Abstract
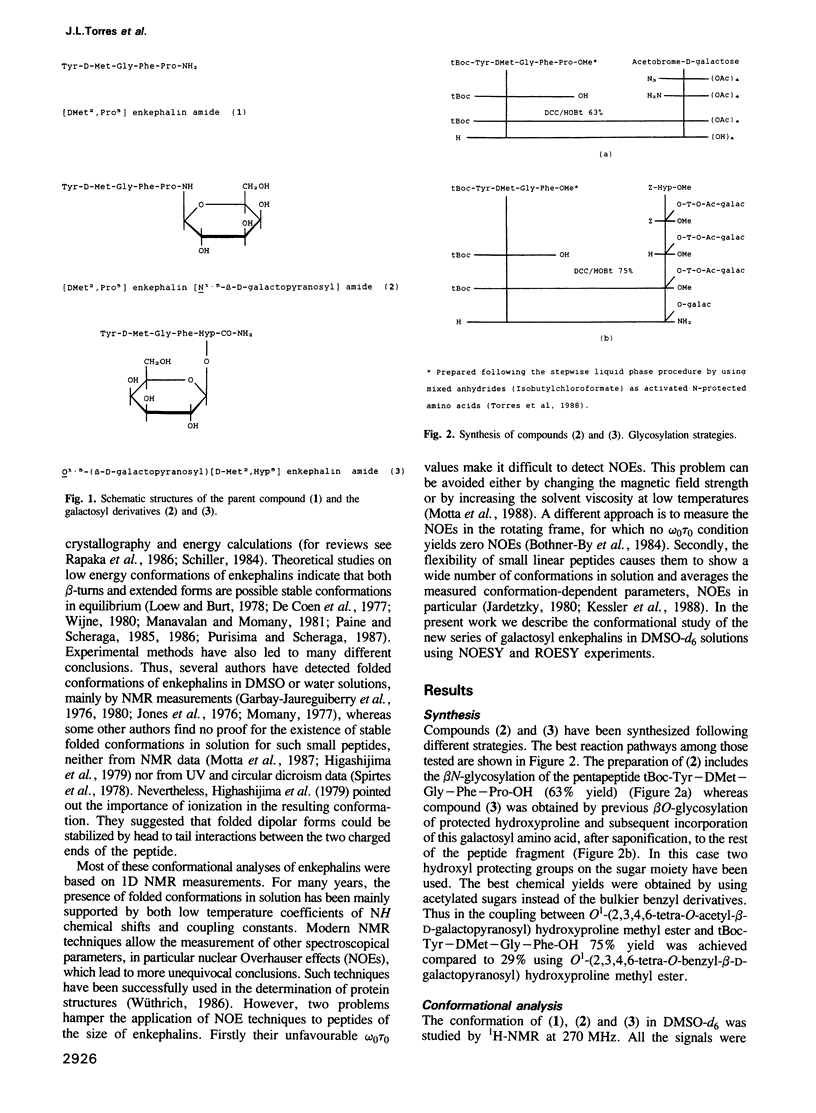
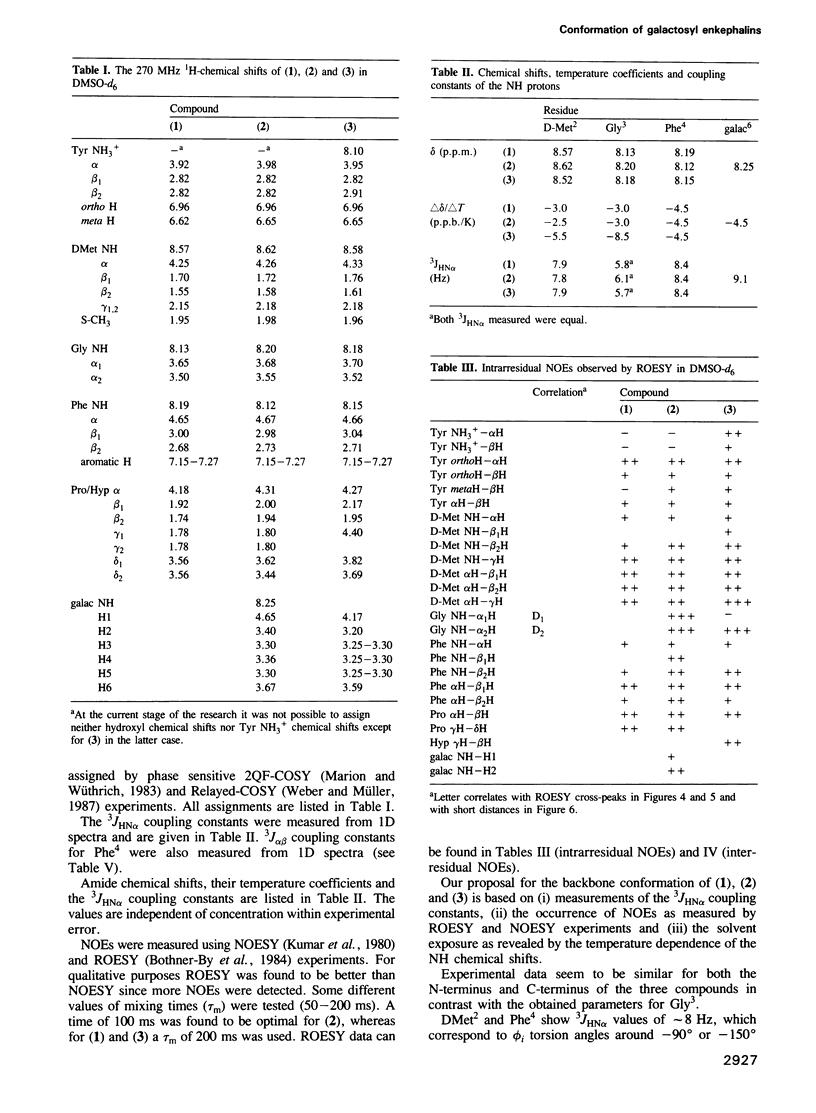
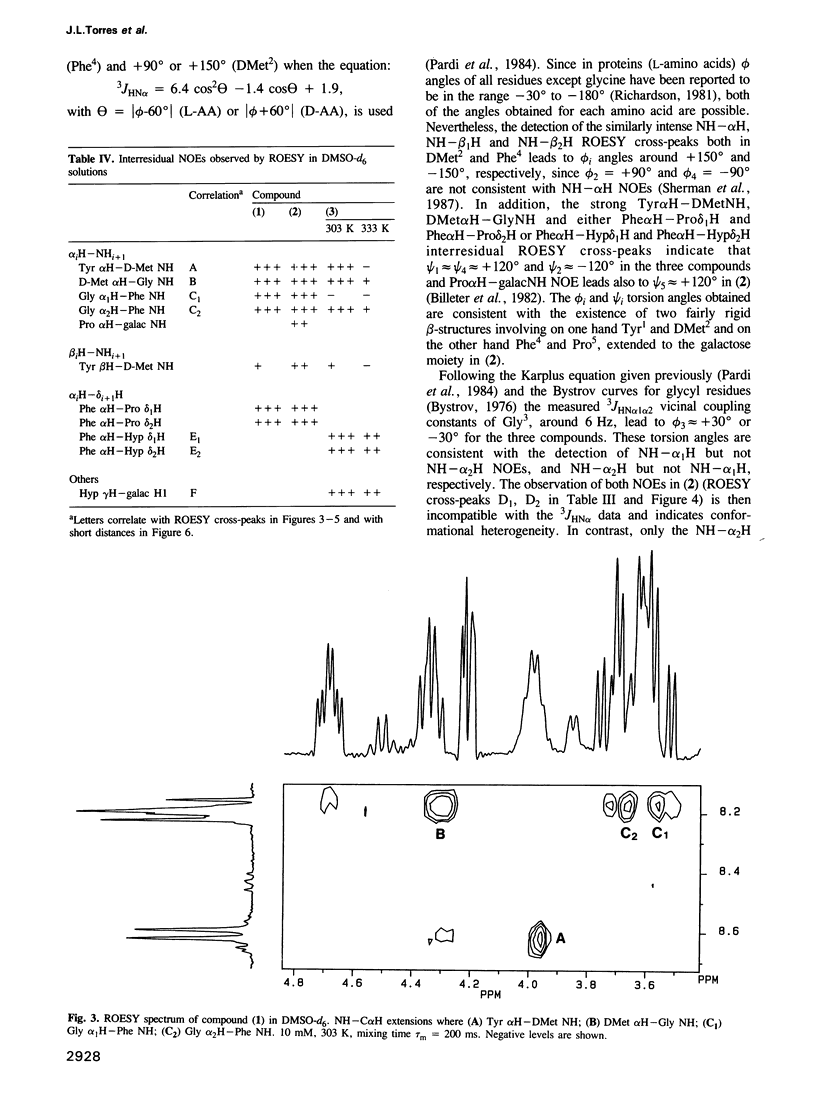
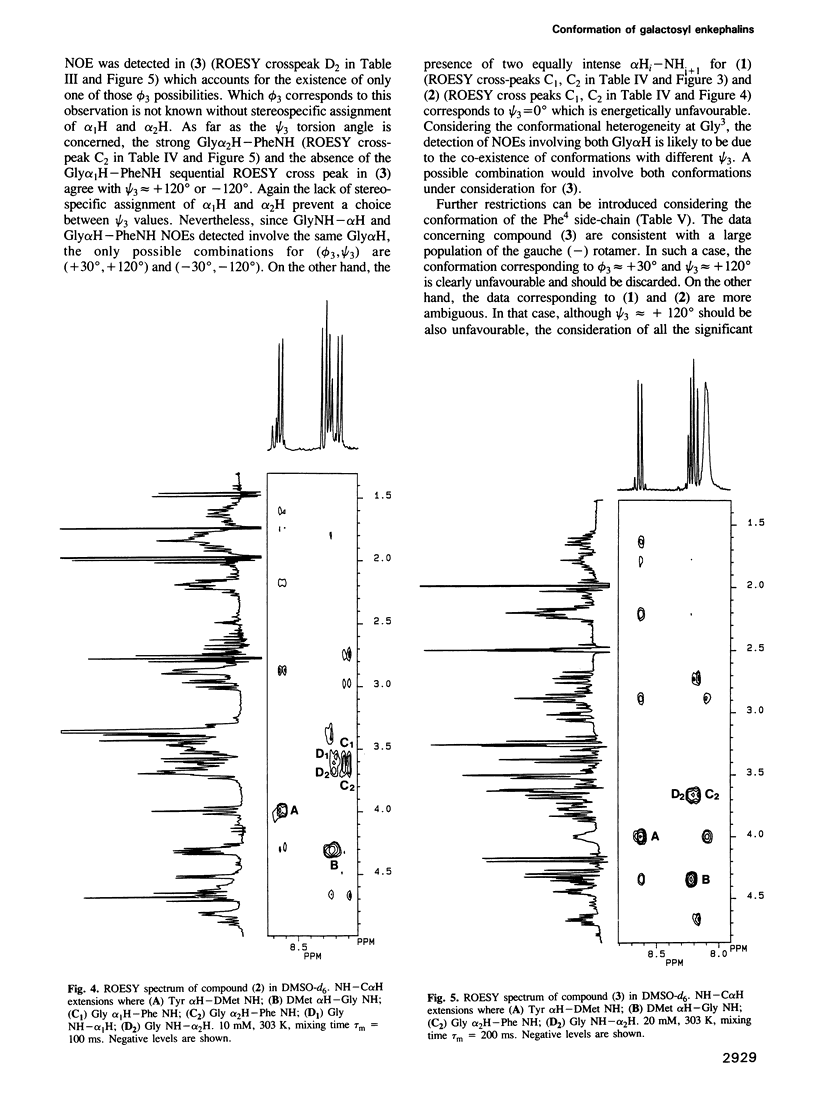
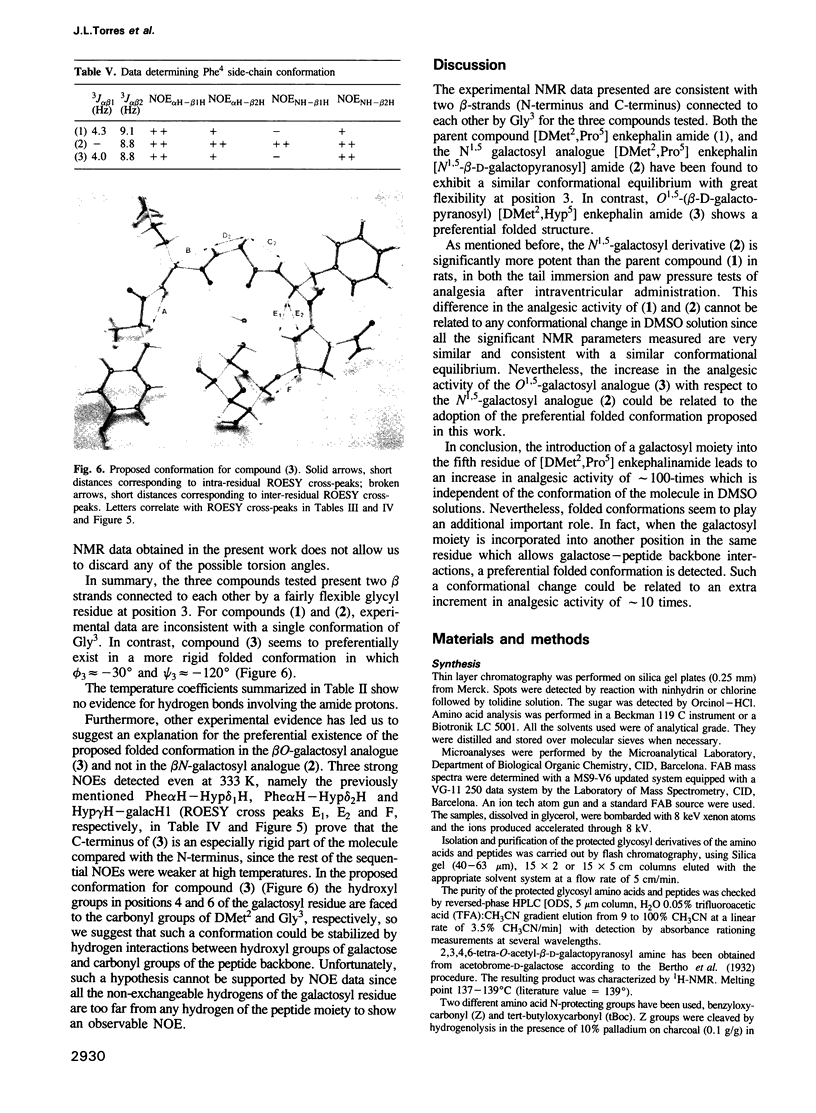
Two galactosyl derivatives of [DMet2,Pro5] enkephalin-amide (compound 1), namely [DMet2,Pro5] enkephalin [N1.5-beta-D-galactopyranosyl] amide (compound 2) and O1.5-(beta-D-galactopyranosyl) [DMet2,Hyp5] enkephalin-amide (compound 3) have been synthesized. Such glycosylpeptides have been shown to be extremely potent analgesic agonists. The conformational analysis of these three compounds in DMSO-d6 solution has been carried out using two-dimensional NMR methods. Both the parent compound (1) and the beta N-galactosyl derivative (2) show similar NMR parameters which are consistent with fairly rigid beta-strands at both the N-terminus and C-terminus, connected by a glycine residue that displays a mixture between multiple conformational states. Thus, although the beta N-galactosyl derivative (2) has been shown to be significantly more potent than the parent compound (1) in the tail immersion and paw pressure tests of analgesia, no correlation can be established between the conformation of (1) and (2) in DMSO and the difference in analgesic activity. In contrast, important conformational differences with