Abstract
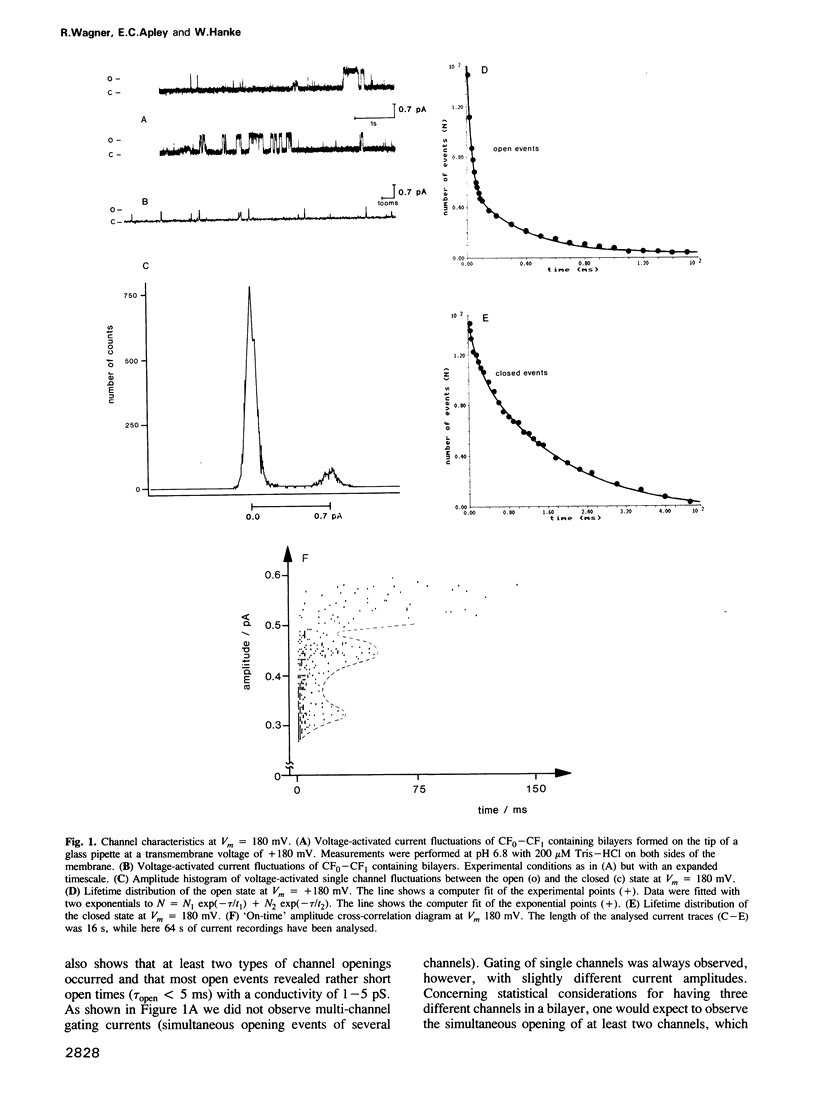
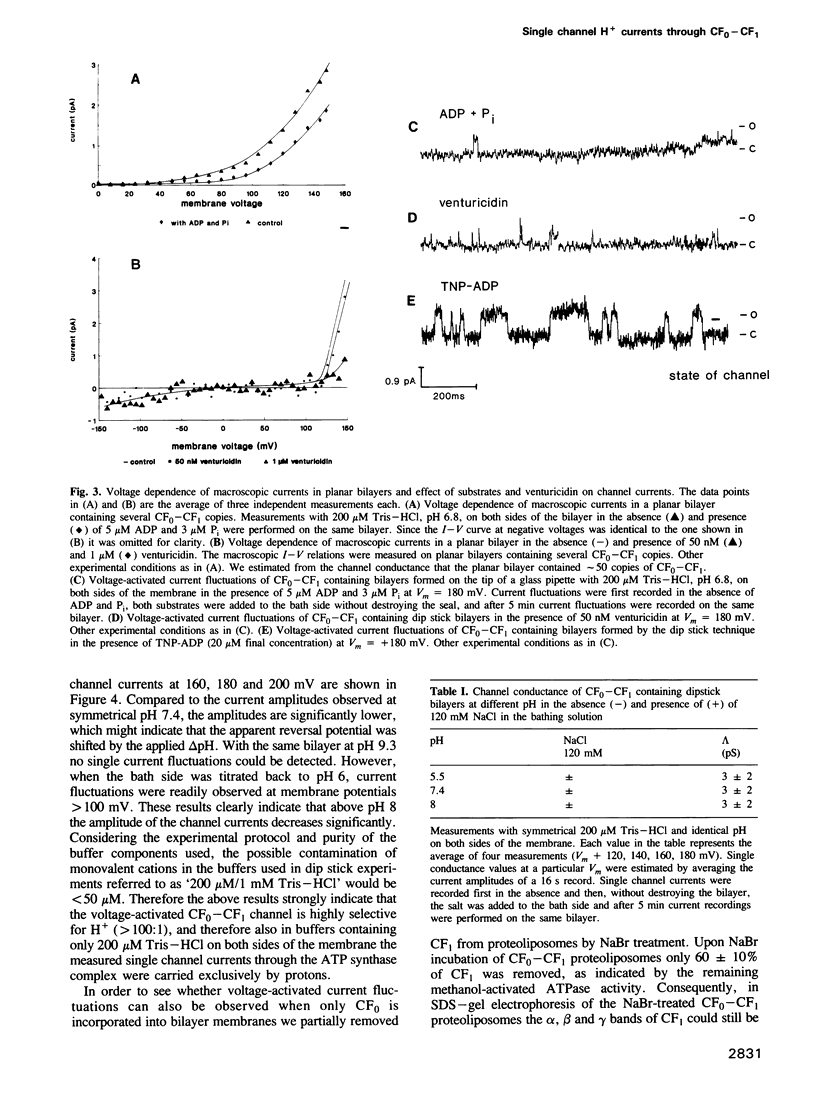
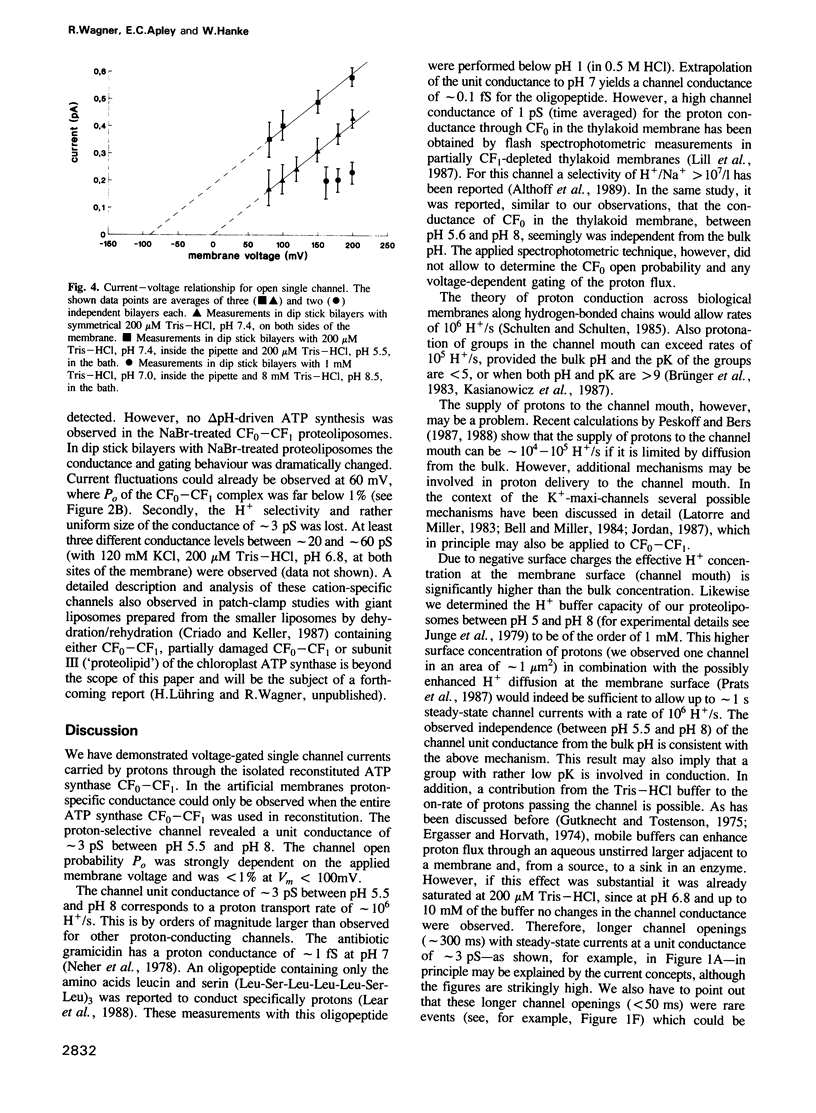
The purified chloroplast ATP synthase (CF(0)-CF(1)) was reconstituted into azolectin liposomes from which bilayer membranes on the tip of a glass pipette ('dip stick technique')and planar bilayer membranes were form ed. The CF(0)-CF(1) facilitated ion conductance through the bilayer membranes. Our results clearly indicated that the observed single channel currents were carried by H+ through the isolated and reconstituted chloroplast ATPase. We demonstrated that in proteoliposomes it is the whole enzyme complex CF(0)-CF(1) and not the membrane sector CF(0) alone that constitutes a voltagegated, proton-selective channel with a high conductance of 1-5 pS at pH 5.5-8.0. After removal of CF(1) from the liposomes by NaBr treatment the membrane sector CF(0) displayed various kinds of channels also permeable to monovalent cations. The open probability P(0) of the CF(0)-CF(1) channel increased considerable with increasing membrane voltage [from P(0) less than or equal to 1% (V(m) less than or equal to 120 mV) to P(0) less than or equal to 30% (120 mV less than or equal to Vm 200 mV)]. In the presence of ADP (3 microM) and P(i) (5 microM), which specifically bind to CF(1), the open probability decreased and venturicidin (1 microM), a specific inhibitor of H+ flow through CF(0) in thylakoid membranes, blocked the channel almost completely. Our results, which reveal a high channel unit conductance, and at membrane voltages less than 100 mV low open probability with concomitant mean open times in the micros timescale (less than 100 micros) for the energy coupling in the enzyme complex. At physiological membrane voltages for photophosphorylation (about 30 mV) the enzyme complex would then display a time-averaged conductance of about 1 fS.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthon G. E., Jagendorf A. T. Evidence for multiple effects in the methanol activation of chloroplast coupling factor 1. Biochim Biophys Acta. 1986 Jan 28;848(1):92–98. doi: 10.1016/0005-2728(86)90164-7. [DOI] [PubMed] [Google Scholar]
