogram of body weight per year for females and males, respectively. However, when potassium content (TBK per weight) for all participants was expressed as a percentage of the value of the first age group (20–29 y), the loss by age for both sexes was described with a single linear fit, indicating that both men and women lose 5% of their original TBK per decade, on average. This observation is a simple and natural demonstration of sarcopenia. In most cases, this loss of muscle mass is accompanied by the decline of other components of FFM, such as protein and bone mineral. In addition, the percentage of body fat increases with age. Using neutron inelastic scattering, the most direct method of in vivo fat assessment (52), Kehayias et al. (44) observed a significant increase in fat percentage with age for female volunteers aged 20–50 y and a continuous increase for males.
Hydration status may also change in the elderly. The importance of age-related dehydration has been recognized but has only recently received great attention. Lean tissue hydration remains normal in free-living healthy elderly (53) but requires monitoring and intervention in many frail elderly (54, 55). We will discuss this approach as a surrogate method for evaluating sarcopenia and frailty later.
Significance.
Both men and women lose 5% of their original TBK per decade, on average. This observation is consistent with previous longitudinal data reported by Flynn et al. (56), who monitored TBK loss over 18 y. The significance of cell mass decline was dramatically demonstrated by the work of Kotler et al. (57), who showed that patients suffering from severe, catabolic end-stage acquired immunodeficiency syndrome (58) cannot sustain a loss of >40% of their normal body cell mass, as measured by TBK. This benchmark is so sensitive that Kotler et al. were able to predict the day on which immunocompromised patients would die based on their rate of potassium loss. We can interpret this observation as the minimum amount of cell mass necessary to maintain the basic metabolic mechanisms to sustain life. Using TBK data vs. age and extrapolating the TBK vs. age linear function to the point of 60% (representing 40% loss), a lifespan limit is calculated at age 110 y (95% CI: 101, 122) based on this simplified TBK content model (44). It is interesting to note that this finding is consistent with other methodologies that have calculated maximal human lifespan, which predict 110–120 y as the upper limit (59, 60).
Methods and Models to Assess Body Mass
There are different approaches to modeling body composition depending on the nature of the compartments used (61). According to the 4-compartment model for body composition, body mass is divided into water, fat, protein, and solid bone. With the exception of extreme obesity (BMI > 45 kg/m2), water is the largest compartment of the body and the main contributor to FFM. In this chemical division of body mass, fat is defined as TGs (62, 63) and differs from adipose tissue, which includes a small amount of water. Body composition methods involve either the measurement of these 4 compartments or a simplified 2-compartment model separating the body into fat and FFM.
A direct (atomic) approach to body composition is elemental partition analysis (EPA). One or more elements of the body are measured by neutron activation analysis, and specific compartments are derived. For example, measurement of body calcium leads to bone mass, nitrogen to body protein, and carbon (after corrections for the contributions due to protein, bone, and glycogen) to body fat (63). A particularly useful application of the EPA method is the assessment of total and regional body fat from the measurement of the carbon-to-oxygen (C/O) ratio in vivo by neutron inelastic scattering (44, 52). The method is based on the mathematical observation that the C/O ratio relates to tissue percentage fat (and lean) in a simple formula that can be derived based only on the stoichiometry of the body compartments. Perhaps the most important benefit of the C/O ratio method is its ability to provide an independent, unbiased measure of FFM. This has direct applications to longitudinal studies in which the quality of lean tissue must be determined and to intervention studies in which the quality of lean tissue must be protected. Sarcopenia is not a simple reduction of FFM but rather a decline in the metabolic capacity of FFM.
The “quality of lean” principle
Historically, body weight has been used as the macroscopic criterion of nutritional status of the catabolic patient. The efficacy of anabolic treatment has been measured by its success in minimizing or reversing weight loss. The availability of body composition devices—such as DXA scanners—in the clinical setting has made the use of FFM possible as a measurable outcome of anabolic treatments. This approach protects against unwanted, or even dangerous, disproportional increases in body fat resulting from aggressive weight-gain treatments.