respect to (1) and (2) have been detected in the beta O-galactosyl derivative (3). In this case, only one of the likely conformations for (1) and (2) are consistent with the experimental data. These data show that the position of the galactose residue in compound (3) causes Gly3 to loose flexibility leading to a more rigid folded conformation. Such a change in conformation could be related to the difference in analgesic activity between (2) and (3).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Billeter M., Braun W., Wüthrich K. Sequential resonance assignments in protein 1H nuclear magnetic resonance spectra. Computation of sterically allowed proton-proton distances and statistical analysis of proton-proton distances in single crystal protein conformations. J Mol Biol. 1982 Mar 5;155(3):321–346. doi: 10.1016/0022-2836(82)90008-0. [DOI] [PubMed] [Google Scholar]
- De Coen J. L., Humblet C., Koch M. H. Theoretical conformational analysis of Met-enkephalin. FEBS Lett. 1977 Jan 15;73(1):38–42. [PubMed] [Google Scholar]
- Garbay-Jaureguiberry C., Baudet J., Florentin D., Roques B. P. Conformational analysis of linear peptides by 15N NMR spectroscopy using the enkephalin-related fragment Tyr-Gly-Gly-Phe as a model compound. FEBS Lett. 1980 Jun 30;115(2):315–318. doi: 10.1016/0014-5793(80)81196-3. [DOI] [PubMed] [Google Scholar]