- Bell J. E., Miller C. Effects of phospholipid surface charge on ion conduction in the K+ channel of sarcoplasmic reticulum. Biophys J. 1984 Jan;45(1):279–287. doi: 10.1016/S0006-3495(84)84154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer P. D. The unusual enzymology of ATP synthase. Biochemistry. 1987 Dec 29;26(26):8503–8507. doi: 10.1021/bi00400a001. [DOI] [PubMed] [Google Scholar]
- Cook N. J., Zeilinger C., Koch K. W., Kaupp U. B. Solubilization and functional reconstitution of the cGMP-dependent cation channel from bovine rod outer segments. J Biol Chem. 1986 Dec 25;261(36):17033–17039. [PubMed] [Google Scholar]
- Criado M., Keller B. U. A membrane fusion strategy for single-channel recordings of membranes usually non-accessible to patch-clamp pipette electrodes. FEBS Lett. 1987 Nov 16;224(1):172–176. doi: 10.1016/0014-5793(87)80442-8. [DOI] [PubMed] [Google Scholar]
- Engasser J. M., Horvath C. Buffer-facilitated proton transport. pH profile of bound enzymes. Biochim Biophys Acta. 1974 Jul 17;358(1):178–192. doi: 10.1016/0005-2744(74)90269-1. [DOI] [PubMed] [Google Scholar]
- Gutknecht J., Tosteson D. C. Diffusion of weak acids across lipid bilayer membranes: effects of chemical reactions in the unstirred layers. Science. 1973 Dec 21;182(4118):1258–1261. doi: 10.1126/science.182.4118.1258. [DOI] [PubMed] [Google Scholar]
- Hanke W. Reconstitution of ion channels. CRC Crit Rev Biochem. 1985;19(1):1–44. doi: 10.3109/10409238509086786. [DOI] [PubMed] [Google Scholar]
- Jordan P. C. How pore mouth charge distributions alter the permeability of transmembrane ionic channels. Biophys J. 1987 Feb;51(2):297–311. doi: 10.1016/S0006-3495(87)83336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge W., Ausländer W., McGeer A. J., Runge T. The buffering capacity of the internal phase of thylakoids and the magnitude of the pH changes inside under flashing light. Biochim Biophys Acta. 1979 Apr 11;546(1):121–141. doi: 10.1016/0005-2728(79)90175-0. [DOI] [PubMed] [Google Scholar]
