Abstract
Purpose
Accurate urine assays for bladder cancer detection would benefit patients and health care systems. Through extensive genomic and proteomic profiling of urine components we previously identified a panel of 8 biomarkers that can facilitate the detection of bladder cancer in voided urine samples. In this study we confirmed this diagnostic molecular signature in a diverse multicenter cohort.
Materials and Methods
We performed a case-control, phase II study in which we analyzed voided urine from 102 subjects with bladder cancer and 206 with varying urological disorders. The urine concentration of 8 biomarkers (IL-8, MMP and 10, PAI-1, VEGF, ANG, CA9 and APOE) was assessed by enzyme-linked immunosorbent assay. Diagnostic performance of the panel of tested biomarkers was evaluated using ROCs and descriptive statistical values, eg sensitivity and specificity.
Results
Seven of the 8 urine biomarkers were increased in subjects with bladder cancer relative to those without bladder cancer. The 7 biomarkers were assessed in a new model, which had an AUROC of 0.88 (95% CI 0.84–0.93), and 74% sensitivity and 90% specificity. In contrast, the sensitivity of voided urine cytology and the UroVysion® cytogenetic test in this cohort was 39% and 54%, respectively. Study limitations include analysis performed on banked urine samples and the lack of voided urine cytology and cytogenetic test data on controls.
Conclusions
The study provides further evidence that the reported panel of diagnostic biomarkers can reliably achieve the noninvasive detection of bladder cancer with higher sensitivity than currently available urine based assays.
Keywords: urinary bladder neoplasms, urine, biological markers, molecular biology, diagnosis
With an estimated 70,980 newly diagnosed cases of BCa and 14,330 deaths from BCa in 2012 cancer of the bladder is the second most common genitourinary malignancy in the United States and among the 5 most common malignancies worldwide.1,2 Urothelial carcinoma, the most prevalent histological subtype, accounts for 90% of all BCa cases in the United States.3 When detected early, the 5-year survival rate of BCa is approximately 94% and, thus, timely diagnosis and intervention dramatically affect the outcome.4
Urine based assays that can noninvasively detect BCa have the potential to improve the rapid diagnosis of BCa as well as augment the invasive diagnostic modality of cystoscopy and bladder biopsy. However, current urine based assays for BCa detection have a limited role in the treatment of patients with BCa.5–7 Most commercially available assays assess the presence of only 1 molecular marker and so have limited accuracy for detecting BCa, especially in an indiscriminate patient population. Considering variation among individuals, crosstalk between molecular pathways and solid tumor heterogeneity, molecular diagnostic or classification signatures composed of multiple biomarkers are likely to be far more accurate than single markers.
Using a range of proteomic and genomic profiling approaches to extensively study voided urine samples, we previously identified a number of promising urine biomarkers and derived diagnostic signatures that show promise for the accurate detection of BCa in voided urine samples. RNA based and protein based signatures were previously validated.8,9 A patented panel of 8 protein biomarkers (IL-8, MMP-9 and 10, PAI-1, VEGF, ANG, CA9 and APOE) that can be measured simply by enzyme-linked immunosorbent assay achieved high accuracy for detecting BCa in a cohort of 127 subjects, including 64 with BCa.10 Previous studies of early results of urine biomarkers tended to lack the breadth of diverse benign urological conditions, eg urolithiasis, voiding symptoms, urinary tract infection and hematuria. This eventually resulted in adversely affecting the specificity of urine based assays in subsequent studies including these benign urological conditions. We tested the promising 8 biomarker panel in a larger, multicenter cohort that included a range of these benign urological conditions.
PATIENTS AND METHODS
This case-control phase II study was approved by the institutional review boards of Orlando Health and the Hospital Clinic, Universitat de Barcelona.
Specimen and Data Collection
Before manipulation or intervention voided urine samples were collected from patients who presented to the outpatient urology clinics at the 2 institutions. From each subject voided urine (50 ml) was collected in a sterile container and immediately refrigerated at the urology clinic. Within 2 hours urine samples were transported to the laboratory on ice. Each urine sample was assigned a unique identifying number before immediate laboratory processing. The sample was centrifuged at 600 × gravity at 4C for 5 minutes. Supernatant was decanted, aliquoted into 1 ml cryovials and stored at −80C before analysis at a genitourinary tissue bank, for which subjects provided written informed consent.