- Garbay-Jaureguiberry C., Roques B. P., Oberlin R. Preferential conformation of the endogenous opiate-like pentapeptide Met-enkephalin in DMSO-D6 solution determined by high field H NMR. Biochem Biophys Res Commun. 1976 Jul 26;71(2):558–565. doi: 10.1016/0006-291x(76)90823-8. [DOI] [PubMed] [Google Scholar]
- Higashijima T., Kobayashi J., Nagai U., Miyazawa T. Nuclear-magnetic-resonance study on Met-enkephalin and Met-enkephalinamide. Molecular association and conformation. Eur J Biochem. 1979 Jun;97(1):43–57. doi: 10.1111/j.1432-1033.1979.tb13084.x. [DOI] [PubMed] [Google Scholar]
- Jardetzky O. On the nature of molecular conformations inferred from high-resolution NMR. Biochim Biophys Acta. 1980 Feb 27;621(2):227–232. doi: 10.1016/0005-2795(80)90174-9. [DOI] [PubMed] [Google Scholar]
- Jones C. R., Gibbons W. A., Garsky V. Proton magnetic resonance studies of conformation and flexibility of enkephalin peptides. Nature. 1976 Aug 26;262(5571):779–782. doi: 10.1038/262779a0. [DOI] [PubMed] [Google Scholar]
- Kumar A., Ernst R. R., Wüthrich K. A two-dimensional nuclear Overhauser enhancement (2D NOE) experiment for the elucidation of complete proton-proton cross-relaxation networks in biological macromolecules. Biochem Biophys Res Commun. 1980 Jul 16;95(1):1–6. doi: 10.1016/0006-291x(80)90695-6. [DOI] [PubMed] [Google Scholar]
