Abstract
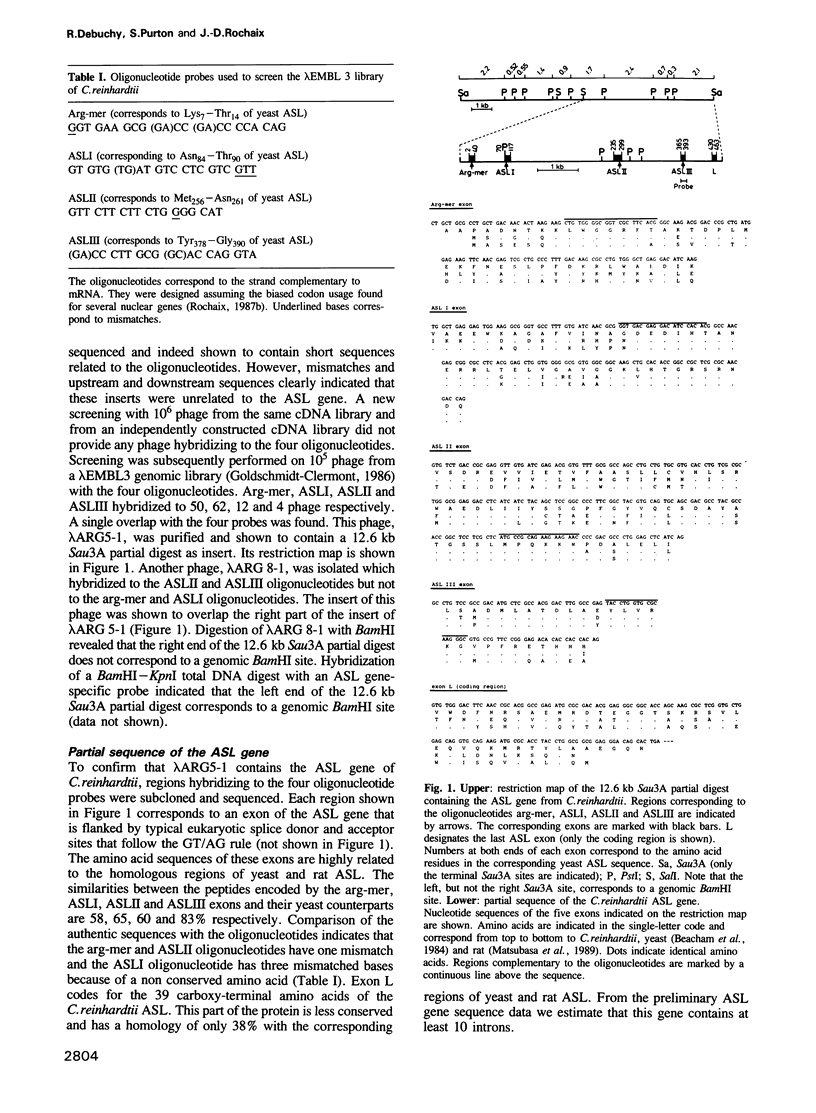
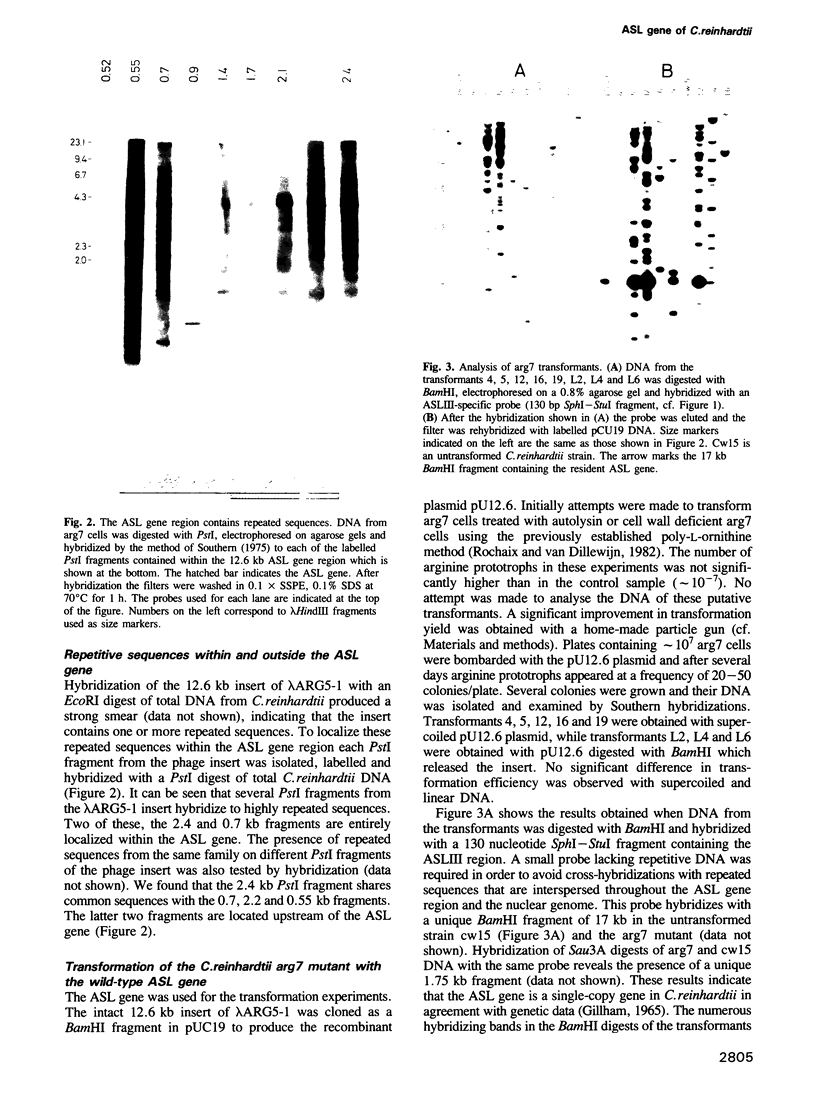
The argininosuccinate lyase (ASL) gene of Chlamydomonas reinhardtii has been cloned using four oligonucleotide probes corresponding to highly conserved regions of the ASL polypeptide sequence. The identity of the gene was confirmed by partial sequencing. It is unique, contains several introns and spans a region less than 7.8 kb that includes highly repetitive sequences. Using a particle gun, a reliable nuclear transformation system has been established by complementing three mutants deficient in ASL activity with the wild-type ASL gene. Analysis of the transformants reveals variable patterns of integration of the transforming DNA into the nuclear genome. Previous work has mapped the mutations in the mutants arg2 and arg7 to either end of the ARG7 locus 1.0 to 1.6 recombination map units apart. Our transformation results show that these two mutations are located within a region of 7.8 kb. This allows for the first correlation of the recombination map and the molecular map at the ARG7 locus and indicates a high recombination frequency in this region of the nuclear genome.