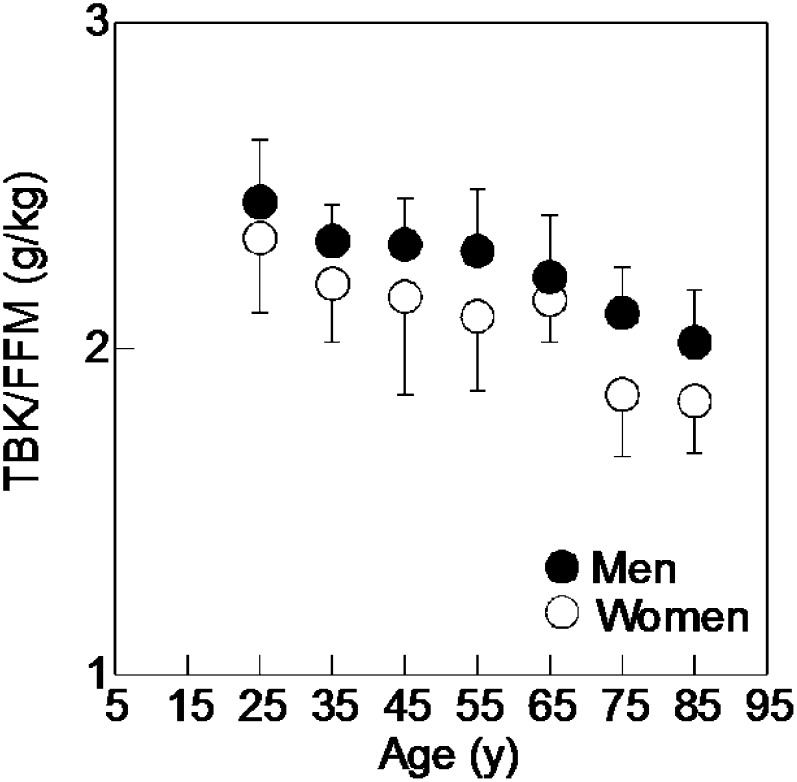
By increasing extracellular fluid, it is possible to increase FFM without an increase in muscle, cell mass, or metabolism. The “quality of lean”—defined as the cell-mass content of FFM—represents the oxygen-consuming, metabolizing fraction of FFM. It is an indirect measure of skeletal muscle and the most sensitive body composition index of nutritional status. In practice, the quality of lean factor can be measured by TBK (assessed by whole-body gamma counting of 40K) divided by FFM (assessed by the C/O ratio method). A true measure of this quality factor was difficult to obtain until the introduction of the EPA approach to measuring FFM. This is because earlier models had to assume a constant composition of lean mass and then use that “constant” to derive FFM directly from TBK, an assumption that is no longer accepted. The potassium content of FFM as a function of age is shown in Figure 1 (adapted from reference 44 with permission). Two characteristics are immediately apparent from this graph: 1) a reduction in TBK/FFM with age; and 2) a large variation of this ratio within each age group. This variation indicates that TBK alone is a poor predictor of lean body mass unless age- and sex-dependent equations are used. The important outcome of an anabolic treatment is the maintenance or increase of the quality of lean body mass. Similarly, the efficacy of treating obesity can be assessed because weight is reduced without compromising the quality of lean.
FIGURE 1.
Results from a cross-sectional study with 188 healthy, free-living volunteers from the Boston area aged 20–89 y. Mean values of potassium content of fat-free mass [total body potassium (grams) to fat-free mass (kilograms)] plotted against the age of each group. Fat-free mass was calculated by subtracting body fat derived by neutron inelastic scattering from body weight. Total body potassium was measured in vivo using passive, non-invasive counting of gamma ray emissions from naturally occurring 40K radioisotope. The error bars represent 1 SD for each age group. FFM, fat-free mass; TBK, total body potassium. (Adapted from reference 44 with permission.)
