Abstract
Background
Eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps (CRSwNP) display distinct patterns of inflammation. However, the pathogenic mechanisms underlying the heterogeneity of CRSwNP need further investigation.
Objective
To investigate local immunoglobulin E (IgE) production and phenotype of mast cells in eosinophilic and non-eosinophilic CRSwNP in Chinese.
Methods
Total and specific IgE levels were analyzed by means of the ImmunoCAP system. The molecular steps involved in class switch recombination to IgE were investigated using RT-PCR assays. Mast cell phenotypes, IgE- and high affinity IgE receptor (FcεRI)-positive cells, and allergen binding to specific IgE in sinonasal mucosa were determined by means of immunohistochemistry.
Results
Compared with controls and non-eosinophilic CRSwNP, local total IgE levels were increased, and local specific IgE to common aeroallergens was more frequently found, in Chinese eosinophilic CRSwNP independent of atopy and without significant association with Staphylococcus aureus enterotoxins. The ε germline gene transcript was also more frequently detected in eosinophilic CRSwNP. The number of IgE- and FcεRI-positive cells was increased in eosinophilic CRSwNP. Most IgE- and FcεRI-positive cells were mast cells. Dust mite antigens could bind to IgE on mast cells in situ. The number of mast cells positive for both tryptase and chymase and activated mast cells was increased in eosinophilic CRSwNP and the number of activated mast cells positively correlated with local IgE level, eotaxin-1 level, and eosinophil count in CRSwNP.
Conclusions and Clinical Relevance
The local IgE induced by common aeroallergens may mediate mast cell activation and contribute to subsequent eosinophilic inflammation in Chinese CRSwNP. This study offers a rationale for considering intervention strategies designed to target “local allergy” in eosinophilic CRSwNP.
Keywords: Chronic rhinosinusitis, nasal polyps, mast cell, eosinophil, immunoglobulin E
INTRODUCTION
Chronic rhinosinusitis (CRS) is one of the most common inflammatory pathologies of the nasal and paranasal sinuses. Eosinophilic inflammation has been considered to be a cardinal feature of CRS with nasal polyps (CRSwNP) in Caucasians for a long time. However, our study demonstrated that Chinese CRSwNP is composed of several subtypes, with up to 50% of CRSwNP showing non-eosinophilic inflammation, indicating unique phenotypes and endotypes in Chinese CRSwNP [1, 2]. Emerging researches show that eosinophilic and non-eosinophilic CRSwNP have different inflammation and tissue remodeling patterns and contrasting responses to corticosteroid treatment [1-5]. Nevertheless, the pathogenic mechanisms leading to these different phenotypes need further investigation.
Although eosinophil-attracting chemokines and cytokines have been demonstrated to promote eosinophilic inflammation in CRSwNP, the initial triggers of mucosal eosinophilia in CRSwNP remain obscure [6]. In Caucasian patients with CRSwNP, it has been reported that staphylococcus aureus enterotoxins (SAE), acting as both antigens and superantigens, may drive local IgE production that is associated with eosinophilic inflammation [7]. In Chinese patients, one study also found increased levels of total IgE and specific IgE to SAE in nasal polyps (NP) [8]; however, the relationship between local IgE and eosinophilic inflammation has not been investigated in Chinese CRSwNP and the role of SAE in local IgE production in CRSwNP in nonwhite population remains to be determined.
IgE is produced after heavy-chain class switch recombination in B cells from IgM, IgG or IgA to IgE. It is generally accepted that class switch recombination occurs in lymphoid tissues [9]. Nevertheless, evidence for local class switch recombination has been found in asthma and allergic rhinitis [10-12]. Whether the local IgE production in NP results from local class switch recombination or B cells already committed to IgE synthesis elsewhere has not been explored extensively. In vitro experiments have demonstrated that specific antigen exposure and anti-IgE stimulation can activate the mast cells derived from NP from Caucasians [13]. However, the role of IgE in the activation of mast cells in CRSwNP in vivo and the relationship between mast cell phenotype and inflammation pattern in CRSwNP have not been established.
The purposes of the current study were (1) to investigate the role of SAE and common aeroallergens in inducing the local IgE production, and (2) to define the relationship between local IgE production, mast cell phenotype, and eosinophilic inflammation in Chinese CRSwNP.
METHODS
Subjects
This study was approved by the Ethics Committee of Tongji Hospital of Huazhong University of Science and Technology and was conducted with written informed consent from patients. The clinical data of patients are summarized in Table 1. Not all samples were included in every study method because of the limited amount for some samples. The diagnosis of CRSwNP was made according to the current European EAACI Position Paper on Rhinosinusitis and Nasal Polyps and American guidelines [14, 15]. CRSwNP was classified as eosinophilic when percent tissue eosinophils exceeded 10% of total infiltrating cells as defined by our previous study [1]. Control subjects were those undergoing septoplasty because of anatomic variations and without other sinonasal diseases. Polyp tissues from CRSwNP patients and inferior turbinate mucosa from control patients were harvested during surgery. Atopic status was evaluated using the skin prick tests with a standard panel of 20 common inhalant allergens in our region that performed in accordance with the WHO guideline [16]. Oral glucocorticoid and intranasal steroid spray were discontinued at least 3 months and 1 month before surgery, respectively. Subjects who had an antrochoanal polyps, cystic fibrosis, fungal sinusitis, primary ciliary dyskinesia, or gastroesophageal reflux disease were excluded from the study. Further information including symptom and physical finding assessment is provided in this article’s Online Supplement.