- Junge W., Rumberg B., Schröder H. The necessity of an electric potential difference and its use for photophosphorylation in short flash groups. Eur J Biochem. 1970 Jul;14(3):575–581. doi: 10.1111/j.1432-1033.1970.tb00326.x. [DOI] [PubMed] [Google Scholar]
- Kasianowicz J., Benz R., McLaughlin S. How do protons cross the membrane-solution interface? Kinetic studies on bilayer membranes exposed to the protonophore S-13 (5-chloro-3-tert-butyl-2'-chloro-4' nitrosalicylanilide). J Membr Biol. 1987;95(1):73–89. doi: 10.1007/BF01869632. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Latorre R., Miller C. Conduction and selectivity in potassium channels. J Membr Biol. 1983;71(1-2):11–30. doi: 10.1007/BF01870671. [DOI] [PubMed] [Google Scholar]
- Lear J. D., Wasserman Z. R., DeGrado W. F. Synthetic amphiphilic peptide models for protein ion channels. Science. 1988 May 27;240(4856):1177–1181. doi: 10.1126/science.2453923. [DOI] [PubMed] [Google Scholar]
- Linnett P. E., Beechey R. B. Inhibitors of the ATP synthethase system. Methods Enzymol. 1979;55:472–518. doi: 10.1016/0076-6879(79)55061-7. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- MUELLER P., RUDIN D. O., TIEN H. T., WESCOTT W. C. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature. 1962 Jun 9;194:979–980. doi: 10.1038/194979a0. [DOI] [PubMed] [Google Scholar]
- Maloney P. C. Energy coupling to ATP synthesis by the proton-translocating ATPase. J Membr Biol. 1982;67(1):1–12. doi: 10.1007/BF01868643. [DOI] [PubMed] [Google Scholar]
