Abstract
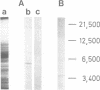
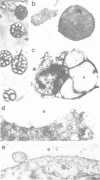
The existence of an insulin-mediated cell-to-cell signaling in the sponge Geodia cydonium is demonstrated in this study by molecular biological and immunological techniques. The sequence of a sponge cDNA clone encoding preproinsulin was analyzed for the first time and determined to comprise a high homology to human preproinsulin (60-80% homology). The predicted polypeptide of preproinsulin from sponge contains two disulfide bridges which link the A- to the B-chain. The intra-A chain disulfide bridge is absent. Applying immunological and electron microscopical techniques it is shown that insulin is produced in specialized cells (spherulous cells). Experimental evidence is presented which indicates that the sponge preproinsulin (predicted Mr 11,850) is processed to insulin (Mr 5600; B-chain, Mr 3700 and A-chain, Mr 1900). Plasma membranes of sponge cells are shown to be provided with an insulin-binding receptor composed of two molecules (Mr 104,000 and Mr 98,000). Heterologous insulin (from bovine pancreas) was found to stimulate gene expression in G. cydonium cells. It is concluded that sponges are provided with an endocrine signaling circuit: signaling cells (spherulous cells), hormone (insulin), and hormone receptor bearing target cells which respond to the hormone stimulus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Takiya S., Suzuki Y., Iwami M., Kawakami A., Takahashi S. Y., Ishizaki H., Nagasawa H., Suzuki A. cDNA structure and expression of bombyxin, an insulin-like brain secretory peptide of the silkmoth Bombyx mori. J Biol Chem. 1989 May 5;264(13):7681–7685. [PubMed] [Google Scholar]
- Banskota N. K., Taub R., Zellner K., Olsen P., King G. L. Characterization of induction of protooncogene c-myc and cellular growth in human vascular smooth muscle cells by insulin and IGF-I. Diabetes. 1989 Jan;38(1):123–129. doi: 10.2337/diab.38.1.123. [DOI] [PubMed] [Google Scholar]
- Blundell T. L., Humbel R. E. Hormone families: pancreatic hormones and homologous growth factors. Nature. 1980 Oct 30;287(5785):781–787. doi: 10.1038/287781a0. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Turner R. S., Kuhns W. J., Weinbaum G. A possible model for cell-cell recognition via surface macromolecules. Philos Trans R Soc Lond B Biol Sci. 1975 Jul 17;271(912):379–393. doi: 10.1098/rstb.1975.0059. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collier E., Watkinson A., Cleland C. F., Roth J. Partial purification and characterization of an insulin-like material from spinach and Lemna gibba G3. J Biol Chem. 1987 May 5;262(13):6238–6247. [PubMed] [Google Scholar]
- Conlon J. M., Reinecke M., Thorndyke M. C., Falkmer S. Insulin and other islet hormones (somatostatin, glucagon and PP) in the neuroendocrine system of some lower vertebrates and that of invertebrates--a minireview. Horm Metab Res. 1988 Jul;20(7):406–410. doi: 10.1055/s-2007-1010849. [DOI] [PubMed] [Google Scholar]
- Falcon C., Pfliegler G., Deckmyn H., Vermylen J. The platelet insulin receptor: detection, partial characterization, and search for a function. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1190–1196. doi: 10.1016/s0006-291x(88)81000-3. [DOI] [PubMed] [Google Scholar]
- Gramzow M., Bachmann M., Uhlenbruck G., Dorn A., Müller W. E. Identification and further characterization of the specific cell binding fragment from sponge aggregation factor. J Cell Biol. 1986 Apr;102(4):1344–1349. doi: 10.1083/jcb.102.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramzow M., Zimmermann H., Janetzko A., Dorn A., Kurelec B., Schröder H. C., Müller W. E. Control of the aggregation factor-aggregation receptor interaction in sponges by protein kinase C. Exp Cell Res. 1988 Nov;179(1):243–252. doi: 10.1016/0014-4827(88)90363-1. [DOI] [PubMed] [Google Scholar]
- HUMPHREYS T. CHEMICAL DISSOLUTION AND IN VITRO RECONSTRUCTION OF SPONGE CELL ADHESIONS. I. ISOLATION AND FUNCTIONAL DEMONSTRATION OF THE COMPONENTS INVOLVED. Dev Biol. 1963 Aug;8:27–47. doi: 10.1016/0012-1606(63)90024-1. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maier V., Steiner P., Fuchs J., Pfeifle B., Mezger M., Pfeiffer E. F. Isolation and partial characterization of insulin of the honeybee (Apis mellifica). Horm Metab Res. 1988 Jul;20(7):421–425. doi: 10.1055/s-2007-1010851. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moscona A. A. STUDIES ON CELL AGGREGATION: DEMONSTRATION OF MATERIALS WITH SELECTIVE CELL-BINDING ACTIVITY. Proc Natl Acad Sci U S A. 1963 May;49(5):742–747. doi: 10.1073/pnas.49.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier H. D., Jr, Spencer E. M., Dearden L. C., Jansons R. A. The effect of glucocorticoids on plasma insulin-like growth factor I concentration in the rat fetus. Pediatr Res. 1987 Jul;22(1):92–95. doi: 10.1203/00006450-198707000-00021. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Zahn R. K., Müller I., Kurelec B., Uhlenbruck G., Vaith P. Cell aggregation of the marine sponge Geodia cydonium. Identification of lectin-producing cells. Eur J Cell Biol. 1981 Apr;24(1):28–35. [PubMed] [Google Scholar]