TABLE 1.
Patients’ clinical data.
| Control | Eos CRSwNP | Non-Eos CRSwNP |
Control vs Eos CRSwNP P value |
Control vs Non-Eos CRSwNP P value |
Eos vs Non-Eos CRSwNP P value |
|
|---|---|---|---|---|---|---|
| Subject, n | 22 | 20 | 29 | |||
| Sex, male, n (%) | 14 (63.6) | 14 (70) | 15 (51.7) | NS | NS | NS |
| Age [years, median(IQR)] | 27.5 (18.5-46.0) | 38.0 (29.3-44.0) | 35.0 (20.0-54.0) | NS | NS | NS |
| Patients with atopy, n (%) | 6 (27.3) | 8 (40) | 7 (24.1) | NS | NS | NS |
| Patients with asthma, n (%) | 0 (0) | 2 (10) | 0 (0) | NS | NS | NS |
| Bilateral CT score [median (IQR)] | 0 (0-0) | 16.0 (9.0-20.0) | 17.0 (13.0-22.0) | < 0.001 | < 0.001 | NS |
| Bilateral NP score [median (IQR)] | 0 (0-0) | 3.0 (2.0-4.0) | 3.0 (2.0- 4.0) | < 0.001 | < 0.001 | NS |
| Nasal obstruction VAS score [median (IQR)] |
4 (4- 5) | 6 (4.5-8) | 6 (5-8) | NS | NS | NS |
| Nasal discharge VAS Score [median (IQR)] |
0 (0- 1) | 4 (2.5-6.5) | 4 (2- 7.5) | < 0.001 | 0.003 | NS |
| Headache VAS score [median (IQR)] |
0 (0- 0) | 2 (0- 4) | 0 (0- 3) | 0.002 | 0.013 | NS |
| Facial pain VAS score [median (IQR)] |
0 (0- 0.75) | 0 (0- 1) | 0 (0-0.5) | NS | NS | NS |
| Hyposmia VAS score [median (IQR)] |
1 (0-2) | 4 (2.5-5.5) | 2 (0-6) | 0.003 | NS | NS |
| Overall discomfort VAS score [median (IQR)] |
4 (2- 4.75) | 5 (4-7) | 5 (4-6.5) | NS | NS | NS |
Eos, eosinophilic; CRSwNP, chronic rhinosinusitis with nasal polyps; Non-Eos, non-eosinophilic; NP, nasal polyps; VAS, visual analogue scale; CT, computer tomography, IQR, interquartile range.
Cytokine and IgE measurement in tissue homogenates
Tissue samples were weighed and homogenized and the supernatants were harvested for later analysis [7]. The protein levels of cytokines and chemokines were detected by means of bio-plex suspension chip technology (BIO-RAD, Hercules, Ca) according to the manufacturer’s instructions. Total IgE and specific IgE levels to common airborne allergens were detected by the ImmunoCAP system (Phadia, Uppsala, Sweden). Specific IgE was determined for house dust mix (Hx2, Dermatophagoides pteronyssinus, Dermatophagoides farinae and Blatella germanica), mould mix (Mx2, Penicillium notatum, Aspergillus fumigatus, Candida albicans and Alternaria), animal epidermal and protein mix (Ex1, Dander from cat, horse, cow and dog), tree pollen mix (Tx4, Oak, Elm, Maple leaf sycamore, Willow and Cottonwood), weed pollen mix (Wx5, Common Ragweed, Mugwort, Marguerite, Dandelion and Golded rod) and staphylococcus aureus enterotoxin A (SEA) and B (SEB) [2, 7, 8]. More information is provided in the Online Supplement.
Histology study
Eosinophil and total inflammatory cell were counted on hematoxylin-eosin stained sections, and immunohistochemical staining was performed as previously described [1]. Primary antibodies used (Table S1 in the Online Supplement) and additional information regarding immunohistochemistry is provided in the Online Supplement. Consecutive serial sections were used to study the relationship between IgE- or FcεRI-positive cells and CD20, CD138, or tryptase-positive cells. Mast cell phenotype was assessed by detecting tryptase-positive and, on a consecutive section, chymase-positive cells. As double labeling was not performed, tryptase-positive mast cells generally reflect the total population of mast cell (MCTot), including mast cells that are tryptase-only positive and those that are both tryptase and chymase positive (MCTC) [17]. Chymase-positive mast cells in this study reflected the MCTC population [17]. Consequently, MCTC/MCTot estimated the proportion of MCTC in the total mast cell population [17]. Degranulated mast cells were identified as cells that have discrete and relatively focal staining, but do not have well-circumscribed staining with anti-tryptase antibody staining. In addition, extracellular staining is often observed in the area surrounding activated cells [18].
To detect allergen binding to specific IgE on mast cells in NP, consecutive sections from the eosinophilic CRSwNP patients with local specific IgE to dust mites were stained with biotinylated Dermatophagoides farinae antigens (DerF 1, 400 ng/mL and DerF 2, 300 ng/mL; Wolwubiotech, Zhejiang, China) and anti-tryptase antibody, respectively. To determine whether the allergen binding was specific to IgE, some consecutive sections were preincubated with or without blocking polyclonal antibody direct against IgE (undiluted; Abcam, Cambridge, UK), respectively.