The 2 tissue banks were queried for suitable specimens for analysis, which included 308 subjects. The study cohort comprised 102 individuals with newly diagnosed, histologically confirmed, urothelial carcinoma only pathology and 206 without urothelial carcinoma, including 14 with urinary tract infection, 9 with gross hematuria, 44 with urolithiasis, 47 with moderate to severe LUTS, and 92 without a history of BCa and none of the mentioned benign conditions. According to the International Consensus Panel on Bladder Tumor Markers,7 this cohort served as a case-control phase II study of our diagnostic signature. Data are reported using STARD (Standards for the Reporting of Diagnostic Accuracy) criteria.11
A voided urine specimen from subjects with BCa and hematuria was sent to a clinical laboratory for VUC and the UroVysion cytogenetic test (111 and 74 samples, respectively). VUC was interpreted by 2 experienced in-house cytopathologists at each institution. A commercial company in the United States performed the cytogenetic tests. Subjects with BCa and hematuria underwent cystoscopy and axial imaging of the abdomen and pelvis, while subjects with urolithiasis and LUTS underwent cystoscopy alone. In the cancer group postoperative histological confirmation of urothelial carcinoma was recorded, including grade and stage. The table lists pertinent information on clinical presentations.
Urine ELISA
Levels of human IL-8 (ab46032), VEGF (100663, Abcam®), MMP-9 (DMP900), CA9 (DCA900), MMP-10 (DMP1000, R&D Systems®) PAI-1 (EA-0207, Signosis, Sunnyvale, California), ANG (CK400, Cell Sciences®), and human APOE (KA 1031, Abnova, Walnut, California) were monitored in urine samples using commercial ELISA according to manufacturer instructions. Calibration curves were prepared using purified standards for each assessed protein. Curves were fitted by linear or 4 parameter logistic regression according to manufacturer instructions. Due to the unavoidable variability of voided urine with respect to total volume and time in the bladder, each biomarker was normalized to urine creatinine and to urine protein.12 Laboratory personnel were blinded to the final diagnosis.
Data Analysis
We investigated the diagnostic performance of the 8 urine biomarkers for BCa detection. We used the Wilcoxon rank sum test to determine the association between each biomarker and BCa. For combinatorial analysis the goal was to identify the most accurate multivariate models overall and also define accurate models using a minimum number of necessary biomarkers. For model selection we applied the logistic regression procedure with BCa status (yes vs no) as the response variable, and the biomarkers as predictive variables. We used the all subset method to evaluate the predictive value of each possible biomarker subset.
The BIC13 was used to compare models. The BIC, a widely used criterion in model selection, balances the model likelihood and the number of biomarkers included in the model. As suggested by Austin and Tu,14 we applied the bootstrap method using 1,000 bootstrap samples to select the most efficient, stable model to predict the presence of BCa. For bootstrap sampling we used the stratification technique. Bootstrap samples were taken from subjects with BCa and without BCa separately. The 2 samples were together to form an overall bootstrap sample. The stratified sampling technique ensured that the number of subjects with and without BCa in a bootstrap sample were the same as in the original data set. For each bootstrap sample all candidate subsets of biomarkers were ranked from top to bottom by BIC. The subset of biomarkers that was ranked at the top most frequently among the 1,000 bootstrap samples was selected as the best combination of biomarkers to predict BCa.
After the predictive model was selected, we performed logistic regression analysis with the biomarkers in the model as explanatory variables. We used the regression line, a linear combination of biomarkers obtained on logistic regression analysis, to generate the nonparametric ROC curve.15 Using an arbitrary cutoff threshold, we defined that a diagnostic test was positive or negative when the linear combination of biomarkers was greater than or equal to, or less than the cutoff. For a given cutoff threshold we calculated test sensitivity and specificity. We generated the ROC curve by plotting values for sensitivity against the false-positive rate (1 – specificity) for various cutoff thresholds. The relative ability of the combination of select biomarkers to indicate BCa was estimated by calculating the AUROC with a higher AUC indicating a stronger predictor. We estimated the sensitivity and specificity of a combination of biomarkers at the optimal cutoff value defined by the Youden Index,16 ie the cutoff that maximizes the sum of sensitivity and specificity. The accuracy of a biomarker to predict BCa was defined as the average value of sensitivity and specificity. Statistical significance was considered at p <0.05 and reported p values are 2 sided. All analysis was done with SAS®, version 9.3.