Review of other body composition methods
Perhaps the most common indirect method for measuring body composition is anthropometry. BMI has been used to indirectly classify individuals according to adiposity amount. This approach, although imprecise regarding changes of lean body mass, is often used by clinicians; generally, these measurements are interpreted together with additional clinical information. In trying to improve the spectrum of anthropometric possibilities, some researchers examined different measures or indices. Based on a representative sample of the population of France, Rolland et al. (64) proposed calf circumference as a good alternative to detect muscle changes due to aging, although not necessarily sarcopenia. However, until now, there has not been a good cutoff value to define sarcopenia when using calf circumference. In a literature review, it was pointed out that a cutoff point of 31 cm presented low sensitivity (44.3%) and specificity (91.4%) to predict sarcopenia (65). The 31-cm value was initially proposed based on the previously noted work of Rolland et al. (64), in which the authors specified the quadriceps as the muscle that could best represent FFM losses. However, this anthropometric measure still lacks reference studies.
DXA was developed to provide precise measurements of bone density for the screening and management of osteoporosis. It works on the principle that, by measuring absorption of photons at 2 distinct energies, projected bone density (bone mass per square centimeter) can be assessed at high precision in the presence of overlaying tissue. As the method developed, it became clear that the composition of overlaying soft tissue should also be measured (rather than assumed) to improve the quality of bone measurements (66). Modern DXA instruments achieve this by analyzing soft tissue in image pixels that do not contain bone and use those measurements to predict the composition of overlaying soft tissue. This feature not only improved the accuracy of bone density measurements but also gave rise to a new body composition tool that can provide soft-tissue composition analysis in vivo. The method works best in the areas of the body that contain well-defined bone areas, such as the arms and legs, and becomes less direct for areas of the trunk (67). Because of the dominance of skeletal muscle in arms and legs, investigators often use DXA measurements of appendicular skeletal muscle (ASM), defined as the sum of the lean soft-tissue masses for the arms and legs (68). Some researchers proposed an index of relative muscle mass, obtained by dividing ASM by body height squared (ASM/ht2) (6, 15) and used this index as a screening tool for sarcopenia. The authors defined sex-specific cutoff values for sarcopenia. An individual was considered sarcopenic when ASM/ht2 was 2 SDs below the reference population data. The calculated cutoff points were <7.26 kg/m2 for men and <5.45 kg/m2 for women. Baumgartner et al. (6) and Janssen et al. (15) used values from the NHANES III study to develop a scale for severity of sarcopenia based on muscle loss. They used bioelectrical impedance analysis (BIA) to derive (indirectly) muscle mass and used as a criterion the skeletal muscle mass index (SMI), defined as skeletal muscle/body mass expressed in percentage by weight. Using their definition, class I sarcopenia was present in individuals whose SMI was 1–2 SDs deviations below the young adult values, and a more severe class II sarcopenia was present when SMI was 2 SDs below the young adult values.
Soft-tissue DXA measurements also provide a measurement of adipose tissue, an important parameter for populations in which BMI or skinfold measurements alone cannot predict adiposity. The presence of fat can reduce muscle quality and/or impair muscle capacity; thus, some authors consider it important to analyze fat mass together with FFM. One author defined sarcopenic obesity as having 2 components: 1) an ASM/ht2 falling below 2 SDs; and 2) a body fat percentage >27% for men and 38% for women (69). Dufour et al. (70) modified these percentages in a study with the Framingham, Massachusetts population, in which obesity was defined as percentage fat mass >30% and 40% for men and women, respectively. Others have proposed the adoption of a residual method of analysis that incorporates fat mass and FFM into the definition and classification of sarcopenia (7, 8, 10, 21). It is not clear whether the inclusion of fat mass makes the utility of the term sarcopenia more useful or just more complicated. However, it serves as a warning that BMI alone or empirical BIA equations can be misleading when evaluating the elderly.