Mast cells both in lamina propria (LP) and epithelium were counted. For other cell types, positive cells in LP were counted. Five randomly selected high-powered fields were examined and the results were expressed as cells per high-powered field (HPF) of LP or as cells per millimeter of epithelium as described elsewhere [1].
RT-PCR
Total RNA was extracted and reverse-transcribed to cDNA, and real-time PCR was performed with appropriate primers (Table S2 in the Online Supplement) as mentioned in previous study [1, 19]. Relative gene expression was calculated by using the 2(-Delta Delta CT) method. The ε germline gene transcript (εGLT) expression was investigated by conventional PCR. The specific circle transcript Iε-Cμ and Iε-Cγ (products resulting from class switch recombination from IgM to IgE and from any IgG to IgE, respectively) were investigated using a nested PCR amplification protocol with appropriate primers constructed from published sequences (Table S3 in the Online Supplement) as previously described [11]. The technical information for PCR assay is provided in this article’s Online Supplement.
Statistical analysis
For continuous variables, results are expressed as medians and interquartile ranges, or in box and whisker plots displaying medians and interquartile ranges. With continuous variables, the Kruskal-Wallis H-test was used to assess significant intergroup variability and the Mann-Whitney U 2-tailed test was used for between-group comparisons. Differences in proportions were tested by the chi-square test. The Spearman test was used to determine correlations. Significance was accepted at P < .05.
RESULTS
Expression of TH2 cytokines and eosinophil chemoattractants
The protein levels of IL-5, IL-13, and eotaxin-1 (CCL11), but not IL-4, regulated upon activation normal T cell expressed and secreted (RANTES, CCL5), and granulocyte macrophage colony stimulating factor (GM-CSF), were significantly higher in eosinophilic CRSwNP than in controls and non-eosinophilic CRSwNP (Fig S1 in the online supplement). The levels of IL-13 (r = 0.892, P < 0.001) and eotaxin-1 (r = 0.606, P = 0.048) were correlated with the number of tissue eosinophil in eosinophilic CRSwNP (Fig S2 in the online supplement).
IgE levels
Compared with those seen in controls and non-eosinophilic CRSwNP, eosinophilic CRSwNP had a marginally significant increase in total serum IgE levels, whereas a significant increase in total IgE levels in tissue homogenates (Fig 1). There was no significant difference in serum or tissue IgE level between non-eosinophilic CRSwNP and controls (Fig 1). Interestingly, non-atopic eosinophilic CRSwNP also had significantly higher local IgE levels than controls and non-atopic non-eosinophilic CRSwNP (Fig 1). Again, a trend toward higher levels of serum IgE was found in non-atopic eosinophilic CRSwNP compared with non-atopic non-eosinophilic CRSwNP (Fig 1). The comparison between atopic subjects was not performed because of the limited number of atopic subjects. Local specific IgE to common inhalant allergen mix was more frequently found in eosinophilic CRSwNP (83.3%) than in non-eosinophilic CRSwNP (30.8%, P < 0.001) and controls (16.7%, P < 0.001). The detailed data of each subject is listed in Table S4 in the Online Supplement. The specific IgE antibodies to different aeroallergen mix were detected in comparable frequency in eosinophilic CRSwNP. Half of CRSwNP patients with positive local IgE production presented local IgE to more than one kind of allergen mix. In our study only 25% of eosinophilic CRSwNP, 7.7% of non-eosinophilic CRSwNP, and none controls had specific IgE to SEA and/or SEB, and there was no significant difference among the different groups. Interestingly and importantly, specific local IgE to aeroallergens were detected in similar frequency in non-atopic and atopic subjects; in addition, the majority of the subjects with local specific IgE to aeroallergens did not have local production of SEA and SEB specific IgE (Table S4 in the online supplement).
FIG 1.
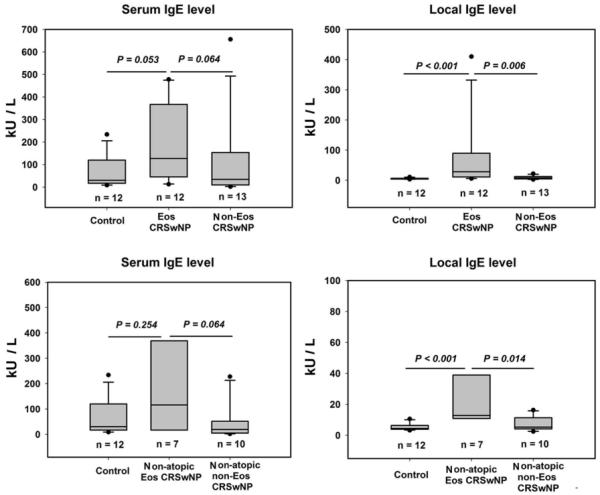
Serum and local total immunoglobulin E (IgE) levels in control and different types of chronic rhinosinusitis with nasal polyps (CRSwNP). Eos CRSwNP, eosinophilic CRSwNP; Non-Eos CRSwNP, non-eosinophilic CRSwNP.