RESULTS
Patient Characteristics
The table lists demographic, clinical and pathological characteristics of the 308 study subjects. As expected, the diverse nonBCa cohort, which included subjects with urolithiasis and urinary tract infection, known confounders of urine based diagnostic assays, was younger and comprised more women and minority subjects than the BCa cohort. VUC achieved 39% sensitivity and the cytogenetic assay achieved 54% sensitivity. Specificity could not be calculated since assays were not performed in all nonBCa cases.
In the cancer cohort all cancers were de novo. Of the subjects 60% had nonMIBC and 38% had low grade BCa. Clinical stage was Tis in 6 cases (6%), Ta in 41 (40%), T1 in 14 (14%) and T2 or greater in 41 (40%). Tumor grade was low in 38 cases (37%) and high in 64 (63%). Median tumor size was 3.0 cm. Median followup in the control cohort was 4 months and in the cancer cohort it was 14 months.
Urine Biomarker Levels
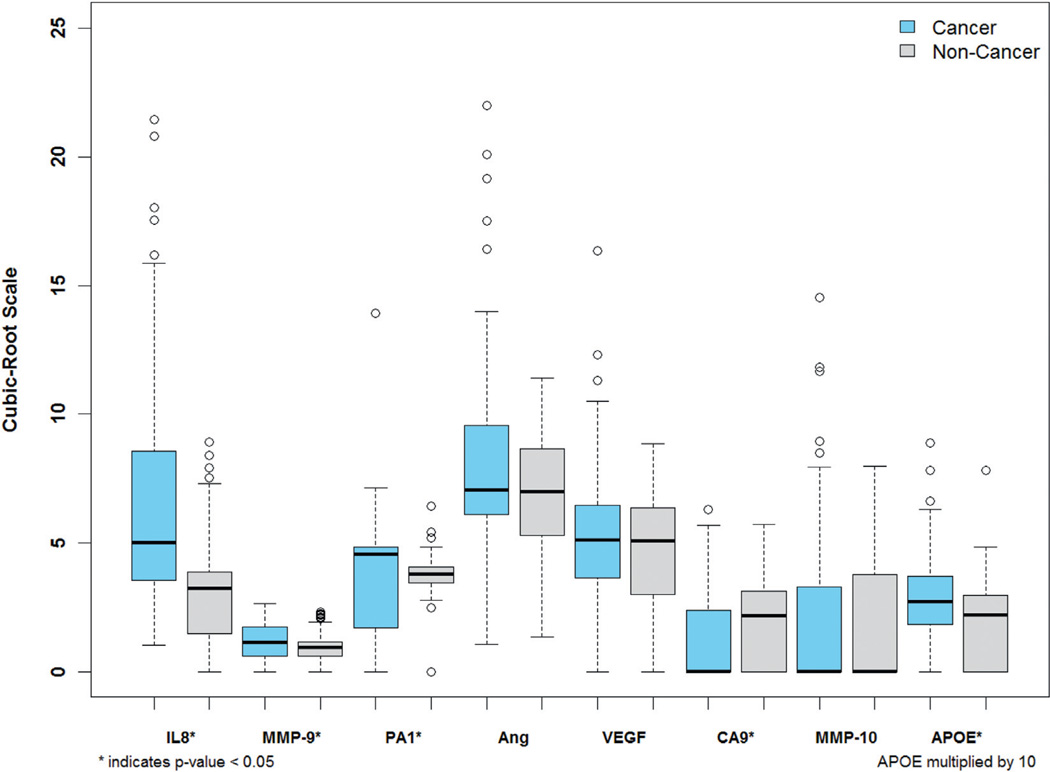
To overcome the variable urine volumes we normalized biomarker data to urine protein and urine creatinine.12 Since normalization to creatinine was more informative, we used it for subsequent analysis. Biomarker urine concentration was visualized as box plot data (fig. 1). The urine concentration of 6 biomarkers (IL-8, MMP-9 and 10, PAI-1, ANG and APOE) was significantly increased in subjects with BCa compared to controls. The observed VEGF increase in BCa cases was not statistically significant. Urine CA9 was significantly decreased in BCa cases. Biomarker levels were also compared with respect to tumor grade and stage. IL-8, MMP-9, PAI-1 and APOE were significantly increased in high compared to low grade tumors as well as in MIBC compared to nonMIBC (data not shown).