It is important to investigate total body water (TBW) when trying to diagnose sarcopenia. Schoeller (53) observed that both men and women lose body water with age. In women, the decrease is small through middle age and becomes more rapid after the age of 60 y. In men, the decrease begins in middle age and continues throughout the rest of the lifespan. Considering that FFM comprises 73% water, the analysis of TBW can be considered as a method for monitoring FFM but not necessarily sarcopenia.
To use body water as a surrogate for muscle loss or sarcopenia, one has to consider its distribution between compartments. There is an important relation between extracellular water (ECW) and intracellular water, inflammation, OS, and sarcopenia. During inflammation, the permeability of capillaries changes as the result of alterations in colloid osmotic pressure and pressure between plasma and interstitial fluids; all of these changes are associated with tissue swelling. Interstitial fluid is an important element of the tissue microenvironment, and therefore extracellular factors contributing to an inflammatory reaction have important clinical implications (71).
Because intracellular water is more metabolically active than ECW, a loss of intracellular water reflects loss in overall metabolic activity. Therefore, it is important to investigate where in the body these changes in water occur. Additionally, it could be important to consider body water changes together with inflammation because of the relation between aging and inflammation.
Total body volumes of intracellular water and ECW, as well as TBW, can be determined by in vivo tracer dilution. The most common way to perform these analyses is to measure TBW with an isotopic tracer of water and ECW with a tracer that does not enter the intracellular space. The difference between the 2 measurements reflects the intracellular water (53). Typical techniques used to perform these types of measurements are dilution of deuterium (TBW) and the dilution and analysis of bromine in plasma (ECW) (72). It follows that the ECW-to-TBW ratio can be an important consideration when seeking the early detection of sarcopenia. This ratio is referred to as the frailty factor by Kehayias et al. (73). The potential clinical value of the frailty factor was validated by comparing data obtained from nursing home residents against healthy, free-living elderly individuals, as well as elderly individuals from a national U.S. database.
When unable to work with stable isotope dilutions, an alternative diagnostic technique might be the in vivo measurement of the electrical properties of the body. BIA is considered a practical, noninvasive and easy-to-perform technique, because single-frequency resistance (R) and reactance (Xc) have been successful in estimating TBW (74, 75). In addition, a simple BIA vector analysis approach has gained attention as a valuable tool to assess hydration status and cellular mass (76). In this technique, R and Xc are obtained at 50 kHz, normalized for the individual’s height, and then plotted as a bivariate vector. This type of analysis is notable for its independence from regression equations and body weight, which often constitute problems when studying elderly populations (77). In a vector representation of impedance, phase angle analysis and changes in Xc have been shown to be of prognostic value in many disease settings. The approach by Piccoli et al. (76) uses R and Xc in a height-calibrated manner, thus avoiding arbitrary assumptions or empirical equations. It has been suggested that the use of BIA vector analysis in gerontology enables the evaluation of typical changes in body compartments related to aging (78).
In conclusion, despite the lack of consensus on an exact definition, the term “sarcopenia” has already served its primary purpose. It has created awareness for this condition, its monitoring, and its management. We are proposing a practical extension of its utility. There is a reason to associate the definition of a clinical condition with what is practical to measure and monitor. We have a good example from the history of the diagnosis of osteoporosis. Before the availability of bone density technology, the definition of osteoporosis included the occurrence of at least 1 bone fracture, and it was diagnosed that way. Today, its diagnosis is associated with bone density values, and its management has become preventive. We hope that age-related changes of soft tissue will follow the same path so that muscle loss and frailty can be delayed and managed, especially in the elderly. Similarly, we suggest that the acceptance of a practical validated measure to easily gauge the prevalence of sarcopenia in the population should be our next step. This will help translate our experiences with the physiologic mechanisms of sarcopenia to a clinical approach for its management.