The mRNA expression of εGLT, Cε, Iε-Cμ and Iε-Cγ and AID
The frequency of εGLT mRNA expression was increased in eosinophilic CRSwNP (42.9%) compared with controls (8.3%, P = 0.048) and non-eosinophilic CRSwNP (6.7%, P = 0.023). Representative results of PCR assay of εGLT are shown in Fig 2A. DNA recombination, producing ε circle transcript, marks an irreversible step in the commitment of B cells to the synthesis of IgE. In this study, we failed to detect the expression of circle transcript Iε-Cμ and Iε-Cγ in sinonasal mucosa in all subjects studied. IgE synthesis by B cells is the final step in the production of IgE. The detection frequency of Cε transcript was significantly higher in eosinophilic CRSwNP (85.7%), but not in non-eosinophilic CRSwNP (64.3%), compared with that seen in controls (30%, P = 0.005). AID catalyses the initial step of class switch recombination. In the present study, we found that AID mRNA expression was increased similarly in both eosinophilic and non-eosinophilic CRSwNP compared with that seen in controls (Fig 2B).
FIG 2.
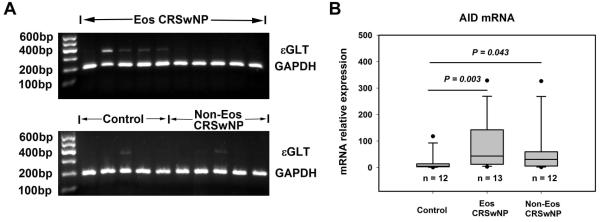
A, The representative pictures of electrophoresis results of RT-PCR assay of ε germline gene transcript (εGLT). The data of εGLT mRNA expression frequency is presented in the results section. B, The relative mRNA expression level of activation-induced cytidine deaminase (AID) is increased in both eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps (CRSwNP). Eos CRSwNP, eosinophilic CRSwNP; Non-Eos CRSwNP, non-eosinophilic CRSwNP.
B cell and plasma cell infiltration, and the expression of B cell chemoattractants, CD40, and CD40L
In line with our previous observations [1], plasma cell and B cell infiltration was enhanced similarly in eosinophilic and non-eosinophilic CRSwNP compared with that seen in controls (Fig S3 in the online supplement). The mRNA expression of B cell chemoattractant, CXCL13, but not CXCL12, was also increased similarly in eosinophilic and non-eosinophilic CRSwNP compared with that seen in controls (Fig S3 in the online supplement). CD40 ligand (CD40L) is transiently expressed on activated CD4+ T cells and is competent to induce IgE synthesis by interaction with CD40 expressed on B cells in the presence of IL-4 or IL-13 [20]. In the present study, no significant difference in CD40 or CD40L mRNA expression was detected among different study groups (Fig S3 in the online supplement). Follicle-like structures were defined as described by Humby [21]. They were more frequently found in eosinophilic CRSwNP (40.0%; P = 0.042) and non-eosinophilic CRSwNP patients (41.7%; P = 0.018) compared with those seen in controls (9.1%). A representative photomicrograph of sinonasal tissue sections showing the follicle-like structure is provided in Fig S4 in the online supplement.
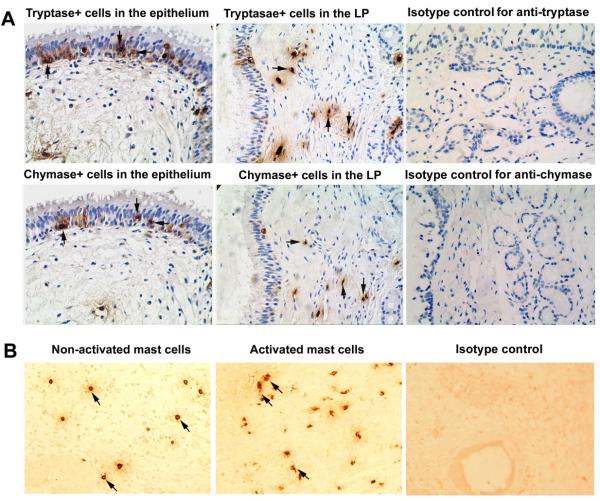
Mast cell phenotype, location, and activation
Mast cells predominately infiltrated LP of sinonasal mucosa and, to a lesser extent, infiltrated epithelium. Mast cell phenotype was assessed by detecting tryptase-positive and, on a consecutive section, chymase-positive cells (Fig 3A). As shown in Fig 4, in the LP, although no significant difference in the number of MCTot was observed among the groups studied, the number of MCTC and the MCTC/MCTot ratio were markedly increased in eosinophilic CRSwNP compared with those seen in non-eosinophilic CRSwNP and controls. In the epithelium, only the number of MCTot was significantly increased in eosinophilic CRSwNP as compared with that seen in controls (Fig 4). Intriguingly, eosinophilic CRSwNP, but not non-eosinophilic CRSwNP, had significantly more degranulated MCTot in LP compared with that seen in controls (Fig 3B and Fig 4). Consistently, the level of mRNA for prostaglandin D synthase (PGDS) was significantly increased in eosinophilic CRSwNP but not in non-eosinophilic CRSwNP compared with that seen in controls (Fig 4). Since only a small number of activated mast cells could be found in epithelium, no quantification in the epithelium was done.
FIG 3.