Figure 1.
Urine concentration of 8 biomarkers in BCa vs nonBCa groups normalized to urine creatinine. Cubic root transformation was applied to each biomarker value to decrease skewness. Horizontal lines indicate median. Significance was assessed by Wilcoxon rank sum test (p <0.05).
Model Development
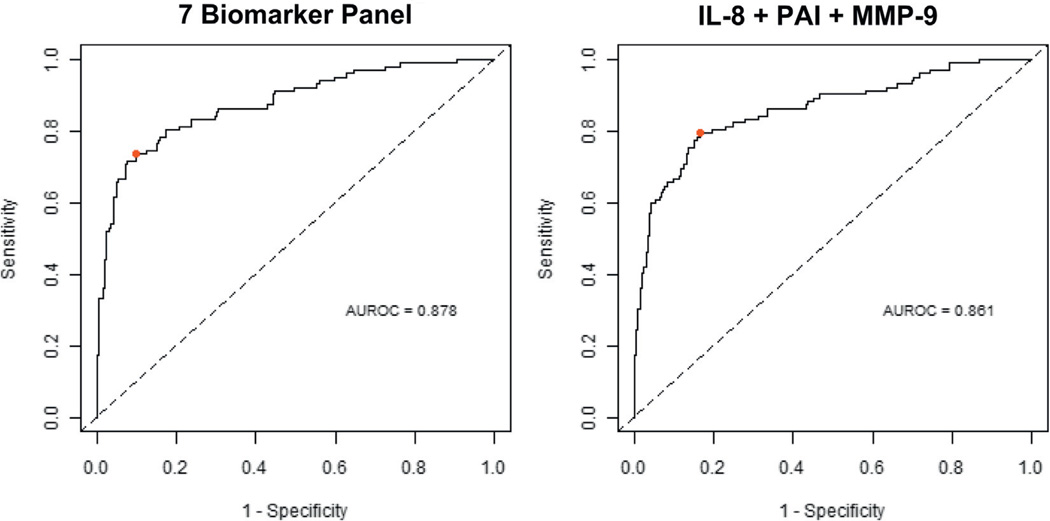
Since CA9 was negatively associated with BCa, we excluded this biomarker and used the remaining 7 for model selection. For these 7 biomarkers the AUROC was 0.88 (95% CI 0.84–0.93) with 74% sensitivity, 90% specificity, 79% positive predictive value and 87% negative predictive value (fig. 2). Biomarker data were evaluated using logistic regression to derive optimal multivariate diagnostic models that could achieve high accuracy with the minimum number of biomarkers. Analysis revealed that the combination of IL-8, PAI-1 and MMP-9 was most frequently ranked as the top multiplex model. The comparison of model performance was visualized using ROC curves (fig. 2). The AUROC for a model that used the 7 biomarkers and the AUROC for the 3-biomarker model (IL-8, PAI-1 and MMP-9) were essentially the same (0.88, 95% CI 0.84–0.92 and 0.86, 95% CI 0.81–0.91, respectively). Using the optimal cutoff values defined by the Youden Index16 calculation, the 3-biomarker model achieved 79% sensitivity, 84% specificity, 70% positive predictive value and 89% negative predictive value. The 7 and 3-biomarker signatures achieved significantly higher sensitivity than VUC (each p <0.001) and the cytogenetic assay (p <0.005 and <0.001, respectively).
Figure 2.
Diagnostic performance of urine biomarker combinations. ROC curves were plotted to compare diagnostic performance of 7 and 3 (IL-8, PAI-1 and MMP-9) biomarker signatures. Based on AUROC, Youden Index cutoffs (red circle) that maximized sum of sensitivity and specificity were determined for each combination.