- Müller W. E., Zahn R. K. Purification and characterization of a species-specific aggregation factor in sponges. Exp Cell Res. 1973 Jul;80(1):95–104. doi: 10.1016/0014-4827(73)90279-6. [DOI] [PubMed] [Google Scholar]
- Nagasawa H., Kataoka H., Isogai A., Tamura S., Suzuki A., Mizoguchi A., Fujiwara Y., Suzuki A., Takahashi S. Y., Ishizaki H. Amino acid sequence of a prothoracicotropic hormone of the silkworm Bombyx mori. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5840–5843. doi: 10.1073/pnas.83.16.5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K. Simultaneous localization of multiple tissue antigens using the peroxidase-labeled antibody method: a study on pituitary glands of the rat. J Histochem Cytochem. 1968 Sep;16(9):557–560. doi: 10.1177/16.9.557. [DOI] [PubMed] [Google Scholar]
- Ocrant I., Valentino K. L., Eng L. F., Hintz R. L., Wilson D. M., Rosenfeld R. G. Structural and immunohistochemical characterization of insulin-like growth factor I and II receptors in the murine central nervous system. Endocrinology. 1988 Aug;123(2):1023–1034. doi: 10.1210/endo-123-2-1023. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paonessa G., Gounari F., Frank R., Cortese R. Purification of a NF1-like DNA-binding protein from rat liver and cloning of the corresponding cDNA. EMBO J. 1988 Oct;7(10):3115–3123. doi: 10.1002/j.1460-2075.1988.tb03178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perler F., Efstratiadis A., Lomedico P., Gilbert W., Kolodner R., Dodgson J. The evolution of genes: the chicken preproinsulin gene. Cell. 1980 Jun;20(2):555–566. doi: 10.1016/0092-8674(80)90641-8. [DOI] [PubMed] [Google Scholar]
- Ricordi C., Lacy P. E., Scharp D. W. Automated islet isolation from human pancreas. Diabetes. 1989 Jan;38 (Suppl 1):140–142. doi: 10.2337/diab.38.1.s140. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robbins D. C., Tager H. S., Rubenstein A. H. Biologic and clinical importance of proinsulin. N Engl J Med. 1984 May 3;310(18):1165–1175. doi: 10.1056/NEJM198405033101807. [DOI] [PubMed] [Google Scholar]
- Rottmann M., Schröder H. C., Gramzow M., Renneisen K., Kurelec B., Dorn A., Friese U., Müller W. E. Specific phosphorylation of proteins in pore complex-laminae from the sponge Geodia cydonium by the homologous aggregation factor and phorbol ester. Role of protein kinase C in the phosphorylation of DNA topoisomerase II. EMBO J. 1987 Dec 20;6(13):3939–3944. doi: 10.1002/j.1460-2075.1987.tb02735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder H. C., Kuchino Y., Gramzow M., Kurelec B., Friese U., Uhlenbruck G., Müller W. E. Induction of ras gene expression by homologous aggregation factor in cells from the sponge Geodia cydonium. J Biol Chem. 1988 Nov 5;263(31):16334–16340. [PubMed] [Google Scholar]
- Shimizu Y., Takabayashi E., Yano S. Y., Shimizu N., Yamada K., Gushima H. Molecular cloning and expression in Escherichia coli of the cDNA coding for rat lipocortin I (calpactin II). Gene. 1988 May 15;65(1):141–147. doi: 10.1016/0378-1119(88)90427-1. [DOI] [PubMed] [Google Scholar]
- Smit A. B., Vreugdenhil E., Ebberink R. H., Geraerts W. P., Klootwijk J., Joosse J. Growth-controlling molluscan neurons produce the precursor of an insulin-related peptide. Nature. 1988 Feb 11;331(6156):535–538. doi: 10.1038/331535a0. [DOI] [PubMed] [Google Scholar]
- Sures I., Goeddel D. V., Gray A., Ullrich A. Nucleotide sequence of human preproinsulin complementary DNA. Science. 1980 Apr 4;208(4439):57–59. doi: 10.1126/science.6927840. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigele M., DeBernardo S., Leimgruber W. Fluorometric assay of secondary amino acids. Biochem Biophys Res Commun. 1973 Jan 23;50(2):352–356. doi: 10.1016/0006-291x(73)90847-4. [DOI] [PubMed] [Google Scholar]
- Weinbaum G., Burger M. M. Two component system for surface guided reassociation of animal cells. Nature. 1973 Aug 24;244(5417):510–512. doi: 10.1038/244510a0. [DOI] [PubMed] [Google Scholar]
- Wetekam W., Groneberg J., Leineweber M., Wengenmayer F., Winnacker E. L. The nucleotide sequence of cDNA coding for preproinsulin from the primate Macaca fascicularis. Gene. 1982 Sep;19(2):179–183. doi: 10.1016/0378-1119(82)90004-x. [DOI] [PubMed] [Google Scholar]
- de Pablo F., Chambers S. A., Ota A. Insulin-related molecules and insulin effects in the sea urchin embryo. Dev Biol. 1988 Nov;130(1):304–310. doi: 10.1016/0012-1606(88)90436-8. [DOI] [PubMed] [Google Scholar]