- McCarty R. E., Fuhrman J. S., Tsuchiya Y. Effects of adenine nucleotides on hydrogen-ion transport in chloroplasts. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2522–2526. doi: 10.1073/pnas.68.10.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. A commentary on alternative hypotheses of protonic coupling in the membrane systems catalysing oxidative and photosynthetic phosphorylation. FEBS Lett. 1977;78(1):1–20. doi: 10.1016/0014-5793(77)80263-9. [DOI] [PubMed] [Google Scholar]
- Nagle J. F., Morowitz H. J. Molecular mechanisms for proton transport in membranes. Proc Natl Acad Sci U S A. 1978 Jan;75(1):298–302. doi: 10.1073/pnas.75.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sandblom J., Eisenman G. Ionic selectivity, saturation, and block in gramicidin A channels. II. Saturation behavior of single channel conductances and evidence for the existence of multiple binding sites in the channel. J Membr Biol. 1978 Apr 26;40(2):97–116. doi: 10.1007/BF01871143. [DOI] [PubMed] [Google Scholar]
- Peskoff A., Bers D. M. Electrodiffusion of ions approaching the mouth of a conducting membrane channel. Biophys J. 1988 Jun;53(6):863–875. doi: 10.1016/S0006-3495(88)83167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats M., Tocanne J. F., Teissie J. Lateral proton conduction at a lipid/water interface. Effect of lipid nature and ionic content of the aqueous phase. Eur J Biochem. 1987 Jan 15;162(2):379–385. doi: 10.1111/j.1432-1033.1987.tb10612.x. [DOI] [PubMed] [Google Scholar]
- Schulten Z., Schulten K. A model for the resistance of the proton channel formed by the proteolipid of ATPase. Eur Biophys J. 1985;11(3):149–155. doi: 10.1007/BF00257393. [DOI] [PubMed] [Google Scholar]
- Senior A. E. ATP synthesis by oxidative phosphorylation. Physiol Rev. 1988 Jan;68(1):177–231. doi: 10.1152/physrev.1988.68.1.177. [DOI] [PubMed] [Google Scholar]
- Wagner R., Ponse G., Strotmann H. Binding of 2'(3')-O-(2,4,6-trinitrophenyl)-adenosine 5'-diphosphate opens the pathway for protons through the chloroplast ATPase complex. Eur J Biochem. 1986 Nov 17;161(1):205–209. doi: 10.1111/j.1432-1033.1986.tb10143.x. [DOI] [PubMed] [Google Scholar]