A successful management program will grant older adults a safe extension to their independent living and will become a central part of preventive medicine approaches. A universally accepted scale for levels of sarcopenia will help us define cutoff points for assisted living and develop a feedback tool for evaluating the efficacy of anabolic treatments and rehabilitation programs.
Loss of muscle with aging opens the window to a number of complications, including frailty. Therefore, it is necessary to investigate the best methods and techniques needed to detect these losses in their initial stages, using inflammation and its consequent effect on water homeostasis as an important aspect for consideration.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ASM, appendicular skeletal muscle; BIA, bioelectrical impedance analysis; C/O, carbon-to-oxygen; ECW, extracellular water; EPA, elemental partition analysis; FFM, fat-free mass; GH, growth hormone; ht2, height squared; OS, oxidative stress; R, resistance; SMI, skeletal muscle index; TBK, total body potassium; TBW, total body water; Xc, reactance.
Literature Cited
- 1.Abate M, Di Iorio A, Di Renzo D, Paganelli R, Saggini R, Abate G. Frailty in the elderly: the physical dimension. Eura Medicophys. 2007;43:407–15 [PubMed] [Google Scholar]
- 2.Cherry D, Lucas C, Decker SL. Population aging and the use of office-based physician services. NCHS Data Brief. 2010(41):1–8 [PubMed] [Google Scholar]
- 3.Jensen GL, McGee M, Binkley J. Nutrition in the elderly. Gastroenterol Clin North Am. 2001;30:313–34 [DOI] [PubMed] [Google Scholar]
- 4.Wilson MM, Morley JE. Invited review: aging and energy balance. J Appl Physiol. 2003;95:1728–36 [DOI] [PubMed] [Google Scholar]
- 5.Bauer JM, Sieber CC. Sarcopenia and frailty: a clinician’s controversial point of view. Exp Gerontol. 2008;43:674–8 [DOI] [PubMed] [Google Scholar]
- 6.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63 [DOI] [PubMed] [Google Scholar]
- 7.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol. 2001;90:2157–65 [DOI] [PubMed] [Google Scholar]
- 8.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137:231–43 [DOI] [PubMed] [Google Scholar]
- 9.Roubenoff R, Castaneda C. Sarcopenia-understanding the dynamics of aging muscle. JAMA. 2001;286:1230–1 [DOI] [PubMed] [Google Scholar]
- 10.Visser M, Pluijm SM, Stel VS, Bosscher RJ, Deeg DJ. Physical activity as a determinant of change in mobility performance: the Longitudinal Aging Study Amsterdam. J Am Geriatr Soc. 2002;50:1774–81 [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(Suppl 5):990S–1S [DOI] [PubMed] [Google Scholar]
- 12.Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S–7S [DOI] [PubMed] [Google Scholar]
- 13.Chumlea WC, Cesari M, Evans WJ, Ferrucci L, Fielding RA, Pahor M, Studenski S, Vellas B. Sarcopenia: designing phase IIB trials. J Nutr Health Aging. 2011;15:450–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics.” Clin Nutr. 2010;29:154–9 [DOI] [PubMed] [Google Scholar]
- 15.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–96 [DOI] [PubMed] [Google Scholar]
- 16.Fearon K, Evans WJ, Anker SD. Myopenia-a new universal term for muscle wasting. J Cachexia Sarcopenia Muscle. 2011;2:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–34 [DOI] [PubMed] [Google Scholar]
- 18.Taaffe DR, Henwood TR, Nalls MA, Walker DG, Lang TF, Harris TB. Alterations in muscle attenuation following detraining and retraining in resistance-trained older adults. Gerontology. 2009;55:217–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12:1995–2004 [DOI] [PubMed] [Google Scholar]