A, Representative photomicrographs of tryptase and chymase immunohistochemical staining of consecutive sections of polyp tissues. Arrows in the same direction indicate the same cells in consecutive serial sections that are positive for both tryptase and chymase and defined as tryptase- and chymase-positive mast cells (MCTC). Tryptase-positive mast cells generally reflect the total population of mast cells (MCTot). LP, lamina propria. B, Representative photomicrographs of tryptase immunohistochemical staining of polyp tissue section showing activated and undegranulated mast cells. Degranulated mast cells were identified as cells that have discrete and relatively focal staining, but do not have well-circumscribed staining. In addition, extracellular staining is often observed in the area surrounding activated cells. The slides for observing activated mast cells were not counterstained with hematoxylin. Representative photomicrographs of immunohistochemical staining with respective isotype controls are also provided. Original magnification × 400.
FIG 4.
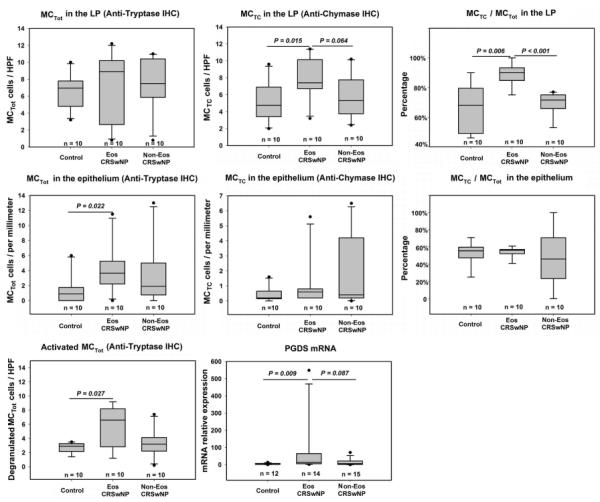
The number of total mast cells (MCTot) and mast cells positive for both tryptase and chymase (MCTC), and the ratio of MCTC/MCTot in the lamina propria (LP) and epithelium, the number of activated mast cells in LP, and the relative mRNA expression level of prostaglandin D synthase (PGDS) in tissues from controls and eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps (CRSwNP). Mast cell phenotype was assessed by detecting tryptase-positive and, on a consecutive section, chymase-positive cells. HPF, high-powered field; IHC, immunohistochemistry; Eos CRSwNP, eosinophilic CRSwNP; Non-Eos CRSwNP, non-eosinophilic CRSwNP.
IgE- and FcεRI-positive cells
A significantly increased number of IgE-positive cells and FcεRI expressing cells were detected in the LP of eosinophilic CRSwNP compared with non-eosinophilic CRSwNP and controls (Fig 5, A and C). Most IgE-positive cells were mast cells and a few were plasma cells; no IgE-positive B cell was found (Fig 5B). Most of the FcεRI expressing cells were mast cells as well (Fig 5D).
FIG 5.
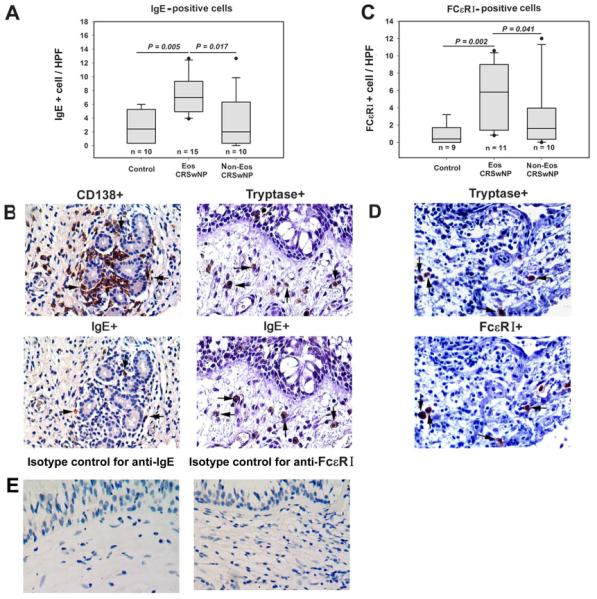
IgE- and FcεRI-positive cells in tissues. A, The number of IgE-positive cells is increased in eosinophilic chronic rhinosinusitis with nasal polyps (CRSwNP). B, IgE-positive cells are mast cells and plasma cells. Representative immunostaining of consecutive sections from an eosinophilic CRSwNP subject with anti-IgE and anti-CD138 antibody, respectively; or with anti-IgE and anti-tryptase antibody, respectively. C, The number of FcεRI-positive cells is increased in eosinophilic CRSwNP. D, FcεRI expressing cells are mast cells. Representative immunostaining of consecutive sections from an eosinophilic CRSwNP subject with anti-FcεRI and anti-tryptase antibody, respectively. E, Representative photomicrographs of immunohistochemical staining with isotype controls for anti-IgE and anti-FcεRI antibody. In (B) and (D), arrows with the same direction indicate same cells in consecutive serial sections, original magnification × 400. HPF, high-powered field; Eos CRSwNP, eosinophilic CRSwNP; Non-Eos CRSwNP, non-eosinophilic CRSwNP.