- Loew G. H., Burt S. K. Energy conformation study of Met-enkephalin and its D-Ala2 analogue and their resemblance to rigid opiates. Proc Natl Acad Sci U S A. 1978 Jan;75(1):7–11. doi: 10.1073/pnas.75.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunney J., Ashwell G. A hepatic receptor of avian origin capable of binding specifically modified glycoproteins. Proc Natl Acad Sci U S A. 1976 Feb;73(2):341–343. doi: 10.1073/pnas.73.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavalan P., Momany F. A. Conformational energy calculations on enkephalins and enkephalin analogs. Classification of conformations to different configurational types. Int J Pept Protein Res. 1981 Sep;18(3):256–275. doi: 10.1111/j.1399-3011.1981.tb02980.x. [DOI] [PubMed] [Google Scholar]
- Marion D., Wüthrich K. Application of phase sensitive two-dimensional correlated spectroscopy (COSY) for measurements of 1H-1H spin-spin coupling constants in proteins. Biochem Biophys Res Commun. 1983 Jun 29;113(3):967–974. doi: 10.1016/0006-291x(83)91093-8. [DOI] [PubMed] [Google Scholar]
- Momany F. A. Conformational analysis of methionine-enkephalin and some analogs. Biochem Biophys Res Commun. 1977 Apr 25;75(4):1098–1103. doi: 10.1016/0006-291x(77)91495-4. [DOI] [PubMed] [Google Scholar]