- 20.Thornell LE. Sarcopenic obesity: satellite cells in the aging muscle. Curr Opin Clin Nutr Metab Care. 2011;14:22–7 [DOI] [PubMed] [Google Scholar]
- 21.Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Nevitt M, Harris TB. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82:872–8, quiz 915–6 [DOI] [PubMed] [Google Scholar]
- 22.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc. 2011;12:249–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56 [DOI] [PubMed] [Google Scholar]
- 25.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286:1206–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilkes EA, Selby AL, Atherton PJ, Patel R, Rankin D, Smith K, Rennie MJ. Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am J Clin Nutr. 2009;90:1343–50 [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Heber D. Sarcopenic obesity in the elderly and strategies for weight management. Nutr Rev. 2012;70:57–64 [DOI] [PubMed] [Google Scholar]
- 28.Chevalier S, Goulet ED, Burgos SA, Wykes LJ, Morais JA. Protein anabolic responses to a fed steady state in healthy aging. J Gerontol A Biol Sci Med Sci. 2011;66:681–8 [DOI] [PubMed] [Google Scholar]
- 29.Di Iorio A, Abate M, Di Renzo D, Russolillo A, Battaglini C, Ripari P, Saggini R, Paganelli R, Abate G. Sarcopenia: age-related skeletal muscle changes from determinants to physical disability. Int J Immunopathol Pharmacol. 2006;19:703–19 [DOI] [PubMed] [Google Scholar]
- 30.Cristofalo VJ, Tresini M, Francis MK, Volker C. Biological theories of senescence. In: Bengston VL, Schaie KW, eds. Handbook of theories of aging. New York: Springer Publishing Company; 1999:98–112 [Google Scholar]
- 31.Thorner MO. Statement by the Growth Hormone Research Society on the GH/IGF-I axis in extending health span. J Gerontol A Biol Sci Med Sci. 2009;64:1039–44 [DOI] [PubMed] [Google Scholar]
- 32.Joseph JA, Shukitt-Hale B, Lau FC. Fruit polyphenols and their effects on neuronal signaling and behavior in senescence. Ann N Y Acad Sci. 2007;1100:470–85 [DOI] [PubMed] [Google Scholar]
- 33.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA. 2005;102:5618–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peake J, Della Gatta P, Cameron-Smith D. Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1485–95 [DOI] [PubMed] [Google Scholar]
- 35.Goto M. Inflammaging (inflammation + aging): a driving force for human aging based on an evolutionarily antagonistic pleiotropy theory? Biosci Trends. 2008;2:218–30 [PubMed] [Google Scholar]
- 36.Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky FA, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visser M, Kritchevsky SB, Newman AB, Goodpaster BH, Tylavsky FA, Nevitt MC, Harris TB. Lower serum albumin concentration and change in muscle mass: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82:531–7 [DOI] [PubMed] [Google Scholar]
- 38.Hunt KJ, Walsh BM, Voegeli D, Roberts HC. Inflammation in aging part 1: physiology and immunological mechanisms. Biol Res Nurs. 2010;11:245–52 [DOI] [PubMed] [Google Scholar]
- 39.Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J Clin Invest. 2006;116:33–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luff AR. Age-associated changes in the innervation of muscle fibers and changes in the mechanical properties of motor units. Ann N Y Acad Sci. 1998;854:92–101 [DOI] [PubMed] [Google Scholar]
- 41.Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. 2010;95:139–59 [DOI] [PubMed] [Google Scholar]