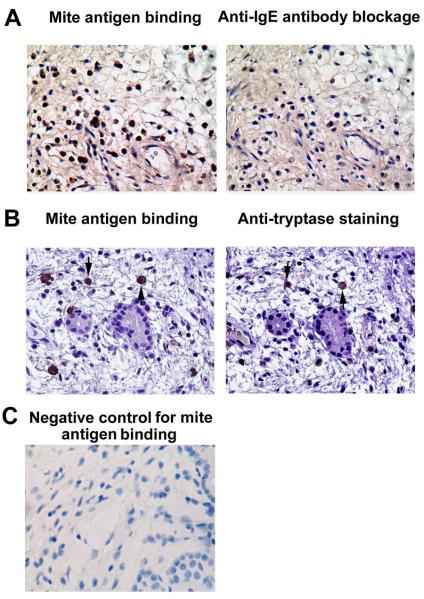
Detection of antigen binding to specific IgE on mast cells
Here, we showed that dust mite antigens could bind to inflammatory cells in polyp tissues which was almost completely abolished by pretreatment with anti-IgE antibody (Fig 6A), indicating that such binding is IgE specific. Next, we demonstrated that many cells bound with dust mite antigens were tryptase positive mast cells (Fig 6B).
FIG 6.
A, Dust mite antigens can bind to inflammatory cells in polyp tissues through IgE. Representative immunostaining of consecutive sections of polyp tissues with biotinylated Dermatophagoides farinae antigens with or without anti-IgE pre-treatment, respectively. The staining is almost completely abolished by pretreatment with anti-IgE antibody. Sections were from an eosinophilic chronic rhinosinusitis with nasal polyps (CRSwNP) subject with local production of specific IgE to duct mites. B, Many cells bound with dust mite antigens are tryptase positive mast cells. Representative immunostaining of consecutive sections of polyp tissues with biotinylated dust mite allergens and anti-tryptase antibody, respectively. Sections were from the same eosinophilic CRSwNP subject. C, A representative photomicrograph of immunohistochemical staining showing negative control for mite antigen binding. Arrows with the same direction indicate same cells in consecutive serial sections, original magnification × 400.
The correlation between IgE, mast cell phenotype, eosinophilic inflammation, and clinical manifestation
As illustrated in Fig S5 in the online supplement, local total IgE concentration positively correlated with total serum IgE level (r = 0.742, P < 0.001) and the number of IgE-positive cells in tissues (r = 0.922, P < 0. 001) in all subjects. Local total IgE level positively correlated with the number of tissue eosinophils (r = 0.644, P < 0.001) and IL-13 (r = 0.576, P = 0.003) protein level in CRSwNP. Although local IgE level did not correlate with the number of MCTot in all three groups (data not shown), it did correlate with the number of activated mast cells (r = 0.642, P = 0.013) in CRSwNP. Interestingly, the number of activated mast cells positively correlated with eotaxin-1 (r =0.652, P = 0.016) and eosinophil count (r = 0.879, P = 0.047) in CRSwNP. There was no significant difference in symptom and physical expression severity between eosinophilic and non-eosinophilic CRSwNP patients, although a trend toward severer olfactory dysfunction was found in eosinophilic CRSwNP (Table1). Local total IgE level only positively correlated with visual analogue scale score of loss of smell (r = 0.885, P = 0.034).
DISCUSSION
CRSwNP encompasses a variety conditions with heterogeneity in inflammation and tissue remodeling patterns [1-3]. Endotyping the immunopathologic features of this disorder may lead to the development of specific therapeutic strategies targeting distinct endotypes [1-5]. In this study, confirming our previous mRNA data [1], we found that the protein levels of IL-5, IL-13, and eotaxin-1 (CCL11) were increased in eosinophilic CRSwNP compared with those seen in non-eosinophilic CRSwNP and controls. Although increased Th2 cytokine and eosinophil chemokine expression may facilitate eosinophil recruitment, survival, and activation, the upstream mechanisms that initiate eosinophilic inflammation in NP remain obscure.
SAE-driven local production of IgE has been linked to eosinophilic inflammation in Caucasian CRSwNP [7]. In this study, we found that local IgE production was also associated with eosinophilic inflammation independent of atopic status in Chinese CRSwNP; however, only 25% of Chinese eosinophilic CRSwNP displayed SAE IgE. On the contrary, specific IgE antibodies to common aeroallergens could be detected in the majority of eosinophilic CRSwNP subjects (83.3%) often in the absence of SAE IgE. These findings suggest a more important role of aeroallergens than SAE in driving local IgE production in Chinese patients. Nevertheless, it should be noted that our current study has a small sample size; in addition, the aeroallergens were not measured individually and only the most common SAE were evaluated due to the limited amount of tissue samples. In our study, a positive correlation was found between local and serum IgE levels even in non-atopic subjects, and IgE levels were higher in tissue than in the corresponding serum after normalization of IgE concentration to the weight of tissue and serum (data not shown), suggesting that the circulating IgE may reflect spillover of IgE from sinonasal mucosa, rather than from lymphoid tissues or bone marrow.