DISCUSSION
The noninvasive detection and monitoring of BCa remains a challenge. VUC is the most often applied and established urine based assay for this purpose. However, while VUC has greater than 93% specificity, it has low sensitivity (25% to 40%) and observer dependent variability.5 Commercial Food and Drug Administration approved tests to measure nuclear matrix protein and bladder tumor antigen have emerged as diagnostic urine protein tests for BCa. Although these single marker assays are more sensitive than VUC, the latter continues to be more specific.17,18
The concept that the presence or absence of a single molecular marker would aid clinical evaluation has not proved to be the case. This is not surprising when considering variation among individuals, crosstalk between molecular pathways and solid tumor heterogeneity. A number of multiplex signatures have been derived and are being made commercially available as clinical assays, especially in the breast cancer field.19,20
A multiplex assay of a panel of BCa associated biomarkers would allow more accurate diagnosis of BCa in voided urine samples. Bartoletti et al previously reported that multiplex microsatellite analysis is an accurate, noninvasive, rapid, inexpensive assay for screening and monitoring superficial transitional cell carcinoma.21 However, to our knowledge we are the only group to date to extensively analyze voided urine samples by global genomics and global proteomics, integrate our data sets and derive a diagnostic signature that was subsequently confirmed in an independent cohort.10
Our current series further expands our study by testing the diagnostic signature in a large cohort partly composed of a diverse nonBCa cohort. Since these diverse, benign subjects adversely affect the accuracy of urine based assays, we were compelled to include such a cohort for analysis before any further development of our multiplex assay. Seven of the 8 urine biomarkers were increased in subjects with BCa relative to subjects without BCa, while CA9 was increased in those without BCa. This unexpected result may be explained by 2 factors. 1) The BCa group in the current study included a lower percent of de novo subjects with MIBC and high grade BCa than our initial cohort.11 2) It is feasible that CA9 would be increased in the urine of individuals with some of these benign conditions, decreasing its diagnostic potential for BCa.
Combinatorial analysis revealed that the 3-biomarker signature composed of IL-8, PAI-1 and MMP-9 generated an AUC of 0.860 for BCa detection (fig. 2). Although the AUC changed little from the 3 to the 7-biomarker signature (0.860 vs 0.880), the 7-biomarker signature was less prone to error and so was considered more robust. IL-8 was the most prominent biomarker (AUC 0.801, 95% CI 0.749–0.853) (data not shown). Increased urine IL-8 levels are associated with increased disease stage22 and recurrence.23 The other 2 biomarkers in our 3-biomarker signature (PAI-1 and MMP-9) were reported in BCa. Hudson and McReynolds noted that noninvasive and invasive human BCa cell lines produce PAI-1,24 while increased PAI-1 gene and protein expression in tissue and plasma was observed in patients with BCa and a poor prognosis.25 In addition, a study of 188 subjects showed that high MMP-9 levels correlated with large tumor size, higher tumor grade and decreased overall survival.26 The results of studies that measured urine MMP-2 and MMP-9 by ELISA and zymography suggest that MMP-9 may be useful as an adjunct to cytology.27
Clinically, accurate BCa assays can have a clear impact on initial diagnostic performance and BCa clinical management. If reliable urine diagnostic biomarker assays can decrease the number of invasive, uncomfortable cystoscopies, improved patient compliance and satisfaction would likely follow. Furthermore, the increased diagnostic efficiency and cost savings would benefit patients and health care systems. The ultimate goal is to detect BCa in a timely manner, such that the patient can expect improved survival as well as improved quality of life.
Our study has several limitations. Processed, banked urine samples were analyzed. We are currently investigating the performance of our select biomarkers in urine specimens processed by a number of protocols, including testing unprocessed, freshly voided urine. Furthermore, VUC results were not available for all nonBCa cases, limiting our ability to accurately report its true specificity as well as its false-positive rate in this cohort. Other maneuvers can be used to normalize the urine protein concentration to creatinine,28 which may be explored in subsequent studies. Additionally, the diagnostic sensitivity of the UroVysion cytogenetic assay in our study was lower than previously reported (54% vs 71%).29 The applicability of these assays outside our laboratory is also uncertain. Thus, we are now facilitating the confirmation of these results at an external laboratory. Lastly, it is impractical to assay a voided urine sample using 8 commercial ELISA kits. Therefore, we developed proprietary antibodies for our diagnostic signature and aim to incorporate them into a single diagnostic kit using ELISA or quantum dot technology.
CONCLUSIONS
In this case-control, phase II biomarker discovery study we validated and further refined a highly accurate diagnostic model that shows promise for development into a clinical assay to non-invasively diagnose BCa. If this multiplex assay is accurate and reliable in subsequent large, multicenter studies, it could conceivably be used to diagnose BCa and screen asymptomatic individuals at risk.