- 42.Carlson ME, Suetta C, Conboy MJ, Aagaard P, Mackey A, Kjaer M, Conboy I. Molecular aging and rejuvenation of human muscle stem cells. EMBO Mol Med. 2009;1:381–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmed T, Haboubi N. Assessment and management of nutrition in older people and its importance to health. Clin Interv Aging. 2010;5:207–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kehayias JJ, Fiatarone M, Zhuang H, Roubenoff R. Total body potassium and fat: relevance to aging. Am J Clin Nutr. 1997;66:904–10 [DOI] [PubMed] [Google Scholar]
- 45.Cetin DC, Nasr G. Obesity in the elderly: more complicated than you think. Cleve Clin J Med. 2014;81:51–61 [DOI] [PubMed] [Google Scholar]
- 46.Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology. 2012;142:1100–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landi F, Cesari M, Onder G, Lattanzio F, Gravina EM, Bernabei R. Physical activity and mortality in frail, community-living elderly patients. J Gerontol A Biol Sci Med Sci. 2004;59:833–7 [DOI] [PubMed] [Google Scholar]
- 48.Barry BK, Carson RG. The consequences of resistance training for movement control in older adults. J Gerontol A Biol Sci Med Sci. 2004;59:730–54 [DOI] [PubMed] [Google Scholar]
- 49.Latham NK, Bennett DA, Stretton CM, Anderson CS. Systematic review of progressive resistance strength training in older adults. J Gerontol A Biol Sci Med Sci. 2004;59:48–61 [DOI] [PubMed] [Google Scholar]
- 50.Barton E, Morris C. Mechanisms and strategies to counter muscle atrophy. J Gerontol A Biol Sci Med Sci. 2003;58:M923–6 [DOI] [PubMed] [Google Scholar]
- 51.Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–75 [DOI] [PubMed] [Google Scholar]
- 52.Kehayias JJ, Zhuang H. Use of zetatron D-T neutron generator for the simultaneous measurement of carbon, oxygen, and hydrogen in vivo in humans. Nucl Instrum Methods Phys Res. 1993;B79:555–9 [Google Scholar]
- 53.Schoeller DA. Changes in total body water with age. Am J Clin Nutr. 1989;50(Suppl 5):1176–81, discussion 231–5 [DOI] [PubMed] [Google Scholar]
- 54.Ferry M, Vellas B. Prevention and treatment of dehydration in the elderly. In: Arnaud MJ, ed. Hydration throughout life. Montrouge, France: John Libbey Eurotext; 1998:137–49. [Google Scholar]
- 55.Morley JE, Miller DK, Zdrodowski C, Gutierrez B. Perry III HM. Fluid intake, hydration and aging. In: Arnaud MJ, ed. Hydration throughout life. Montrouge, France: John Libbey Eurotext; 1998:107–15. [Google Scholar]
- 56.Flynn MA, Nolph GB, Baker AS, Martin WM, Krause G. Total body potassium in aging humans: a longitudinal study. Am J Clin Nutr. 1989;50:713–7 [DOI] [PubMed] [Google Scholar]
- 57.Kotler DP, Tierney A, Wang J, Pierson R. Magnitude of body-cell-mass depletion and the timing of death from wasting in AIDS. Am J Clin Nutr. 1989;50:444–7 [DOI] [PubMed] [Google Scholar]
- 58.Wheeler DA, Gibert CL, Launer CA, Muurahainen N, Elion RA, Abrams DI, Bartsch G. Weight loss as a predictor of survival and disease progression in HIV infection. Terry Beirn Community Programs for Clinical Research on AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:80–5 [DOI] [PubMed] [Google Scholar]
- 59.Walford RL. The extension of maximum life-span. Clin Geriatr Med. 1985;1:29–35 [PubMed] [Google Scholar]
- 60.Geokas MC. The aging process. Ann Intern Med. 1990;113:455–66 [DOI] [PubMed] [Google Scholar]
- 61.Wang ZM, Pierson RN, Jr, Heymsfield SB. The five-level model: a new approach to organizing body-composition research. Am J Clin Nutr. 1992;56:19–28 [DOI] [PubMed] [Google Scholar]
- 62.Elia M, Livesey G. Theory and validity of indirect calorimetry during net lipid synthesis. Am J Clin Nutr. 1988;47:591–607 [DOI] [PubMed] [Google Scholar]