Full text
PDF
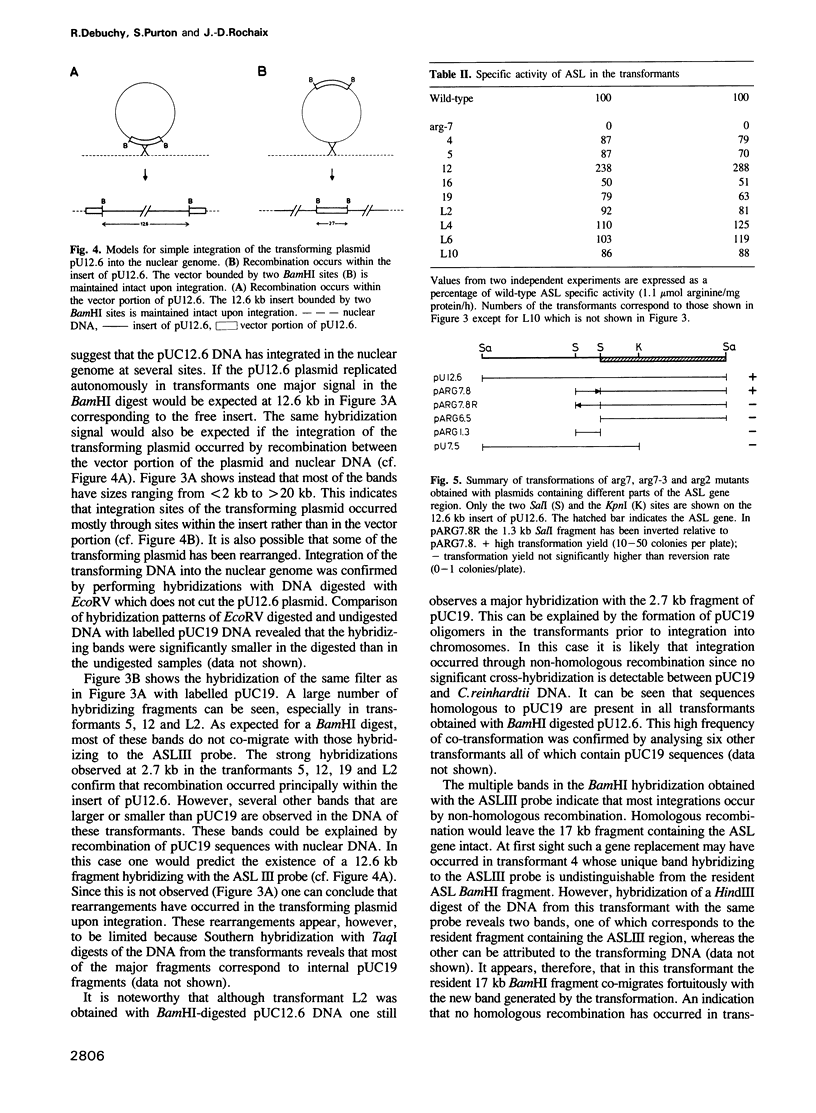



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaya Y., Matsubasa T., Takiguchi M., Kobayashi K., Saheki T., Kawamoto S., Mori M. Amino acid sequence of rat argininosuccinate lyase deduced from cDNA. J Biochem. 1988 Jan;103(1):177–181. doi: 10.1093/oxfordjournals.jbchem.a122227. [DOI] [PubMed] [Google Scholar]
- Beacham I. R., Schweitzer B. W., Warrick H. M., Carbon J. The nucleotide sequence of the yeast ARG4 gene. Gene. 1984 Sep;29(3):271–279. doi: 10.1016/0378-1119(84)90056-8. [DOI] [PubMed] [Google Scholar]
- Charlier D., Piette J., Glansdorff N. IS3 can function as a mobile promoter in E. coli. Nucleic Acids Res. 1982 Oct 11;10(19):5935–5948. doi: 10.1093/nar/10.19.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faragó A., Dénes G. Mechanism of arginine biosynthesis in Chlamydomonas reinhardti. II. Purification and properties of N-acetylglutamate 5-phosphotransferase, the allosteric enzyme of the pathway. Biochim Biophys Acta. 1967 Feb 7;136(1):6–18. doi: 10.1016/0304-4165(67)90315-7. [DOI] [PubMed] [Google Scholar]
- Fedoroff N., Wessler S., Shure M. Isolation of the transposable maize controlling elements Ac and Ds. Cell. 1983 Nov;35(1):235–242. doi: 10.1016/0092-8674(83)90226-x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gillham N. W. Induction of chromosomal and nonchromosomal mutations in Chlamydomonas reinhardi with N-methyl-N'-nitro-N-nitrosoguanidine. Genetics. 1965 Sep;52(3):529–537. doi: 10.1093/genetics/52.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M., Rahire M. Sequence, evolution and differential expression of the two genes encoding variant small subunits of ribulose bisphosphate carboxylase/oxygenase in Chlamydomonas reinhardtii. J Mol Biol. 1986 Oct 5;191(3):421–432. doi: 10.1016/0022-2836(86)90137-3. [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasnain S. E., Manavathu E. K., Leung W. C. DNA-mediated transformation of Chlamydomonas reinhardi cells: use of aminoglycoside 3'-phosphotransferase as a selectable marker. Mol Cell Biol. 1985 Dec;5(12):3647–3650. doi: 10.1128/mcb.5.12.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Johnston S. A., Anziano P. Q., Shark K., Sanford J. C., Butow R. A. Mitochondrial transformation in yeast by bombardment with microprojectiles. Science. 1988 Jun 10;240(4858):1538–1541. doi: 10.1126/science.2836954. [DOI] [PubMed] [Google Scholar]
- Khandjian E. W. UV crosslinking of RNA to nylon membrane enhances hybridization signals. Mol Biol Rep. 1986;11(2):107–115. doi: 10.1007/BF00364822. [DOI] [PubMed] [Google Scholar]