Demographic and clinicopathological characteristics of study subjects
| Control | |||||||
|---|---|---|---|---|---|---|---|
| BCa | Overall | Urinary Tract Infection | Hematuria | Urolithiasis | LUTS | Normal | |
| No. pts | 102 | 206 | 14 | 9 | 44 | 47 | 92 |
| Median age (range) | 69 (20–93) | 56 (18–89) | 59 (20–79) | 66 (38–78) | 51 (19–75) | 63 (19–89) | 51 (18–83) |
| No. male/female | 84/18 | 152/54 | 8/6 | 7/2 | 24/20 | 35/12 | 78/14 |
| No. race (%): | |||||||
| White | 91 (90) | 135 (66) | 12 (86) | 7 (78) | 31 (70) | 38 (81) | 47 (51) |
| Black | 5 (5) | 20 (10) | 1 (7) | 1 (11) | 4 (9) | 2 (4) | 13 (14) |
| Other | 6 (5) | 51 (24) | 1 (7) | 1 (11) | 9 (21) | 7 (15) | 32 (35) |
| No. pos fluorescence in situ hybridization/total No. (%) | 40/74 (54) | 2/22 (9) | 0/2 | 2/9 (22) | 0/1 | 0/3 | 0/7 |
| No. suspicious/pos cytology (%) | 37/94 (39) | 2/22 (9) | 0/2 | 2/9 (22) | 0/1 | 0/3 | 0/7 |
| Median followup (mos) | 14 | 4 | 5 | 1 | 4 | 5 | 3 |
Acknowledgments
Supported by research grants from the Flight Attendant Medical Research Institute (CJR), Florida Department of Health James and Esther King Team Science Award 10KT-01 (CJR) and National Cancer Institute RO1 CA116161 (SG).
Abbreviations and Acronyms
- ANG
angiogenin
- APOE
apolipoprotein E
- BCa
bladder cancer
- BIC
Bayesian information criterion
- CA9
carbonic anhydrase 9
- ELISA
enzyme-linked immunosorbent assay
- IL-8
interleukin-8
- LUTS
lower urinary tract symptoms
- MIBC
muscle invasive BCa
- MMP
matrix metalloproteinase
- PAI-1
plasminogen activator inhibitor 1
- VEGF
vascular endothelial growth factor
- VUC
voided urine cytology
Footnotes
Financial interest and/or other relationship with Nonagen Bioscience.
Study received approval from the Orlando Health and Hospital Clinic, Universitat de Barcelona institutional review boards.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Aben KK, Kiemeney LA. Epidemiology of bladder cancer. Eur Urol. 1999;36:660. doi: 10.1159/000020069. [DOI] [PubMed] [Google Scholar]
- 3.Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, et al. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol. 2000;164:680. doi: 10.1016/s0022-5347(05)67280-1. [DOI] [PubMed] [Google Scholar]
- 4.Herr HW. Tumor progression and survival of patients with high grade, noninvasive papillary (TaG3) bladder tumors: 15-year outcome. J Urol. 2000;163:60. [PubMed] [Google Scholar]
- 5.Tetu B. Diagnosis of urothelial carcinoma from urine. Mod Pathol, suppl. 2009;22:S53. doi: 10.1038/modpathol.2008.193. [DOI] [PubMed] [Google Scholar]
- 6.van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol. 2005;47:736. doi: 10.1016/j.eururo.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Lokeshwar VB, Habuchi T, Grossman HB, et al. Bladder tumor markers beyond cytology: International Consensus Panel on bladder tumor markers. Urology, suppl. 2005;66:35. doi: 10.1016/j.urology.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 8.Urquidi V, Goodison S, Cai Y, et al. A candidate molecular biomarker panel for the detection of bladder cancer. Cancer Epidemiol Biomarkers Prev. 2012;21:2149. doi: 10.1158/1055-9965.EPI-12-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang N, Feng S, Shedden K, et al. Urinary glycoprotein biomarker discovery for bladder cancer detection using LC/MS-MS and label-free quantification. Clin Cancer Res. 2011;17:3349. doi: 10.1158/1078-0432.CCR-10-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodison S, Chang M, Dai Y, et al. A multi-analyte assay for the non-invasive detection of bladder cancer. PLoS One. 2012;7:e47469. doi: 10.1371/journal.pone.0047469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Fam Pract. 2004;21:4. doi: 10.1093/fampra/cmh103. [DOI] [PubMed] [Google Scholar]