- 63.Kehayias JJ, Heymsfield SB, LoMonte A, Wang J, Pierson RN. In vivo determination of body fat by measuring total body carbon. Am J Clin Nutr. 1991;53:1339–44 [DOI] [PubMed] [Google Scholar]
- 64.Rolland Y, Lauwers-Cances V, Cournot M, Nourhashemi F, Reynish W, Riviere D, Vellas B, Grandjean H. Sarcopenia, calf circumference, and physical function of elderly women: a cross-sectional study. J Am Geriatr Soc. 2003;51:1120–4 [DOI] [PubMed] [Google Scholar]
- 65.Visser M. Towards a definition of sarcopenia–results from epidemiologic studies. J Nutr Health Aging. 2009;13:713–6 [DOI] [PubMed] [Google Scholar]
- 66.Heymsfield SB, Wang J, Lichman S, Kamen Y, Kehayias JJ, Pierson RN. Body composition in elderly subjects: a critical appraisal of clinical methodology. Am J Clin Nutr. 1989;50:1167–75 [DOI] [PubMed] [Google Scholar]
- 67.Roubenoff R, Kehayias JJ, Dawson-Hughes B, Heymsfield SB. Use of dual-energy x-ray absorptiometry in body-composition studies: not yet a “gold standard.” Am J Clin Nutr. 1993;58:589–91 [DOI] [PubMed] [Google Scholar]
- 68.Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, Pierson RN., Jr Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52:214–8 [DOI] [PubMed] [Google Scholar]
- 69.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–48 [DOI] [PubMed] [Google Scholar]
- 70.Dufour AB, Hannan MT, Murabito JM, Kiel DP, McLean RR. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: the Framingham Study. J Gerontol A Biol Sci Med Sci. 2013;68:168–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wiig H. Pathophysiology of tissue fluid accumulation in inflammation. J Physiol. 2011;589:2945–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kehayias J, Stamatelatos I, Sheahan C, Neill M. A field method for assessing body composition by portable XRF Bromine analysis: validation against instrumental neutron activation. Proceedings of the International Conference on Isotpoic and Nuclear Analytical Techniques for Health and Environment. 2003 Jun 10–13, IAEA; Vienna, Austria.
- 73.Kehayias JJ, Ribeiro SM, Skahan A, Itzkowitz L, Dallal G, Rogers G, Khodeir M. Water homeostasis, frailty and cognitive function in the nursing home. J Nutr Health Aging. 2012;16:35–9 [DOI] [PubMed] [Google Scholar]
- 74.Chumlea WC, Guo SS, Baumgartner RN, Siervogel RM. Determination of body fluid compartments with multiple frequency bioelectric impedance. Basic Life Sci. 1993;60:23–6 [DOI] [PubMed] [Google Scholar]
- 75.Simpson JA, Lobo DN, Anderson JA, Macdonald IA, Perkins AC, Neal KR, Allison SP, Rowlands BJ. Body water compartment measurements: a comparison of bioelectrical impedance analysis with tritium and sodium bromide dilution techniques. Clin Nutr. 2001;20:339–43 [DOI] [PubMed] [Google Scholar]
- 76.Piccoli A, Nescolarde LD, Rosell J. Conventional and vectorial analysis of bioimpedance in clinical practice (in Spanish). Nefrologia. 2002;22:228–38 [PubMed] [Google Scholar]
- 77.Nescolarde L, Piccoli A, Roman A, Nunez A, Morales R, Tamayo J, Donate T, Rosell J. Bioelectrical impedance vector analysis in haemodialysis patients: relation between oedema and mortality. Physiol Meas. 2004;25:1271–80 [DOI] [PubMed] [Google Scholar]
- 78.Buffa R, Floris G, Marini E. Migration of the bioelectrical impedance vector in healthy elderly subjects. Nutrition. 2003;19:917–21 [DOI] [PubMed] [Google Scholar]
- 79.Evans WJ. What is sarcopenia? J Gerontol A Biol Sci Med Sci. 1995;50(Special Issue):5–8 [DOI] [PubMed] [Google Scholar]
- 80.Binkley N, Buehring B. Beyond FRAX: it’s time to consider “sarco-osteopenia.” J Clin Densitom. 2009;12:413–6 [DOI] [PubMed] [Google Scholar]