- Konvalinkova V., Matagne R. F., Loppes R. Induction and analysis of revertants from various arg-7 mutants lacking argininosuccinate lyase in Chlamydomonas reinhardi. Mutat Res. 1974 Jul;24(1):69–72. doi: 10.1016/0027-5107(74)90048-7. [DOI] [PubMed] [Google Scholar]
- LEVINE R. P., EBERSOLD W. T. The genetics and cytology of Chlamydomonas. Annu Rev Microbiol. 1960;14:197–216. doi: 10.1146/annurev.mi.14.100160.001213. [DOI] [PubMed] [Google Scholar]
- Matagne R. F. Fine structure of the arg-7 ciston in chlamydomonas reinhardi. Complementation between arg-7 mutants defective in argininosuccinate lyase. Mol Gen Genet. 1978 Mar 20;160(1):95–99. [PubMed] [Google Scholar]
- Matsubasa T., Takiguchi M., Amaya Y., Matsuda I., Mori M. Structure of the rat argininosuccinate lyase gene: close similarity to chicken delta-crystallin genes. Proc Natl Acad Sci U S A. 1989 Jan;86(2):592–596. doi: 10.1073/pnas.86.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuo S., Tatsuno M., Kobayashi K., Saheki T., Miyata T., Iwanaga S., Amaya Y., Mori M. Isolation of cDNA clones of human argininosuccinate lyase and corrected amino acid sequence. FEBS Lett. 1988 Jul 18;234(2):395–399. doi: 10.1016/0014-5793(88)80124-8. [DOI] [PubMed] [Google Scholar]
- O'Brien W. E., McInnes R., Kalumuck K., Adcock M. Cloning and sequence analysis of cDNA for human argininosuccinate lyase. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7211–7215. doi: 10.1073/pnas.83.19.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak M., Shillito R. D., Hohn T., Potrykus I. Expression in plants of two bacterial antibiotic resistance genes after protoplast transformation with a new plant expression vector. Nucleic Acids Res. 1986 Jul 25;14(14):5857–5868. doi: 10.1093/nar/14.14.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochaix J. D., van Dillewijn J., Rahire M. Construction and characterization of autonomously replicating plasmids in the green unicellular alga Chlamydomonas reinhardii. Cell. 1984 Apr;36(4):925–931. doi: 10.1016/0092-8674(84)90042-4. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D., van Dillewijn J. Transformation of the green alga Chlamydomonas reinhardii with yeast DNA. Nature. 1982 Mar 4;296(5852):70–72. doi: 10.1038/296070a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
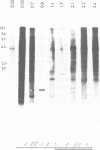
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., Strijkert P. J. Arginine metabolism in Chlamydomonas reinhardi. On the regulation of the arginine biosynthesis. Eur J Biochem. 1969 Apr;8(3):403–407. doi: 10.1111/j.1432-1033.1969.tb00541.x. [DOI] [PubMed] [Google Scholar]
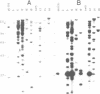
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]