- 12.Reid CN, Stevenson M, Abogunrin F, et al. Standardization of diagnostic biomarker concentrations in urine: the hematuria caveat. PLoS One. 2012;7:e53354. doi: 10.1371/journal.pone.0053354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McQuarrie ADR, Tsai CL. Regression and Time Series Model Selection. Singapore: World Scientific Publishing; 1998. [Google Scholar]
- 14.Austin PC, Tu JV. Bootstrap methods for developing predictive models. Am Stat. 2004;58:131. [Google Scholar]
- 15.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 17.Grossman HB, Messing E, Soloway M, et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA. 2005;293:810. doi: 10.1001/jama.293.7.810. [DOI] [PubMed] [Google Scholar]
- 18.Heicappell R, Wettig IC, Schostak M, et al. Quantitative detection of human complement factor H-related protein in transitional cell carcinoma of the urinary bladder. Eur Urol. 1999;35:81. doi: 10.1159/000019822. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen B, Cusumano PG, Deck K, et al. Comparison of molecular subtyping with BluePrint, MammaPrint, and TargetPrint to local clinical subtyping in breast cancer patients. Ann Surg Oncol. 2012;19:3257. doi: 10.1245/s10434-012-2561-6. [DOI] [PubMed] [Google Scholar]
- 20.Malo TL, Lipkus I, Wilson T, et al. Treatment choices based on OncotypeDx in the breast oncology care setting. J Cancer Epidemiol. 2012;2012:941495. doi: 10.1155/2012/941495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartoletti R, Cai T, Dal Canto M, et al. Multiplex polymerase chain reaction for microsatellite analysis of urine sediment cells: a rapid and inexpensive method for diagnosing and monitoring superficial transitional bladder cell carcinoma. J Urol. 2006;175:2032. doi: 10.1016/S0022-5347(06)00283-7. [DOI] [PubMed] [Google Scholar]
- 22.Sheryka E, Wheeler MA, Hausladen DA, et al. Urinary interleukin-8 levels are elevated in subjects with transitional cell carcinoma. Urology. 2003;62:162. doi: 10.1016/s0090-4295(03)00134-1. [DOI] [PubMed] [Google Scholar]
- 23.Sagnak L, Ersoy H, Ozok U, et al. Predictive value of urinary interleukin-8 cutoff point for recurrences after transurethral resection plus induction bacillus Calmette-Guerin treatment in non-muscle-invasive bladder tumors. Clin Genitourin Cancer. 2009;7:E16. doi: 10.3816/CGC.2009.n.016. [DOI] [PubMed] [Google Scholar]
- 24.Hudson MA, McReynolds LM. Urokinase and the urokinase receptor: association with in vitro invasiveness of human bladder cancer cell lines. J Natl Cancer Inst. 1997;89:709. doi: 10.1093/jnci/89.10.709. [DOI] [PubMed] [Google Scholar]
- 25.Span PN, Witjes JA, Grebenchtchikov N, et al. Components of the plasminogen activator system and their complexes in renal cell and bladder cancer: comparison between normal and matched cancerous tissues. BJU Int. 2008;102:177. doi: 10.1111/j.1464-410X.2008.07568.x. [DOI] [PubMed] [Google Scholar]
- 26.Offersen BV, Knap MM, Horsman MR, et al. Matrix metalloproteinase-9 measured in urine from bladder cancer patients is an independent prognostic marker of poor survival. Acta Oncol. 2010;49:1283. doi: 10.3109/0284186X.2010.509109. [DOI] [PubMed] [Google Scholar]
- 27.Eissa S, Ali-Labib R, Swellam M, et al. Noninvasive diagnosis of bladder cancer by detection of matrix metalloproteinases (MMP-2 and MMP-9) and their inhibitor (TIMP-2) in urine. Eur Urol. 2007;52:1388. doi: 10.1016/j.eururo.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Newman DJ, Pugia MJ, Lott JA, et al. Urinary protein and albumin excretion corrected by creatinine and specific gravity. Clin Chim Acta. 2000;294:139. doi: 10.1016/s0009-8981(00)00181-9. [DOI] [PubMed] [Google Scholar]
- 29.Sarosdy MF, Schellhammer P, Bokinsky G, et al. Clinical evaluation of a multi-target fluorescent in situ hybridization assay for detection of bladder cancer. J Urol. 2002;168:1950. doi: 10.1016/S0022-5347(05)64270-X. [DOI] [PubMed] [Google Scholar]