- Motta A., Tancredi T., Temussi P. A. Nuclear Overhauser effects in linear peptides. A low-temperature 500 MHz study of Met-enkephalin. FEBS Lett. 1987 May 11;215(2):215–218. doi: 10.1016/0014-5793(87)80148-5. [DOI] [PubMed] [Google Scholar]
- Paine G. H., Scheraga H. A. Prediction of the native conformation of a polypeptide by a statistical-mechanical procedure. I. Backbone structure of enkephalin. Biopolymers. 1985 Aug;24(8):1391–1436. doi: 10.1002/bip.360240802. [DOI] [PubMed] [Google Scholar]
- Paine G. H., Scheraga H. A. Prediction of the native conformation of a polypeptide by a statistical-mechanical procedure. II. Average backbone structure of enkephalin. Biopolymers. 1986 Aug;25(8):1547–1563. doi: 10.1002/bip.360250812. [DOI] [PubMed] [Google Scholar]
- Pardi A., Billeter M., Wüthrich K. Calibration of the angular dependence of the amide proton-C alpha proton coupling constants, 3JHN alpha, in a globular protein. Use of 3JHN alpha for identification of helical secondary structure. J Mol Biol. 1984 Dec 15;180(3):741–751. doi: 10.1016/0022-2836(84)90035-4. [DOI] [PubMed] [Google Scholar]
- Purisima E. O., Scheraga H. A. An approach to the multiple-minima problem in protein folding by relaxing dimensionality. Tests on enkephalin. J Mol Biol. 1987 Aug 5;196(3):697–709. doi: 10.1016/0022-2836(87)90041-6. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. E., Rodriguez F. D., Sacristán M. P., Torres J. L., Valencia G., Garcia Antón J. M. New glycosylpeptides with high antinociceptive activity. Neurosci Lett. 1989 Jun 5;101(1):89–94. doi: 10.1016/0304-3940(89)90446-1. [DOI] [PubMed] [Google Scholar]
- Shaw J. S., Turnbull M. J. In vitro profile of some opioid pentapeptide analogues. Eur J Pharmacol. 1978 Jun 1;49(3):313–317. doi: 10.1016/0014-2999(78)90109-7. [DOI] [PubMed] [Google Scholar]
- Sherman S. A., Andrianov A. M., Akhrem A. A. Method of determining protein conformations by the two-dimensional nuclear Overhauser enhancement spectroscopy data. J Biomol Struct Dyn. 1987 Apr;4(5):869–884. doi: 10.1080/07391102.1987.10507684. [DOI] [PubMed] [Google Scholar]
- Spirtes M. A., Schwartz R. W., Mattice W. L., Coy D. H. Circular dichroism and absorption study of the structure of methionine-enkephalin in solution. Biochem Biophys Res Commun. 1978 Mar 30;81(2):602–609. doi: 10.1016/0006-291x(78)91578-4. [DOI] [PubMed] [Google Scholar]
- Torres J. L., Reig F., Valencia G., Rodríguez R. E., García-Antón J. M. [D-Met2,Pro5] enkephalin [N1.5-beta-D-glucopyranosyl] amide: a glycosylpeptide with high antinociceptive activity. Int J Pept Protein Res. 1988 May;31(5):474–480. doi: 10.1111/j.1399-3011.1988.tb00906.x. [DOI] [PubMed] [Google Scholar]