In the present study, the finding of IgE-positive plasma cells in situ represents new evidence for local IgE production in CRSwNP. Nevertheless, whether B cells undergo class switch recombination to IgE locally in polyps has not been studied extensively. Recently, Gevaert et al have reported increased expression of εGLT and recombinant activating genes in Caucasian CRSwNP [22]. In the present study, we also demonstrated increased expression of εGLT in Chinese eosinophilic CRSwNP, suggesting a local class switch recombination to IgE in polyps. B cell chemokine CXCL13 has been found to be increased in CRSwNP as compared with controls previously [23]. Interestingly, in this study, CXCL13 expression, B cell and plasma cell infiltration, follicle-like structure formation, and AID expression were enhanced similarly in eosinophilic and non-eosinophilic CRSwNP, suggesting that local class switch recombination to immunoglobulins other than IgE might happen in non-eosinophilic CRSwNP. Indeed, our unpublished data demonstrated increased local IgA and IgG levels in non-eosinophilic CRSwNP. Nevertheless, specific up-regulation of IL-13 expression may drive the B cells towards specific local class switch recombination to IgE in eosinophilic CRSwNP, which is reflected by the increased expression of εGLT and Cε. In this study, direct evidence of ongoing class switching, as measured by the presence of specific circle transcripts, could not be detected. Circle transcripts are very rare, as each B cell only produces one during chromosomal rearrangement. In addition, the expression of CD40L was not upregulated in eosinophilic CRSwNP. Failure to detect these markers may also relate to the transient expression pattern of these markers.
In this study, we provide the first in-depth description of the IgE- and FcεRI-positive cells, and the relation between IgE and mast cell activation and eosinophilic inflammation in CRSwNP. We found that the number of IgE- and FcεRI-positive cells was significantly increased in eosinophilic CRSwNP compared with non-eosinophilic CRSwNP and controls, and most IgE- and FcεRI-positive cells were mast cells. Mast cells are heterogeneous cells that exist as two major subtypes, mast cell with tryptase positive only and MCTC phenotypes [24, 25]. Prostaglandin D2 production is more pronounced in MCTC [17]. The distribution, phenotype, and regulation of mast cells in CRS have not been extensively explored in vivo. A previous study has demonstrated increased mast cells in CRSwNP by means of flow cytometry [26]. In this study, we provided the evidence for the alterations in phenotypic profile and activity of mast cells in eosinophilic CRSwNP. In eosinophilic CRSwNP, the submucosal total mast cell population (MCTot) was comparable to controls and non-eosinophilic CRSwNP, but extremely dominated by MCTC and characterized by activated mast cells. The change of mast cell phenotype was associated with an increased expression of PGDS, the enzyme responsible for prostaglandin D2 synthesis. Importantly, we found that the number of activated mast cells correlated with local IgE levels, eotaxin-1 levels, and eosinophil count, providing evidence for a contribution of IgE to the regulation of mast cell degranulation and an involvement of activated mast cells, mostly MCTC, in the development of eosinophilic inflammation in CRSwNP. Activated mast cells can synthesize and release numerous proinflammatory mediators and cytokines, such as prostaglandin D2, and lead to subsequent recruitment and activation of Th2 cells and eosinophils, and edema formation [27]. In this study, we also provided the first evidence that allergen could bind to specific IgE on mast cells in polyp tissues. Collectively, these data strongly underline a role of IgE-mediated mast cell activation in the pathogenesis of eosinophilic CRSwNP.
Since most nasal polyps are originated from ethmoid sinus, uncinate process tissue might be more suitable than inferior turbinate tissue to be a control. However, limited amount of uncinate process tissue does not allow a number of measurements required in this study, especially the total and specific IgE detection in tissue homogenates; therefore, we used inferior turbinate tissue as control. It is possible that the differences observed between controls and CRSwNP might be influenced by the difference of tissue localizations. Nevertheless, when we repeated immunohistochemistry and local total IgE level detection experiments using normal uncinate process tissues from 8 patients (age, years, mean ± SD, 34.8 ± 11.2; 5 male) suffering from nasal trauma, mucocele of sphenoid sinus, and nasal tumors, and without ethmoid sinus inflammation on coronal CT scanning and endoscopy, no difference was found between inferior turbinate and normal uncinate process mucosa and our results keep unchanged comparing the difference between polyps and uncinate process mucosa (Fig S6 in the online supplement). More importantly, clear differences were observed between eosinophilic and non-eosinophilic polyps in this study.
In conclusion, in this study, we provide comprehensive evidence for the local production of IgE in subjects of eosinophilic CRSwNP even in those determined to be non-atopic by standard methodologies. Importantly, the local IgE may be induced by common aeroallergens in addition to SAE in Chinese patients. We also demonstrate a role of local IgE-mediated mast cells activation in the induction of eosinophilic inflammation in CRSwNP. Our data support a concept previously described for rhinitis termed “local allergy” or “entopy” in the pathogenesis of eosinophilic CRSwNP [28]. Obviously, this concept needs to be proved in vivo; however, such attempt has been hampered by a lack of a validated and standardized methodology for nasal provocation test in subject with CRSwNP. Our data provide evidence that although eosinophilic and non-eosinophilic CRSwNP have similar clinical manifestations regarding the symptom and polyp severity, they may have different drivers of the disease and may need different therapeutic strategies targeting distinct pathways.
Supplementary Material
Acknowledgments
Grant Support: This study was supported by the National Natural Science Foundation of China (NSFC) grants 81325006, 81020108018 and 81200733, and a grant from the Ministry of Health of China (201202005) as well as grants R01HL078860 and R37HL068546 from the NIH.
Footnotes
Disclosure of conflict of interest: None for every author.
AUTHORS’ CONTRIBUTIONS Study idea and design: ZL, PPC; clinical samples: HW, MZ, WHL; experiment: PPC, YNZ, BL; data analysis: PPC, YNZ, BL, JM, BFW; manuscript: PPC, ZL; critical review of the manuscript: RPS; final approval: all authors.
REFERENCES
- 1.Cao PP, Li HB, Wang BF, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009;124:478–484. doi: 10.1016/j.jaci.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Bachert C, Zhang N, Holtappels G, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010;126:962–968. doi: 10.1016/j.jaci.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Shi LL, Xiong P, Zhang L, et al. Features of airway remodeling in different types of Chinese chronic rhinosinusitis are associated with inflammation patterns. Allergy. 2013;68:101–109. doi: 10.1111/all.12064. [DOI] [PubMed] [Google Scholar]
- 4.Wen W, Liu W, Luo Z, et al. Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol. 2012;129:1522–1528. doi: 10.1016/j.jaci.2012.01.079. [DOI] [PubMed] [Google Scholar]
- 5.Payne SC, Early SB, Huyett P, et al. Evidence for distinct histologic profile of nasal polyps with and without eosinophilia. Laryngoscope. 2011;121:2262–2267. doi: 10.1002/lary.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao T, Kojima Y, Koyanagi A, et al. Eotaxin-1, -2, and -3 immunoreactivity and protein concentration in the nasal polyps of eosinophilic chronic rhinosinusitis patients. Laryngoscope. 2009;119:1053–1059. doi: 10.1002/lary.20191. [DOI] [PubMed] [Google Scholar]
- 7.Bachert C, Gevaert P, Holtappels G, Johansson SG, van Cauwengerge P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol. 2001;107:607–614. doi: 10.1067/mai.2001.112374. [DOI] [PubMed] [Google Scholar]
- 8.Zhang N, Holtappels G, Claeys C, Huand G, van Cauwenberge P, Bachert C. Pattern of inflammation and impact of staphylococcus aureus enterotoxins in nasal polyps from southern China. Am J Rhinol. 2006;20:445–450. doi: 10.2500/ajr.2006.20.2887. [DOI] [PubMed] [Google Scholar]
- 9.Gray D. Immunological memory. Annu Rev Immunol. 1993;11:49–77. doi: 10.1146/annurev.iy.11.040193.000405. [DOI] [PubMed] [Google Scholar]
- 10.Takhar P, Corrigan CJ, Smurthwaite L, et al. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. J Allergy Clin Immunol. 2007;119:213–218. doi: 10.1016/j.jaci.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 11.Takhar P, Smurthwaite L, Coker HA, et al. Allergen drives class switching to IgE in the nasal mucosa in allergic rhinitis. J Immunology. 2005;174:5024–5032. doi: 10.4049/jimmunol.174.8.5024. [DOI] [PubMed] [Google Scholar]
- 12.Fiset PO, Cameron L, Hamid Q. Local isotype switching to IgE in airway mucosa. J Allergy Clin Immunol. 2005;116:233–236. doi: 10.1016/j.jaci.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Zhang N, Holtappels G, Gevaert P, et al. Mucosal tissue polyclonal IgE is functional in response to allergen and SEB. Allergy. 2011;66:141–148. doi: 10.1111/j.1398-9995.2010.02448.x. [DOI] [PubMed] [Google Scholar]
- 14.Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114:155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fokken WJ, Lund VJ, Mullol J, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012;23:1–298. [PubMed] [Google Scholar]
- 16.Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–562. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 17.Balzar S, Fajt ML, Comhair SA, et al. Mast cell phenotype, location, and activation in severe asthma. Data from the severe asthma research program. Am J Respir Crit Care Med. 2011;183:299–309. doi: 10.1164/rccm.201002-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abonia JP, Blanchard C, Butz BB, et al. Involvement of mast cell in eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126:140–149. doi: 10.1016/j.jaci.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Lu X, Wang H, You XJ, Gao QX, Cui YH. Group II subfamily secretory phospholipase A2 enzymes: expression in chronic rhinosinusitis with and without nasal polyps. Allergy. 2007;62:999–1006. doi: 10.1111/j.1398-9995.2007.01381.x. [DOI] [PubMed] [Google Scholar]
- 20.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18:673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 21.Humby F, Bombardieri M, Manzo A, et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 2009;6:e1. doi: 10.1371/journal.pmed.0060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gevaert P, Nouri-Aria KT, Wu H, et al. Local receptor revision and class switching to IgE in chronic rhinosinusitis with nasal polyps. Allergy. 2013;68:55–63. doi: 10.1111/all.12054. [DOI] [PubMed] [Google Scholar]
- 23.Patadia M, Dixon J, Conley D, et al. Evaluation of the presence of B-cell attractant chemokines in chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24:11–16. doi: 10.2500/ajra.2010.24.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takabavashi T, Kato A, Peters AT, et al. Glandular mast cells with distinct phenotype are highly elevated in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012;130:410–420. doi: 10.1016/j.jaci.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ifani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LT. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci USA. 1986;83:4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw JL, Ashoori F, Fakhri S, Citardi MJ, Luong A. Increased percentage of mast cells within sinonasal mucosa of chronic rhinosinusitis with nasal polyps patients independent of atopy. Int Forum Allergy Rhinol. 2012;2:233–240. doi: 10.1002/alr.21021. [DOI] [PubMed] [Google Scholar]
- 27.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rondon C, Canto G, Blanca M. Local allergic rhinitis: a new entity, characterization and further studies. Cuurent Opinion in Allergic and Clinical Immunology. 2010;10:1–7. doi: 10.1097/ACI.0b013e328334f5fb. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.